Disorders of the brain-gut interaction and eating disorders
Mihaela Fadgyas Stanculete, Giuseppe Chiarioni, Dan Lucian Dumitrascu, Dinu Iuliu Dumitrascu, Stefan-Lucian Popa
Abstract
Key Words: Eating disorders; Dyspepsia; Constipation; Irritable bowel syndrome,Anorexia; Gastroparesis
INTRODUCTION
Eating disorders (ED) are psychiatric disorders defined by abnormal eating habits that negatively affect a person's physical or mental health and frequently begin in late childhood or early adulthood[1 ]. The pathogenesis of ED is still unclear, although it has been demonstrated that both genetic and environmental factors are involved. The most frequent ED include binge ED (eating a large amount of food in a short time),anorexia nervosa (eating an extremely small quantity of food due to a fear of gaining weight in contrast with low body weight in reality), bulimia nervosa (characterized by binge eating followed by purging, an attempt to get rid of the food consumed by vomiting or taking laxatives), pica (eating non-food items like hair, paper, sharp objects, metal objects, soil or glass), night ED (delayed circadian pattern of food intake)and avoidant/restrictive food intake disorder (eating only within an extremely narrow repertoire of foods)[1 ,2 ].
Epidemiological studies show that in the developed world, binge eating disorder affects about 1 .6 % of women and 0 .8 % of men, anorexia nervosa about 0 .4 %, and bulimia about 1 .3 % of young women[3 ,4 ]. At some point in their lifetime, more than 4 % of women have anorexia, 2 % have bulimia, and 2 % have a binge eating disorder. In less developed countries, the rates of ED are lower[3 ,4 ]. Females are nine times more frequent than males to suffer from ED. ED do not include obesity. Notwithstanding visible progress in the current therapeutic methods[4 ], ED result in more than 7000 deaths a year, covering the highest mortality rate in all mental illnesses.
Disorders of the brain-gut interaction (DGBI) previously known as functional gastrointestinal disorders, involve visceral hypersensitivity and motility disturbances of different parts of the gastrointestinal tract[5 ]. DBGI are defined by several variable combinations of chronic or recurrent gastrointestinal symptoms that do not have an identified underlying pathophysiology. In the absence of any biological marker or endoscopic modifications, the identification and classification of DGBIs is based on symptoms[6 ,7 ]. Patients suffering from DGBIs typically present with various symptoms as early satiety, postprandial fullness, bloating, nausea, emesis, and epigastric pain[6 -8 ]. Gastrointestinal motility disorders are the result of the dysfunction of the extrinsic nervous system, enteric nervous system, interstitial cells of Cajal(or intestinal pacemakers), or smooth muscle[6 -8 ]. The type of disorder in transit(delay or acceleration) and the region of the gastrointestinal tract affected are the main criteria that are used for the classification. The neural control of the gastrointestinal tract is a subject that is still generating interest and debates[9 ]. The close connections between the central nervous system and the enteric system are realized by ascending and descending fibers[10 ]. The ascending tract sends information from the digestive tube to the spine, hypothalamus, diencephalon and the cerebral cortex[9 ,10 ]. The descending tracts are responsible for the motility of the muscular layers of the digestive tract as well as glandular secretion[9 ]. A large part of the descending neurons has its origin in the prefrontal cerebral cortex[10 ]. This area is responsible for emotions and personality. Therefore, gastrointestinal motility is influenced by psychological factors[11 -14 ].
MATERIALS AND METHODS
A thorough literature search was undertaken. PubMed, Cochrane Library, EMBASE,and WILEY databases were screened for relevant publications regarding DGBI in ED.The search terms included: (“Eating disorders” OR “disorders of gut-brain interaction”OR “functional gastrointestinal disorders” OR “neurogastroenterological disorders”OR “gastrointestinal motility disorders”) AND (“anorexia nervosa” OR “bulimia nervosa” OR “binge eating disorders”) AND (“gastroparesis” OR “functional dyspepsia” OR “irritable bowel syndrome” OR “functional bowel disorders”).Inclusion criteria of original articles in this systematic review were as follows:Observational cohort population-based or hospital-based and case-control studies,examining the relationship between gastrointestinal disorders and ED. Exclusion criteria were: Studies written in languages other than English, abstracts, conference presentations, letters to the editor and editorials (Figure 1 ). The articles included in this review refer only to DGBI present in ED. Searches were carried out by two independent investigators.
RESULTS
We found 29 articles analyzing the relation between DGBI and ED comprising 13 articles regarding gastroparesis (Table 1 ), 5 articles on functional dyspepsia (FD)(Table 2 ), 7 articles regarding functional constipation (FC) (Table 3 ) and 4 articles on irritable bowel syndrome (IBS).
Gastroparesis
Gastroparesis is a neuromuscular disorder of the upper gastrointestinal tract characterized by delayed gastric emptying in the absence of mechanical obstruction of the stomach[15 ,16 ]. The clinical outcome of the delayed gastric emptying is a combination of symptoms, including early satiety, postprandial fullness, nausea, vomiting,belching, and bloating. The complicated overlap between upper gastrointestinal symptoms leads to a difficult diagnosis between gastroparesis and other DGBIs, such as FD, gastroparesis like syndrome and other organic gastrointestinal disorders[17 ]. A precise diagnosis requires measurement of gastric emptying using gastric scintigraphy, which is considered a gold standard diagnosis technique or using breath testing[15 -17 ].
The etiology of gastroparesis is multifactorial: Diabetes mellitus, infectious,connective tissue diseases, prior gastric surgery, visceral ischemia, myopathic diseases,neurological diseases (most frequent Parkinson disease), coma or artificial ventilation and medications (especially opiate narcotic analgesics and anticholinergic agents)[17 ].Nevertheless, the etiology cannot be established in more than 50 % cases, representing idiopathic gastroparesis. The pathogenesis of gastroparesis is also complex, and abnormalities in fundic tone, gastroduodenal dyscoordination, a weak antral pump,gastric dysrhythmias, abnormal duodenal feedback justify the reason why gastroparesis is considered a part of a broader spectrum of gastric neuromuscular dysfunction that includes impaired gastric accommodation[15 -17 ]. Epidemiological evidence is still lacking. The number of gastroparesis patients in the United States is estimated to be more than 4 million[17 ]. Gastroparesis is associated with ED. A study performed by Szmukleret al[18 ] investigated 20 patients presenting ED using gastric scintigraphy[18 ]. From a total of 20 patients, 8 had anorexia nervosa, 10 had both anorexia nervosa and bulimia nervosa, and 2 with bulimia nervosa alone. In this group, gastric half-emptying time (HET) showed a significant negative correlation with body mass and no correlation with age, gender, duration of illness or use of psychiatric medication. In the second part of the study, 12 patients with delayed gastric emptying were retested after one month, and HET had improved in 9 of 12 (P = 0 .0005 )[18 ]. Further, the normalization of the majority of scintigraphy parameters occurred while the body mass index was still subnormal (less than 20 .3 ) and with amenorrhea still present. The conclusion of the study was that delayed gastric emptying is present in ED and improves quite rapidly after restarting normal feeding. Therefore, the gastrointestinal motility disorder is secondary to insufficient food intake and is not the primary disorder.
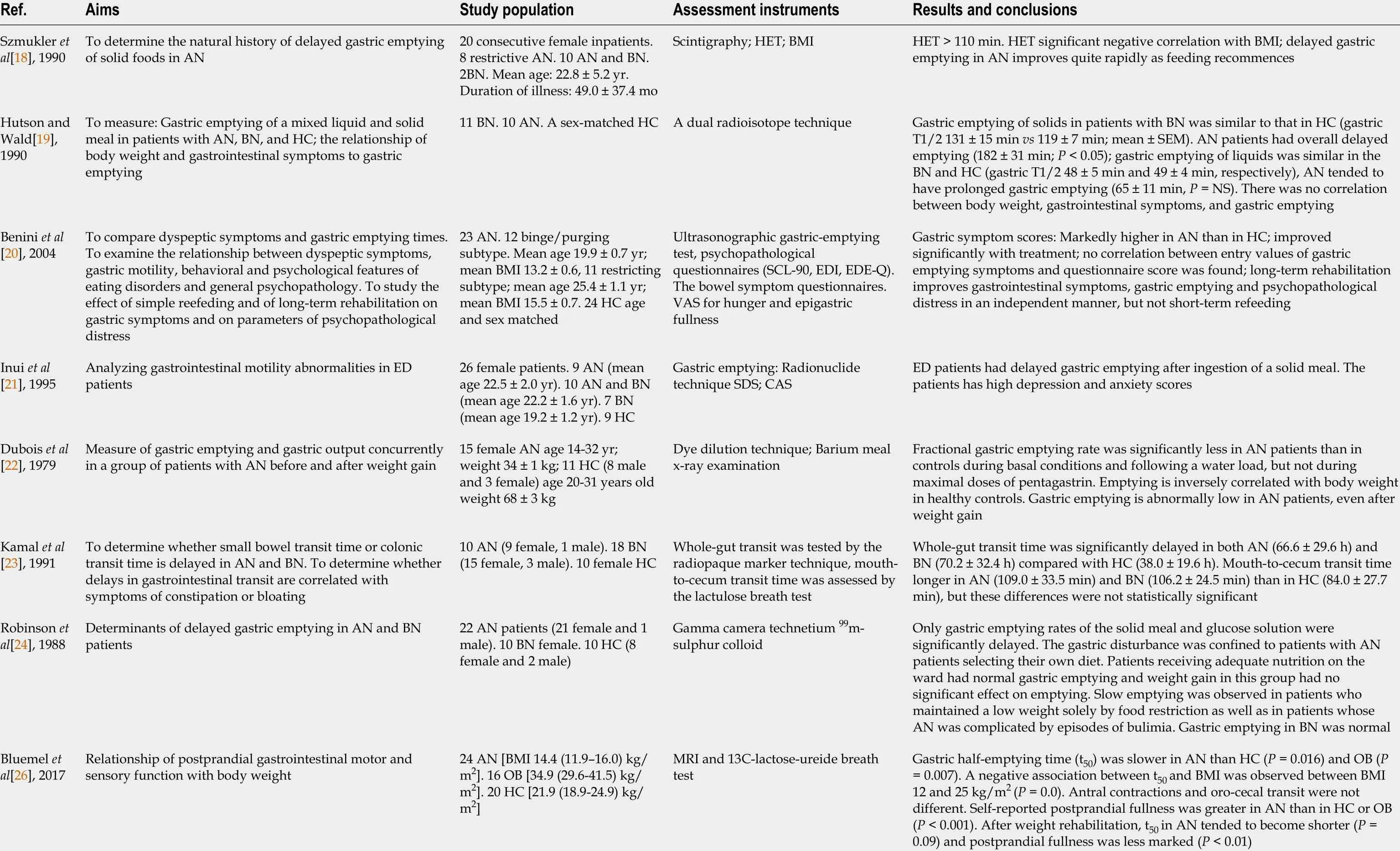
Table 1 Studies investigating the association between gastroparesis and eating disorders

GP: Gastroparesis; GI: Gastrointestinal; AN: Anorexia nervosa; BN: Bulimia nervosa; ED: Eating disorders; HC: Healthy controls; HET: Initial gastric half-emptying time; BMI: Body mass index; SCL-90 : Symptom Check List-90 ; EDI: Eating disorders inventory; EDE-Q: Eating disorders examination-questionnaire; VAS: Visual analogue scale; SDS: Self-rating depression scale; CAS: Cattell anxiety scale; OB: Obesity; MRI: Magnetic resonance imaging; DTPA:Diethylenetriaminepentacetic acid; GCSI: Gastroparesis cardinal symptom index; PAGI-SYM: Patient assessment of upper GI symptoms; GCSI: Gastroparesis cardinal symptom inventory; EDDS: Eating disorder diagnostic scale; FED:Feeding or eating disorder; NIAS: Nine item avoidant/restrictive food intake disorder survey; GES: Gastric emptying scintigraphy; ARFID: Avoidant/restrictive food intake disorder.
A study performed by Hutsonet al[19 ] analyzed gastric emptying of a mixed liquid and solid meal in 11 patients with bulimia nervosa and was compared with ten patients with anorexia nervosa and a sex-matched control population[19 ]. The authors of the study decided to use a dual radioisotope technique in order to measure gastricemptying. Similar to previous studies, the relationship between body mass index and gastrointestinal symptoms were also examined. Authors reported at gastric emptying of solids in patients with bulimia nervosa was similar to that in controls (gastric T1 /2131 ± 15 min vs 119 ± min; mean ± SEM) and anorexia nervosa patients had overall significantly delayed emptying (182 ± 31 min[19 ]. Gastric emptying of liquids was similar in bulimic patients and healthy controls[19 ]. The study did not find any correlation between body mass index, gastrointestinal symptoms, and gastric emptying, indicating that gastrointestinal symptoms are unreliable criteria of gastric emptying in patients with ED[19 ]. Although gastric scintigraphy is the gold standard technique for measuring gastric emptying, not all studies use it. A study performed byBeniniet al[20 ] analyzed 23 anorexic patients using an ultrasonographic gastricemptying test and psychopathological questionnaires, before and after 4 and 22 wk rehabilitation[20 ]. The result was that gastric symptom scores were markedly higher in patients than in controls and improved significantly with treatment. Further, no correlation between entry values of gastric emptying symptoms and questionnaire score was found[20 ]. The study summarized that long-term rehabilitation improves gastrointestinal symptoms, gastric emptying, and psychopathological distress, and short-term does not.

Table 2 Studies analyzing the association between functional dyspepsia and eating disorders
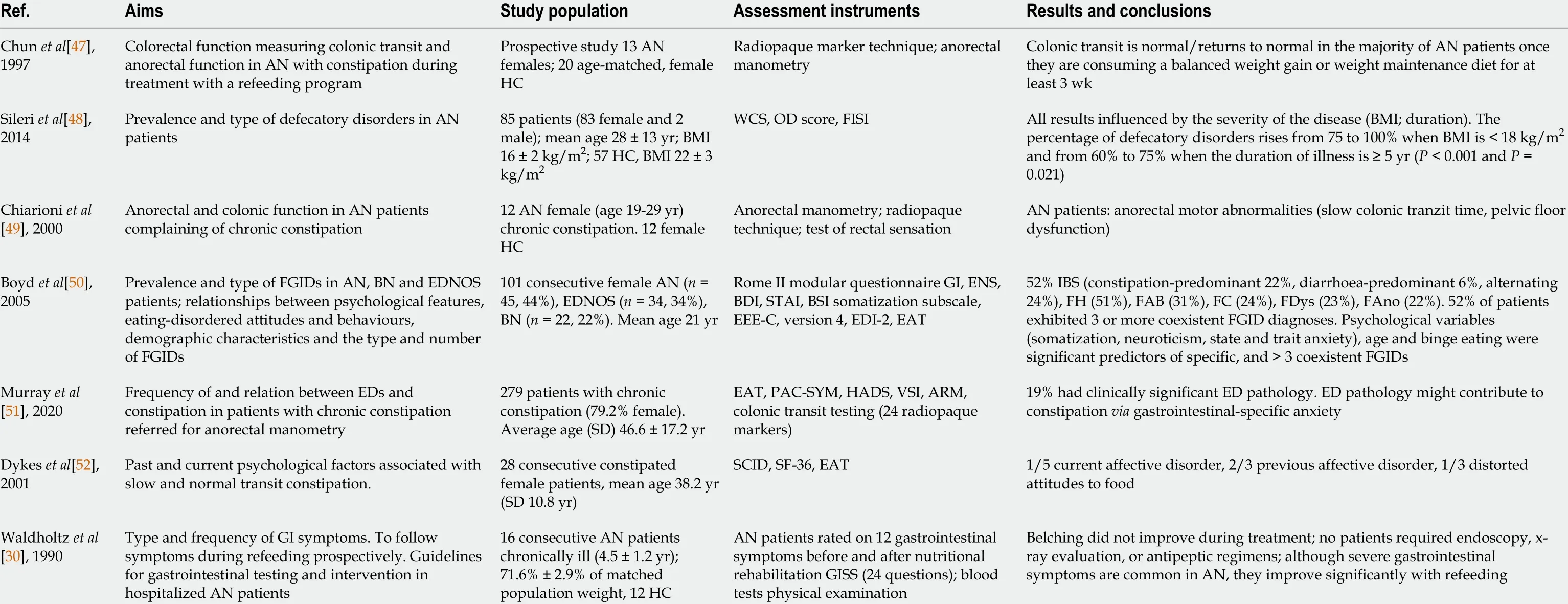
Table 3 Studies analyzing the association between functional constipation and eating disorders
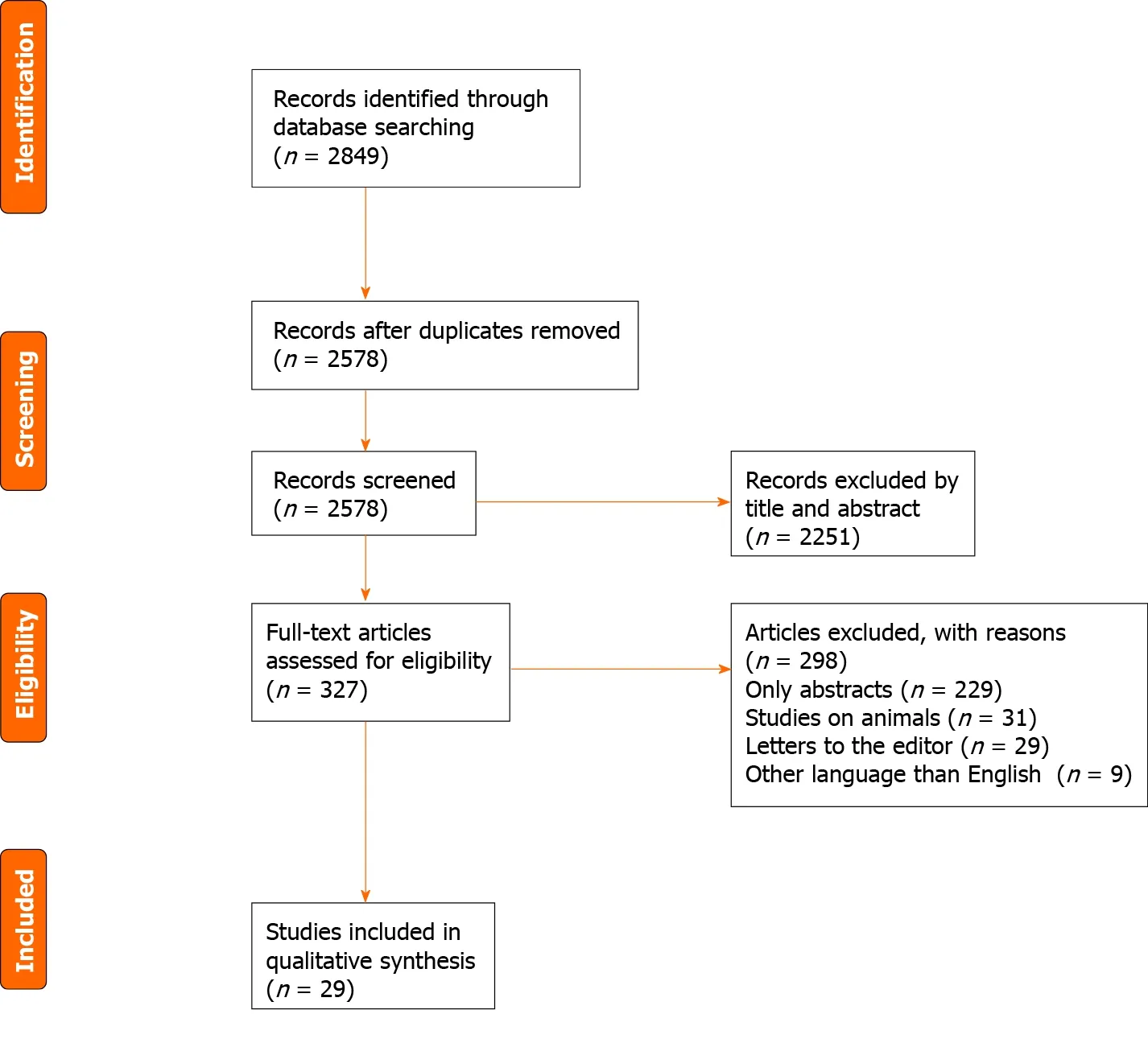
Figure 1 PRISMA flow diagram for study selection.
A study performed by Inuiet al[21 ] analyzed gastrointestinal motility abnormalities in 26 female patients who met the DSM-III-R criteria for ED[21 ]. Gastric emptying was measured using a radionuclide technique, and all patients were additionally evaluated using a self-rating depression scale and the Cattell anxiety scale. Nine patients were diagnosed with anorexia nervosa, 10 with anorexia nervosa and bulimia nervosa, and 7 with bulimia nervosa. In addition, the time expressed in minutes at which half the meal was emptied from the stomach was measured. Patients with anorexia nervosa,anorexia nervosa with bulimia nervosa, and bulimia nervosa all had delayed gastric emptying as compared to nine normal healthy controls and had delayed gastric emptying after ingestion of a solid meal, regardless of DSM-III-R classification[21 ]. The authors concluded that impaired gastric motility might be caused not only by food restriction or emesis but also by other independent factors from the patient nutritional state[21 ].
The intricate effect of depression and anxiety shows that gastroparesis pathogenesis is more complex, and psychotherapy may have an essential role in the nutritional rehabilitation of patients with ED. Anorexia nervosa and bulimia nervosa were the most frequent ED associated with gastroparesis, a fact demonstrated by numerous studies over the last 6 decades[22 -30 ]. Limited evidence regarding the association of gastroparesis and pica, night eating disorder and avoidant/restrictive food intake disorder was found[22 -30 ].
治疗后,2组患者的VAS评分较治疗前均显著降低(均P<0.05),观察组VAS评分更低于对照组(P<0.05)。见表2。
FD
FD is one of the most frequent DGBIs and is defined using the Rome IV criteria as any combination of the following symptoms: Postprandial fullness, early satiety, epigastric pain, and epigastric burning that are severe enough to interfere with the usual activities and occur at least three daysperweek over the past three months with an onset of at least six months before presentation[31 ].
FD includes three syndromes: (1 ) Postprandial distress syndrome; (2 ) Epigastric pain syndrome; and (3 ) Overlapping postprandial distress syndrome and epigastric pain syndrome[31 -33 ]. The pathophogensis of FD is multifactorial and incompletely understood. Dysfunctional gastrointestinal motility (antral hypomotility, delayed or rapid gastric emptying, impaired gastric accommodation), psychological stress,visceral hypersensitivity, psychiatric disorders (depressive disorder, anxiety disorder),gastric or duodenal hypersensitivity to specific types of food and gastric distension,have been incriminated as pathogenesis mechanisms[34 -44 ]. Smokers, women,patients with Helicobacter pylori infection, ED patients, and nonsteroidal anti-inflammatory drug users have an increased risk of developing FD[34 -44 ].
The dyspeptic symptoms may be encountered in ED. Thus, a study performed by Santonicolaet al[37 ] analyzed the prevalence of FD in ED patients, constitutional thinner subjects with no pathology, obese patients, and healthy volunteers[37 ]. The patients were recruited from a clinic specialized in treating ED, and the study groups included 20 anorexia nervosa patients, six bulimia nervosa patients, ten unspecified eating disorder patients, nine constitutional thinner subjects, 32 obese patients and 22 healthy controls[37 ]. The presence of epigastric pain syndrome and postprandial distress syndrome were diagnosed according to Rome III criteria. The intensity and frequency score of early satiety, epigastric fullness, epigastric pain, epigastric burning,epigastric pressure, belching, nausea, and vomiting were measured by a standardized questionnaire[37 ]. The result was that 90 % of anorexia nervosa patients, 83 .3 % of bulimia nervosa patients, 90 % of unspecified eating disorder patients, 55 .6 % of constitutionally thin subjects and 18 .2 % of the healthy volunteers met the postprandial distress syndrome criteria (χ2, P < 0 .001 ) and only one bulimia nervosa patient met the epigastric pain syndrome criteria[37 ]. Emesis was present in 100 % of bulimia nervosa patients, in 20 % of ED not otherwise specified patients, in 15 % of anorexia nervosa patients, in 22 % of constitutional thinner subjects, and, in 5 .6 % healthy volunteers (χ2 ,P< 0 .001 )[37 ]. The pathologic eating behavior causes dysfunctional gastrointestinal sensitivity and motility, and after a variable period of time, the resulting DGBIs can persist independently of the eating disorder that originally caused the motility dysfunction[38 ].
A study performed by Cremoniniet al[39 ] analyzed the gastrointestinal symptoms associated with binge ED[39 ]. A population-based survey of community residents through a mailed questionnaire was performed, and a total of 4096 subjects were included in the study. Binge eating disorder was present in 6 .1 % of subjects and was associated with the following gastrointestinal symptoms: Acid regurgitation,heartburn, dysphagia, bloating and epigastric pain, diarrhea, constipation and feeling the sensation of anal blockage[39 ]. The study demonstrated that both upper and lower gastrointestinal symptoms appear in binge ED.
Although the number of studies analyzing ED and FD is small, dyspeptic symptoms are more common in anorexia nervosa and bulimia nervosa patients.
A limitation of most studies regarding FD in ED is that a clear delimitation between meal-related gastrointestinal symptoms is lacking, and future studies analyzing the theoretical and clinical implications might help in developing a more efficient diagnosis and therapeutic scheme[40 ,41 ].
FC
FC is defined according to the Rome IV criteria as a change in bowel habit, or defecatory behavior that results in acute or chronic symptoms or diseases that would be resolved with the relief of constipation and a patient must have experienced at least two of the symptoms over the preceding three months[45 ,46 ]. FC is present also in ED.Thus, a study by Chunet al[47 ] analyzed the prevalence of FC in anorexic patients by measuring colonic transit and anorectal function[47 ]. The first study group consisted of 13 anorexic females, and the second study group consisted of 20 healthy female control subjects. Colonic transit was measured using a radiopaque marker technique,and anal sphincter function, rectal sensation, expulsion dynamics, and rectal compliance were measured with anorectal manometry[47 ]. The result showed that colonic transit returns to normal in the majority of patients after they start to finish a specialized refeeding program[47 ].
A study performed by Sileriet al[48 ] analyzed the prevalence of defecatory disorders in anorexic patients[48 ]. The Wexner constipation score (WCS), Altomare's obstructed defecation score (ODS), and the fecal incontinence severity index were used to evaluate constipation and incontinence of 83 female anorexic patients and 57 healthy volunteers. The result showed that constipation (defined as WCS ≥ 5 ) was present in 83 % of anorexic patients and in 12 % of healthy controls (P = 0 .001 ), while obstructed defecation syndrome (defined as ODS ≥ 10 ) was present in 84 % of anorexic patients and in 12 % of healthy controls (P < 0 .001 )[48 ].
A study performed by Chiarioniet al[49 ] evaluated the prevalence and pathogenetic mechanisms of FC in 12 female anorexic patients and 12 healthy female controls[49 ].Pelvic floor dysfunction was analyzed using an anorectal manometry, and colonic transit time was measured by a radiopaque marker technique.
A subgroup of 8 patients was retested after a specialized refeeding program[49 ].The results showed that 66 .7 % of anorexic patients had slow colonic transit times, and 41 .7 % had pelvic floor dysfunction. The specialized refeeding program normalized the colonic transit time in the subgroup of 8 patients, but pelvic floor dysfunction did not normalize in these patients[49 ]. The study demonstrated that anorectal motility dysfunctions and delayed colonic transit are frequent in anorexic patients, a result also shown by other studies[30 ,50 -52 ].
Although, the relation between FC and ET was analyzed in a limited number of studies, a significant association between the disorders was found.
IBS
IBS is one of the most frequent DGBIs characterized by abdominal pain and altered bowel habit in the absence of a specific organic pathology[53 ,54 ]. Epidemiologic studies show that the prevalence of irritable bowel syndrome is 10 %-20 % and the incidence of 1 %-2 % per year[53 ,54 ]. Perkins et al[55 ] performed a study about the prevalence of IBS in a large sample of ED patients analyzing the timing of onset of the ED symptomatology and assessing if they are predictors of IBS. The result of the study was that 64 % of ED patients met the Manning criteria for IBS and 87 % had developed their ED before the onset of IBS symptoms, with a mean period of time of 10 years between the onset of ED and IBS demonstrating that EDs increase the risk of IBS[55 ].
A study performed by Dejonget al[56 ] examined the prevalence of IBS in patients with bulimia nervosa and showed a high prevalence of IBS in the bulimia nervosa group (68 .8 %) [b]. IBS diagnosis was assessed using the Manning criteria and even if the study demonstrated a high incidence of IBS in patients with BN, the relationship between those disorders remain unclear[56 ]. A study performed by Sullivan et al[57 ]on 48 IBS patients, 32 ED patients, 31 inflammatory bowel disease (IBD) patients and 28 healthy controls analyzed the relationship between IBS, IBD and ED. The results showed that the eating Attitudes Test score for the IBS group was higher than IBD and control group (P= 0 .05 ), demonstrating the correlation between IBS and ED[57 ].
The relation between clinical characteristics of ED and IBS was investigated in a study performed by Tanget al[58 ] on 60 IBS patients. The result was that the severity of IBS symptomatology was correlated with Perfectionism and Ineffectiveness and severe episodes of emesis were associated with self-reported binge-purge behaviors measured by the Bulimia subscale of the EDI[58 ]. Bulimia nervosa was more common in IBS patients, but no data about the effect of psychiatric therapy on IBS symptoms was found.
DISCUSSION
In this review we looked for current evidence of the association of neurogastroenterological disorders with ED. We identified 29 studies, focusing on four gastrointestinal disorders associated with ED (gastroparesis, FD, FC and irritable bowel syndrome).Despite progress in the field of neurogastroenterology, we were not able yet to identify a causative relation between neurogastroenterological disorders and ED.
One of the strengths of this study is that it highlights the overlap between ED and neurogastroenterological disorders, a fact of paramount importance for the management of those patients who often are affected with both type of disorders, but,just as often, are treated for only one disorder, depending on whether they visit the psychiatrist or the gastroenterologist.
No relation between gastroesophageal reflux disease and ED was found[11 -14 ]. A limited number of studies investigated the rumination syndrome but we were not able to find supportive data about the association between rumination and ED.
The small number of patients included in the majority of studies represent the main limitation and future studies under the support of international collaboration might help in developing a more efficient therapy. Another limit of most studies regarding FD is that a clear delimitation between meal-related gastrointestinal symptoms is lacking, and future studies using an animal model might help in developing precise diagnosis tools[40 -44 ]. Anorexia nervosa and bulimia nervosa were the most frequent ED associated with FD[37 -39 ]. Most studies about FC showed that anorectal motility dysfunctions and delayed colonic transit are frequent in anorexic patients and anorexia nervosa was the most frequent eating disorder associated with FC[47 -53 ]. IBS was associated with anorexia nervosa and bulimia nervosa[55 -58 ].
Anorexia nervosa and bulimia nervosa were the most frequent ED associated with gastroparesis[18 -21 ]. A major limit in studies concerning gastroparesis was the fact that not all studies use gastric scintigraphy for diagnosis, although it is the gold standard technique for measuring gastric emptying. Thereby, a lack of a clear distinction between gastroparesis and FD was present[20 ,21 ,59 ].
Clear evidence for a cause effect relationship between ED and DGBI, still does not exist. More powerful studies are required. In respect to therapy of DGBI in ED, the absence of randomized controlled trials (RCTs) is the main reason why no guidelines for gastrointestinal symptomatology in ED exist. These limitations can be overcome by projecting larger RCTs with significant samples of ED and DGBI patients.
CONCLUSION
There is no evidence for a cause-effect relationship between DGBI and ED. Their common symptomatology required correct identification and a tailored therapy of each disorder.
ARTICLE HIGHLIGHTS
 World Journal of Gastroenterology2021年24期
World Journal of Gastroenterology2021年24期
- World Journal of Gastroenterology的其它文章
- COVID-19 and its effects on the digestive system
- Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: A systematic review and meta-analysis
- Stem cell injection for complex anal fistula in Crohn’s disease: A single-center experience
- Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis
- Chronic intestinal failure and short bowel syndrome in Crohn’s disease
- Early genetic diagnosis of clarithromycin resistance in Helicobacter pylori
