Study on the Plasticizing Properties of TMETN in Casting CMDB Propellants by Molecular Dynamics Simulation and Experiment
QU Bei,TANG Qiu-fan,ZHANG Ya-jun,ZHANG Zheng-zhong,ZHAO Yu,FAN Xue-zhong,FU Xiao-long,DENG Chong-qing,LI Ji-zhen
(Xi′an Modem Chemistry Research Institute,Xi′an 710065,China)
Abstract:In order to implement the application of trimethylolethane trinitrate (TMETN)in the casting composite modified double-base (CMDB)propellants to reduce propellant sensitivity,the interaction between different plasticizers (NG,TMETN and NG/BTTN)and NC was simulated by molecular dynamics,and the parameters such as gyration radius,volume distribution and radial distribution function were obtained.The simulation results were verified by curing experiments,mechanical properties and mechanical sensitivity tests.The results show that the interaction between NG and NC is the strongest and the gyration radius is the largest among the different plasticizers.The plasticizing property of TMETN is worse,so the assistant plasticizer should be added.The propellant with only TMETN can not be cured even at 75℃,while the propellant with mass ratio of 1∶1 for NG and TMETN can be cured well at 70℃.At the same time,TMETN can make the impact sensitivity of propellant up to 58.6 cm,and the friction sensitivity reduce to less than 40%,which is consistent with the theoretical calculation results of NC plasticizing effect in the order of NG>NG/BTTN>TMETN.
Keywords:CMDB propellants;TMETN;plasticization;sensitivity;molecular dynamics simulation
Introduction
With the development of high-cost weapon systems,the desire for insensitive munitions becomes much stronger/(urgent)in order to enhance their survivability[1].For decades,composite modified double base (CMDB)propellants have been widely used as power source of rockets and missiles[2].The formulation of CMDB propellants included nitrocellulose (NC)as binder matrix and nitroglycerin (NG)as an energetic plasticizer which displays extremely high sensitivity,making it vulnerable to be attacked in battlefields.It′s known that the application of insensitive energetic materials has been proved as an efficient way to reduce the sensitivity of CMDB propellants[3-4].Trimethylolethane trinitrate (TMETN)is a representative energetic plasticizer[5-6],owing to its similar molecular structure to that of nitrocellulose (NG),while the sensibility,hygroscopicity and toxicity of TMETN are much lower than those of NG[7].The insensitive and green extrusion double based propellants have been studied successfully by researchers[8-9].In these propellants,NG has been completely substituted by TMETN,and the sensitivity of the propellants obviously decreased.However,the application of TMETN in the casting CMDB propellants has not been investigated since the plastication of TMETN on NC plays an important role in the casting and curing process of CMDB propellants.Simulation is an quite useful method to predict the miscibility and compatibility of a polymer-solvent system[10].
In this study,both simulation and experimental methods were applied to investigate the interactions between NC and three plasticizers,including NG,TMETN,and mixed ester NG/1,2,4-butanetriol trinitrate (BTTN),respectively.Then the radius of gyration,volume distribution and radial distribution function have been studied to reveal the interactions between NC and different plasticizers,which can be useful to judge the feasibility of NG being replaced by TMETN in a casting CMDB propellant.The curing effect,mechanical properties and sensitivity of the propellants with different plasticizers were further tested by experiments to compare the plasticating effects of TMETN and those of NC,so the computational results were verified.This study can provide technical guidance and reference for insensitive casting CMDB propellants formulation design.
1 Methods
1.1 Simulation details
The molecular dynamics simulations were carried out using the Materials Studio 7.0 program package with the COMPASS force field[11-12].This force field is suitable for MD simulation of the nitrate ester plasticizers and binders,which is applicable for NG[13-14],NC[15-16]and BTTN[17]as the previous researches show.
The molecular structures of NG,TMETN,BTTN and NC were built with the Visualizer module,and then their structures were optimized by discover module using the Smart minimization method.The chemical structure of NG,TMETN,BTTN and NC were shown in Fig.1.
The molecular models of NG/NC,TMETN/NC and NG/BTTN/NC were prepared by amorphous cell with the mass ratios were 32∶28,32∶28 and 16∶16∶28,respectively.Then,these amorphous systems (NG/NC,TMETN/NC and NG/BTTN/NC)were subsequently optimized by a 5 000 step energy minimization to eliminate the useless contacts by smart minimization with convergence level of medium.Afterward,400ps MD equilibration on these amorphous systems was performed to obtain a module in isothermal-isobaric (NPT)ensemble.The temperature started from 293 K to 343 K with an increasing step of 50 K,and the pressure was kept at 101.325 kPa.The standard of the system to achieve equilibrium needs to consider the state of temperature and energy.When the fluctuation of temperature is within 10 K or the energy is invariable of small fluctuation around the average energy value,these systems were equilibrated.
1.2 Experiments
1.2.1Preparationofsamples
The CMDB propellants containing different plasticizers were prepared by casting technology and the basic formulations were shown in Table 1.

Table 1 The basic formulations of the casting CMDB propellants
1.2.2CuringExperiment
These casting CMDB propellant slurries were cured at different temperatures (65,70,75)for three days after mixing.
1.2.3Mechanicalpropertiestest
The mechanical properties of the casting CMDB propellants were measured by INSTRON 4 505 (USA)tensile tester.Cured propellants were cut into slices,from which JANNAF dog bones were stamped.The tests were carried out at temperatures of -40,+20 and +50℃ with 100 mm/min cross-head speed,respectively.
1.2.4Sensitivitiestest
The impact sensitivities of all propellants were tested by WL-1 impact sensitivity tester from Xi′an Modern Chemistry Research Institute.The drop hammer of 2 kg was used with a sample mass of 30 mg.The impact sensitivity was indicated by the 50% explosion probability of a sample (characteristic height,H50)with the impact of the drop hammer.The friction sensitivities were measured by WM-1 friction sensitivity tester from Xi′an Modern Chemistry Research Institute.The vector was 66° and the pressure was 2.45 MPa with a sample mass of 20 mg.The results were expressed by the explosion probability of the sample with friction.
2 Results and Discussion
2.1 Molecular models
The molecular models of NG/NC,TMETN/NC and NG/BTTN/NC were shown in Fig.2-Fig.4.From Fig.2(a)-Fig.4(a),the NC chains were more extended in the molecular models of NG/NC and NG/BTTN/NC than in the TMETN/NC model,which indicated that the plasticizing properties of NG and mixed ester NG/BTTN for the microstructure of NC were better than that of TMETN.In addition,the NC chains in Fig.2(b)were more expanded than the chains in Fig.2(a).When the temperature was increased from 293 K to 343 K,nearly all of the NC chains ran through the molecular models both in Fig.2 and Fig.4.This meant that the higher the temperature,the more likely the microstructure of NC in the plasticizing process would be affected by NG.However,in Fig.3(a)and Fig.3(b),the temperature had little effect on the microstructure of NC in the plasticizing process.In comparison to the systems of NG/NC and NG/BTTN/NC,the microstructures of NC at different temperatures changed slightly.A similar conclusion was found that the plasticizing properties of NG and NG/BTTN were better than that of TMETN.

Fig.2 Molecular models of NG/NC(The blue,red and yellow long chains are the three chains of NC)

Fig.3 Molecular models of TMETN/NC(The blue,red and yellow long chains are the three chains of NC)
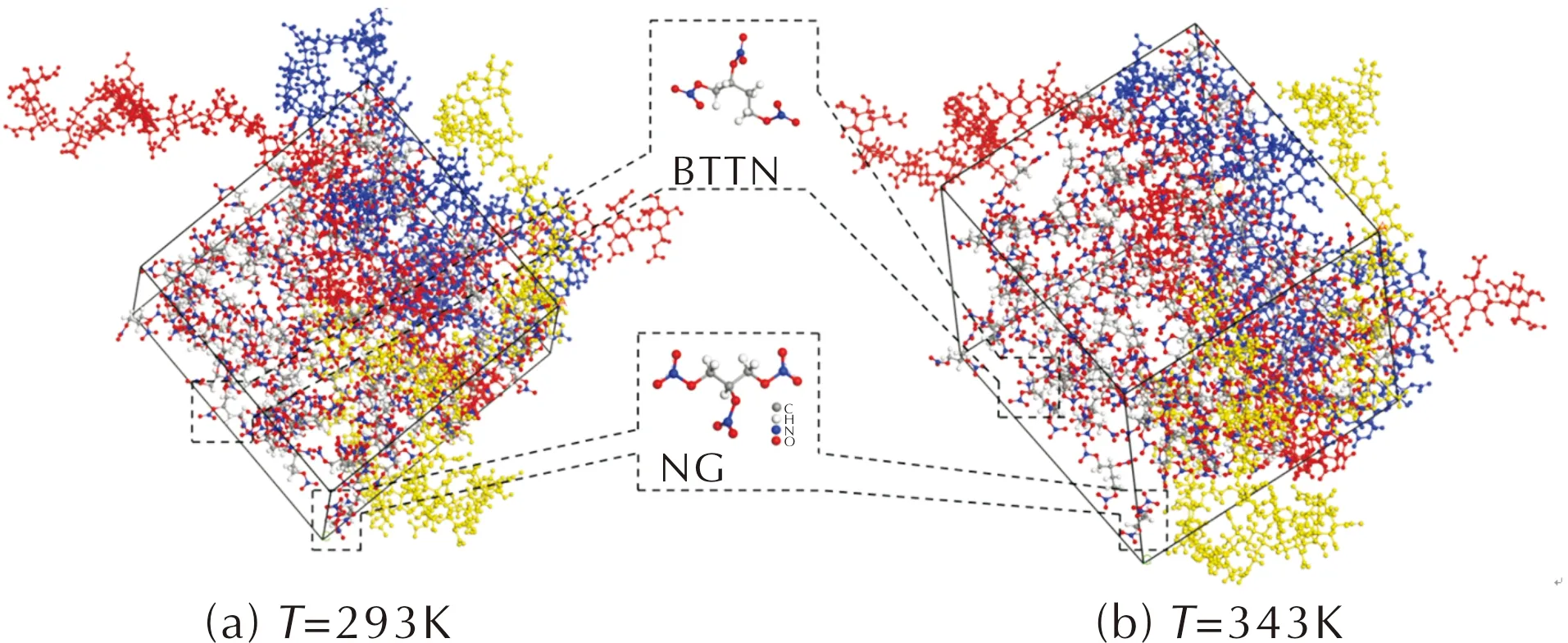
Fig.4 Molecular models of NG/BTTN/NC (The blue,red and yellow long chains are the three chains of NC)
2.2 Radius of gyration
The radius of gyration was defined as the distance between an axis and the point of maximum inertia in a rotating system.It is widely used in structural,mechanical and molecular simulations of energetic materials[18-19].In order to study the influences of different plasticizers on the microstructure of NC in the plasticizing process,the radius of gyration was calculated.The results were shown in Table 2.

Table 2 Gyration radius of NC at different models and temperatures
As a polymer,the flexibility and internal degrees of freedom of NC chains were limited by the intramolecular interactions.It was found that the radius of gyration was significantly increased by adding NG,NG/BTTN and TMETN,and the largest increase was adding NG at the same temperature in Table 2.Most radiuses of rotation were also increased with increasing the temperature.However,comparing with adding plasticizers,the temperature had less effect on the microstructure of NC in the plasticizing process.According to the changes in the radius of gyration,it could be inferred that the internal force in the NC chains could be weakened by rising temperature or adding plasticizers,such as NG,TMETN and NG/BTTN.Then the internal degree of freedom of NC chains was increased,which lead to an increase in the radius of gyrations.This meant that the increase of the radius of gyration reflected the degree of the interaction between NC and different plasticizers.Therefore,the interaction between NC and NG was strongest with the highest radius of gyration.
2.3 Volume distribution
The size distribution of free volume in energetic materials is accessible to estimate by molecular dynamics simulations and experimental studies by the probe methods[20-23].The occupied volumes of NC,NG,TMETN and BTTN were simulated,and the results were 7.778,0.161,0.204 and 0.183 nm3,respectively.The volume distributions of NC,NG,TMETN and BTTN were shown in Fig.5.The volume distribution of NG/NC,TMETN/NC and NG/BTTN/NC at different temperatures were shown in Fig.6 and the occupied volume and free volume of these molecules models were listed in Table 3.In Fig.6,the grey section was occupied volume (Vo)and the blue was free volume (Vf).

Fig.5 Volume distribution of NC,NG,TMETN and BTTN

Fig.6 Volume distribution of NG/NC,TMETN/NC and NG/BTTN/NC at different temperatures
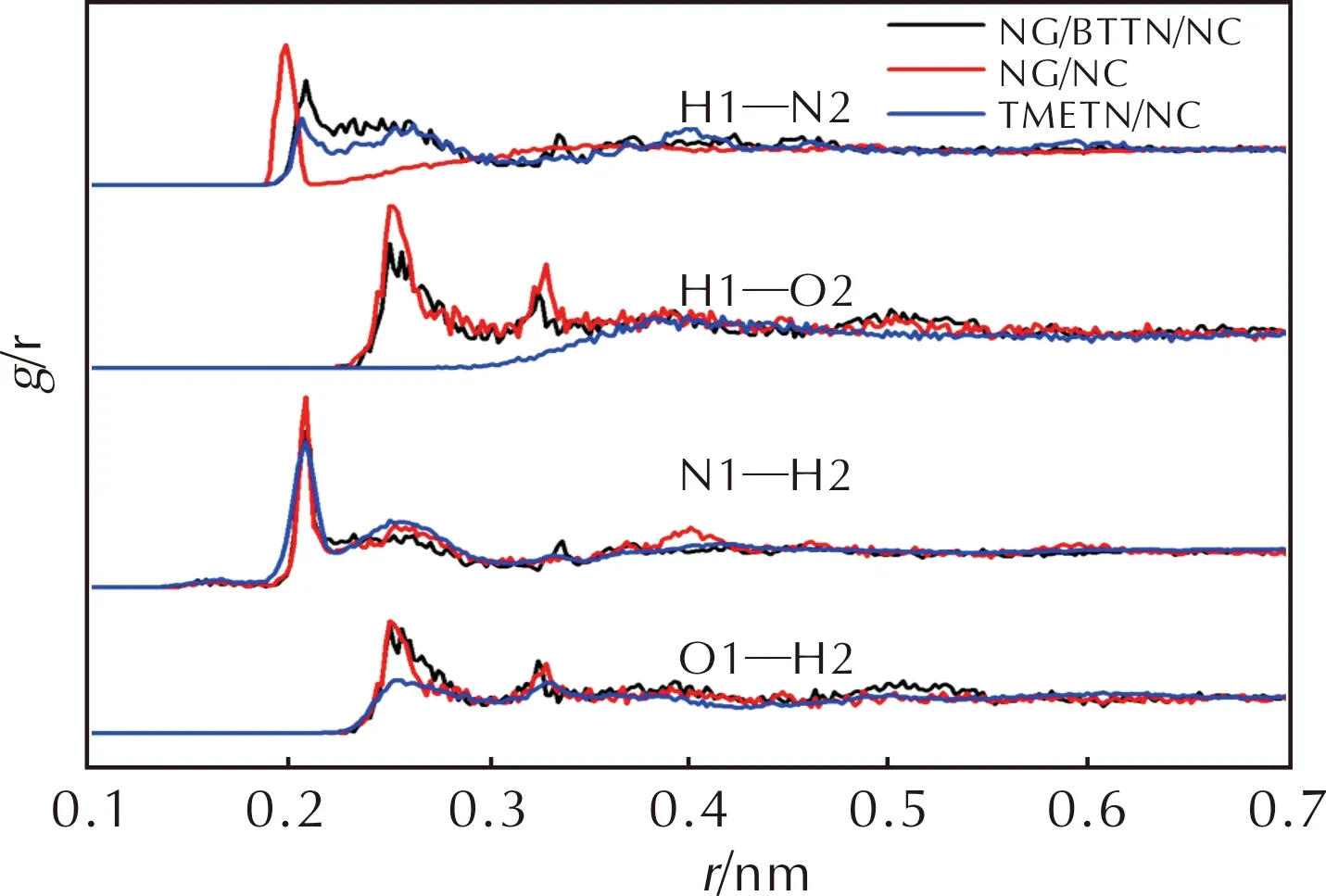
As can be seen from Fig.6 and Table 3,the occupied volumes of TMETN/NC blend are 56.878 nm3(293 K)and 57.273 nm3(343 K),higher than those of NG/NC and NG/BTTN/NC blends at the same calculating temperature.The reason is due to the occupied volume of TMETN being 0.204 nm3,higher than those of NG (0.161 nm3)and BTTN (0.183 nm3).It was worth noting that the free volume of TMETN/NC blend were 4.931 nm3(293 K)and 5.229 nm3(343 K),much lower than those of NG/NC and NG/BTTN/NC blends at the same temperature.It indicated that the plasticizing ability of TMETN for NC was bad,because the higher the free volume is,the more space the chains segments of NC have for rotation,jumping or slipping.As a result,the order of the plasticizers for the plastication of NC was TMETN Table 3 Occupied volume and free volume of molecular models at different temperatures The radial distribution function (RDF)is a useful physical tool to describe the structure of a system because it gives a measure of the probability densityg(r)of finding a particle in the distance r from another particle[24-25].To further reveal the interactions between NC chains and different plasticizers,the RDF had been analyzed.Here,two kinds of atom pairs (H…O and H…N)were considered.H,O and N (negatively charged)atoms in NC molecules are denoted as H(1),O(1)and N(1)and those in different plasticizers molecules were named as H(2),O(2)and N(2),respectively.The RDF for H…O and H…N atomic pairs in the NG/NC,TMETN/NC and NG/BTTN/NC blends at different temperatures were shown in Fig.7. Fig.7 The RDF for H…O and H…N atomic pairs in the NG/NC,TMETN/NC and NG/BTTN/NC blends at different temperatures Generally,the interaction distance range (r)for a hydrogen bond was 0.20—0.31 nm and for strong van der Waals (vdW)interaction was 0.31—0.50 nm.Whenrwas longer than 0.50 nm,vdW interaction was very weak[26].In Fig.7,the RDF curves of NG/NC,TMETN/NC and NG/BTTN/NC blends show a sharp strong peak at a distance of 0.2 nm for atom pairs of H1…N2 and N1…H2,0.26 nm for atom pairs of H1…O2 and O1…H2,which were within the range of hydrogen bonds.Besides,there was a sharp peak at a distance of 0.33 nm for atom pairs of H1…O2 and O1…H2 in NG/NC and NG/BTTN/NC blends,belonging to the strong vdW force.Other wide peaks occurred at a farther distance for atom pairs of H1…N2 and N1…H2,respectively,within the range of vdW forces.As a summary,the N atoms in NC,NG,BTTN and TMETN had a great influence on the intermolecular interactions when NC was blended with these plasticizers.Moreover,by comparing the same pairs,the g(r)of NG/NC were higher than those of TMETN/NC and NG/BTTN/NC in all pairs.It is demonstrated that there was a stronger interaction between NG and NC,just in agreement with the former discussion. The dynamic mechanical properties can be used to judge the plastication of different plasticizers for NC.It can also be applied to compare the plastication effect between different plasticizers in the plasticizing process for a polymer-plasticizer system.In order to judge the accuracy of the simulation,the CMDB propellants containing different polymer-plasticizer systems were prepared by casting technology and the slurries of the propellants S-2 and S-3 were cured at 65℃,70℃ and 75℃,respectively.Because the NG/NC and NG/BTTN/NC systems were mature formulation systems in the casting CMDB propellants,the propellants S-1 and S-4 were cured at 70℃ for comparison.The sections of the blocks of all the cured propellants were shown in Fig.8. Fig.8 The section of different cured propellants The slurries of propellants S-2,S-3 at 65℃ and propellant S-2 at 70℃ were no curing.The propellant components distributed unevenly on the sections of the propellant S-3 at 70℃ and the propellant S-2 at 75℃ illustrated in Fig.8(b)and Fig.8(d),which indicated that these propellants were incompletely solidified.It was demonstrated that the propellant with TMETN as the sole plasticizer could not cure even if the temperature rose to 75℃.Therefore,TMETN could not be used in casting CMDB propellants alone as a plasticizer.However,Fig.8(e)showed an even-distributed surface of the propellant S-3 at 75℃,indicating that the propellant with the mixed ester NG/TMETN could be well cured by rising temperature to more than 70℃.This result was consistent with the conclusion of the theoretical simulation.The plasticizing properties of NG and NG/BTTN were much better than that of TMETN.Therefore,if TMETN is applied in the casting CMDB propellants,it is necessary that NG should be added as a secondary plasticizer or the curing process should be optimized by raising the curing temperature. 2.6.1Mechanicalproperties Mechanical properties of the casting CMDB propellants can judge the plastication effect of different plasticizers for NC.The plasticizing properties of a plasticizer will increase when the mechanical properties of the propellants were enhanced.The mechanical properties of propellants S-1,S-3 S-4 were tabulated in Table 4.From Table 4,it can be seen that the mechanical properties of propellant S-1 and S-4 with ester NG and NG/BTTN as plasticizers were better than that of propellant S-3 with mixed ester NG/TMETN as a plasticizer.This was because the plasticizing properties of TMETN/NC were not as excellent as that of NG/NC,which is in good agreement with the simulation results. Table 4 The mechanical properties of propellants 2.6.2Mechanicalsensitivities The mechanical sensitivity results of the propellants S-1,S-3,S-4 were tabulated in Table 5.From Table 5,it can be seen that,compared with those of S-1,S-4,both impact and friction sensitivities of S-3 decrease distinctly,owing to the order of the sensitivities of the plasticizers was:NG>NG/BTTN>NG/TMETN.Therefore,it was demonstrated that partial replacing NG with TMETN in the casting CMDB propellants can obviously decrease the mechanical sensitivities.TMETN can be used as the main plasticizer in the CMDB propellants by optimizing the process parameters,such as adding a secondary plasticizer or raising the curing temperature. Table 5 The mechanical sensitivities of propellants (1)Molecular dynamics simulations and experimental study are applied to investigate the interactions between NC and three plasticizers,including NG,TMETN,and mixed ester NG/BTTN.It was found that the plasticizing property of TMETN was worse than those of NG and NG/BTTN for NC by MD simulations. (2)The propellant with TMETN as sole plasticizer could not be cured and was confirmed further in the curing experiment.However,this problem can be solved by adding NG or increasing the curing temperature.TMETN can be used as the main plasticizer in the CMDB propellants by adding a secondary plasticizer or optimizing the parameters in the curing process,and the mechanical sensitivities of the propellants can be significantly reduced.These findings were of great significance to the development of insensitive casting CMDB propellants.
2.4 Radial distribution function
2.5 Curing experiment

2.6 Properties of propellants


3 Conclusion

