MPTP诱导PD小鼠黑质及前额叶皮质铁相关蛋白表达变化
宋立梅 肖志新 谢俊霞 徐华敏
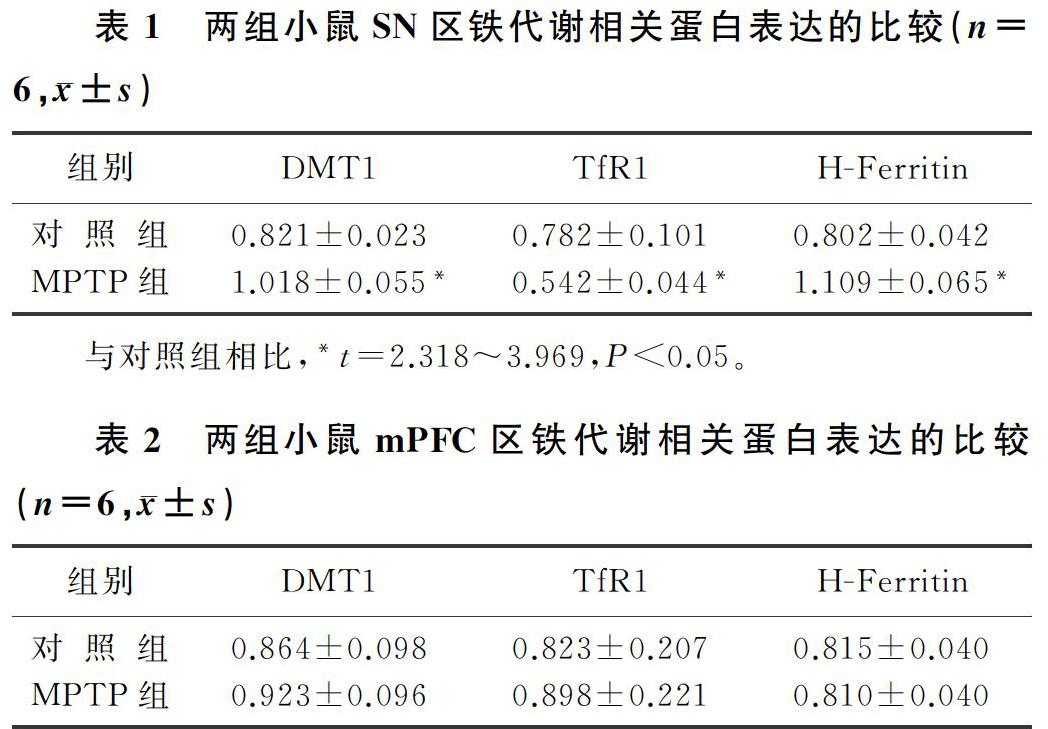
[摘要]目的 研究1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)诱导的帕金森病(PD)小鼠模型黑质(SN)及内侧前额叶皮质(mPFC)铁代谢相关蛋白的表达变化。方法 将12只9周龄C57BL/6J雄性小鼠随机分为对照组和MPTP组。对照组小鼠给予生理盐水腹腔注射,MPTP组小鼠给予MPTP 30 mg/kg腹腔注射,5 d后进行爬杆实验检测小鼠的运动协调能力,Western Blot检测铁代谢相关蛋白的表达变化。结果 爬杆实验结果显示,与对照组相比,MPTP组小鼠转头时间和下杆时间均明显增加,差异有统计学意义(t=2.420、3.464,P<0.05)。Western Blot结果显示,与对照组相比,MPTP组小鼠SN区的重链铁蛋白(H-Ferritin)和二价金属离子转运蛋白1(DMT1)表达明显增加(t=3.969、3.333,P<0.01),转铁蛋白受体1(TfR1)表达明显下降(t=2.318,P<0.05);两组mPFC区的DMT1、TfR1和H-Ferritin表达差异均无显著性。结论 MPTP能够改变小鼠SN区的铁代谢相关蛋白的表达,但并不影响mPFC区铁代谢相关蛋白的表达。
[关键词]帕金森病;1-甲基-4-苯基-1,2,3,6-四氫吡啶;铁蛋白质类;受体,转铁蛋白;额叶前皮质;黑质;小鼠
[中图分类号]R338.2
[文献标志码]A
[文章编号]2096-5532(2021)02-0198-04
[ABSTRACT]Objective To investigate the changes in the expression of iron metabolism-related proteins in the substantia nigra (SN) and the medial prefrontal cortex (mPFC) of mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson disease (PD). Methods A total of 12 male C57BL/6J mice, aged 9 weeks, were randomly divided into control group and MPTP group. The mice in the control group were given intraperitoneal injection of normal saline, and those in the MPTP group were given intraperitoneal injection of MPTP 30 mg/kg. After 5 days, the pole test was used to observe motor coordination ability, and Western blot was used to measure the expression of iron metabolism-related proteins. Results The results of the pole test showed that compared with the control group, the MPTP group had significant increases in the time to turn around and the time to climb down the pole (t=2.420,3.464;P<0.05). The results of Western blot showed that compared with the control group, the MPTP group had significant increases in the expression of heavy-chain ferritin and divalent metal transporter 1 (DMT1) (t=3.969,3.333;P<0.01) and a significant reduction in the expression of transferrin receptor 1 (TfR1) (t=2.318,P<0.05) in the SN of mice, while there were no significant differences between the two groups in the expression of DMT1, TfR1, and heavy-chain ferritin in the mPFC of mice. Conclusion MPTP can change the expression of iron metabolism-related proteins in the SN of mice, but it does not affect the expression of iron metabolism-related proteins in the mPFC of mice.
[KEY WORDS]Parkinson disease; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; ferritins; receptors, transferring; prefrontal cortex; substantia nigra; mice
帕金森病(PD)是中老年人神经退行性疾病中仅次于阿尔兹海默病的第二大神经系统疾病,其主要的临床表现为运动障碍,主要的病理变化为黑质(SN)多巴胺能神经元受损,但其病因和发病机制目前尚未完全阐明。众多研究结果已证实,铁代谢障碍参与了PD的发病[1-3]。1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)是由1-甲基-4-苯基-4-丙氧基哌啶(MPPP)合成的副产物[4]。MPTP本身不具有毒性,但进入大脑后在单胺氧化酶B(MAO-B)的作用下转化成具有毒性的甲基-苯基-吡啶离子(MPP+),被多巴胺转运体(DAT)特异性转运到SN多巴胺能神经元内诱导PD的发生[5-8]。有研究表明,MPTP能够诱导小鼠SN铁的沉积[9]。最新研究发现,脑内铁运输具有两条途径,其中一条途径为腹侧海马(vHip)到内侧前额叶皮质(mPFC)再到SN[10]。但是,此途径异常是否是导致PD模型SN区铁沉积的重要原因,目前尚未有报道。本研究采用MPTP诱导PD小鼠模型,检测其SN区及mPFC区铁代谢相关蛋白的表达变化。
1 材料与方法
1.1 实验材料
1.1.1 实验动物 SPF级8周龄雄性C57BL/6J小鼠購自北京维通利华实验动物公司。饲养条件:温度(21±2)℃,湿度(50±5)%,12-12 h循环昼夜光照,自由饮水进食。实验前适应实验室环境1周。
1.1.2 实验药品 MPTP(Sigma-Aldrich),用生理盐水稀释成6 g/L,注射量为30 mg/kg。
1.2 实验方法
1.2.1 动物分组及处理 将12只雄性C57BL/6J小鼠随机分为对照组和MPTP组,每组6只小鼠。MPTP组小鼠连续5 d给予MPTP 30 mg/kg腹腔注射构建亚急性PD动物模型,对照组小鼠给予等量生理盐水腹腔注射。
1.2.2 爬杆实验 自制直径1.2 cm、高50 cm的直木杆,杆顶部有一小木球,用纱布包裹防止小鼠打滑。实验前1 d训练小鼠,实验时在安静的实验环境下将小鼠头向上放于爬杆顶端,用秒表记录小鼠开始运动至完全转为头向下的时间(转头时间)和小鼠爬下杆至四肢落地的时间(下杆时间)。每次检测间隔1 min,共检测5次,取平均值。
1.2.3 Western Blot检测SN和mPFC区铁代谢相关蛋白的表达变化 行为学检测结束后,给予小鼠联合麻药(水合氯醛+乌拉坦)40 g/L腹腔注射麻醉,在冰上迅速解剖SN和mPFC区,将剖出组织按每4 mg加入100 μL裂解液充分研磨后冰上裂解30 min,4 ℃下以12 000 r/min离心20 min,提取上清。用BCA蛋白测定试剂盒测定蛋白浓度。按1∶4加入Loading buffer,金属浴100 ℃煮5 min。蛋白经 SDS-PAGE 电泳(电压80~120 V)后湿转至 PVDF膜上,用含有50 g/L脱脂奶粉的TBST溶液室温封闭2 h,再分别加入二价金属离子转运蛋白1(DMT1,1∶800)、转铁蛋白受体1(TfR1,1∶1 000)、铁蛋白重链(H-Ferritin,1∶1 000)和β-actin(1∶10 000)一抗,4 ℃摇床过夜。加HRP偶联的二抗(用TBST稀释至1∶10 000),室温孵育1 h。用UVP BioDoc-It成像系统(美国Upland)和ECL高灵敏化学发光液试剂盒(Milipore)显影,采用Image J分析软件进行灰度值分析。
1.3 统计学分析
应用SPSS 18.0软件进行统计学分析,计量资料结果以x2±s表示,两组数据间比较用Students t检验,P<0.05表示差异有统计学意义。
2 结 果
2.1 MPTP对小鼠运动功能的影响
爬杆实验结果显示,对照组和MPTP组小鼠转头时间分别为(1.925±0.182)和(3.242±0.513)s,下杆时间则分别为(6.001±0.396)和(9.872±1.045)s,MPTP组小鼠转头时间和下杆时间与对照组相比较均明显增加,差异具有统计学意义(t=2.420、3.464,P<0.05)。
2.2 MPTP对小鼠SN区铁代谢相关蛋白表达的影响
本文实验结果表明,与对照组相比较,MPTP组小鼠SN区H-Ferritin、DMT1蛋白的表达明显增加(t=3.969、3.333,P<0.01),TfR1蛋白的表达明显下降(t=2.318,P<0.05)。见表1。
2.3 MPTP对小鼠mPFC区铁代谢相关蛋白表达的影响
两组小鼠mPFC区DMT1、TfR1和H-Ferritin蛋白表达比较,差异均无显著性(t=0.096~0.4279,P>0.05)。见表2。
3 讨 论
PD是中老年人常见的神经系统变性疾病,其临床特征为静止性震颤、肌强直、运动减少等运动障碍。目前PD的病因和发病机制尚不明确,可能与环境因素、遗传因素、氧化应激以及炎症反应等有关。有研究表明,SN区的铁沉积参与了PD的发病[11-12]。在MPTP和6-羟基多巴胺(6-OHDA)构建的PD小鼠模型中均出现了明显的铁沉积及铁代谢相关蛋白的表达异常[13-18]。
铁代谢对于正常脑功能的发育至关重要。铁摄取主要通过转铁蛋白(Tf)-转铁蛋白受体(TfR)途径和非转铁蛋白结合铁(NTBI)转运途径。Tf-TfR途径被认为是大脑摄取铁的主要途径,而TfR1是该途径的主要受体。NTBI途径主要通过DMT1来摄取二价铁离子。铁的储存主要由铁蛋白来完成,铁蛋白由H-Ferritin和轻链铁蛋白(L-Ferritin)组成,H-Ferritin具有亚铁氧化酶的活性,能将二价铁离子转为三价铁离子;L-Ferritin存在成核位点,能促进铁核的形成。在脑铁代谢过程中任何环节异常均可能引起铁代谢障碍,导致疾病的发生。有病例对照研究结果表明,Tf-TfR可能参与了PD的发病,其机制可能与多巴胺能神经元内的铁代谢失调或线粒体功能障碍有关[19-21]。本实验室研究证实,DMT1表达增加和铁转运蛋白(Fpn)表达下降可能是导致SN区铁沉积的重要原因[14,22-23]。在PD病人和PD模型SN区均发现,铁转入蛋白DMT1的表达明显升高[22-27]。有研究结果表明,PD动物模型SN区的铁转出蛋白Fpn表达下降[14,23,28-29],但这一结果在PD病人中并未得到证实。
最新的研究证实了脑内存在两条铁转运途径:一条是从vHip到mPFC再到SN的铁转运途径;另一条则是从丘脑(Tha)到杏仁核(AMG)再到mPFC的铁转运途径[10]。并且证实vHip到mPFC再到SN的铁转运途径异常是焦虑发生的关键环节[10]。PD病人的临床表现中,除运动功能障碍外,还存在非运动症状的发生如焦虑、抑郁及记忆力障碍等[30]。mPFC区主要负责情绪调节,与焦虑、抑郁等有关,但是PD病人mPFC区铁代谢相关蛋白的表达是否发生改变尚未有文献报道。本实验选用MPTP诱导的亚急性PD小鼠模型,进行行为学和铁代谢相关蛋白表达检测。行为学实验结果显示,MPTP组小鼠转头时间和下杆时间与对照组相比均明显延长,提示PD小鼠模型构建成功。Western
Blot结果显示,与对照组相比,MPTP组SN区铁代谢相关蛋白H-Ferritin和DMT1表达水平明显增加,TfR1蛋白表達水平明显下降。提示DMT1的表达上调可能是SN铁沉积的重要原因,而经典铁转运途径Tf-TfR途径可能并不参与SN铁沉积。此外,SN区H-Ferritin表达上调,可能与SN铁水平增加有关。但mPFC区铁代谢相关蛋白DMT1、TfR1、H-Ferritin的表达与对照组相比均无明显变化。表明MPTP诱导的PD小鼠模型铁代谢蛋白的表达异常仅出现在SN,而mPFC区并无明显的改变,提示mPFC区可能本身不具有储存铁的能力,与有关研究结果一致[10]。
综上所述,MPTP能够诱导小鼠运动障碍及SN区铁代谢相关蛋白的表达改变,但并不会引起mPFC区铁代谢相关蛋白的表达改变。脑铁代谢的机制十分复杂且目前尚未完全阐明,仍需进一步实验研究。
[参考文献]
[1]SCHWEITZER K J, BRSSEL T, LEITNER P, et al. Transcranial ultrasound in different monogenetic subtypes of Parkinsons disease [J]. Journal of Neurology, 2007,254(5):613-616.
[2]HAGENAH J M, KNIG I R, BECKER B, et al. Substantia nigra hyperechogenicity correlates with clinical status and number of Parkin mutated alleles[J]. Journal of Neurology, 2007,254(10):1407-1413.
[3]XIE W, LI X, LI C, et al. Proteasome inhibition modeling nigral neuron degeneration in Parkinsons disease[J]. Journal of Neurochemistry, 2010,115(1):188-199.
[4]LANGSTON J W. The MPTP story[J]. Journal of Parkin-sons Disease, 2017,7(s1):S11-S19.
[5]LANGSTON J W, IRWIN I, LANGSTON E B, et al. 1-Methyl-4-phenylpyridinium ion (MPP+): identification of a metabolite of MPTP, a toxin selective to the substantia nigra[J]. Neuroscience Letters, 1984,48(1):87-92.
[6]CHIBA K, TREVOR A, CASTAGNOLI N. Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase[J]. Biochemical and Biophysical Research Communications, 1984,120(2):574-578.
[7]CASTAGNOLI N, CHIBA K, TREVOR A J. Potential bioactivation pathways for the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)[J]. Life Sciences, 1985,36(3):225-230.
[8]HEIKKILA R E, MANZINO L, CABBAT F S, et al. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors[J]. Nature, 1984,311(5985):467-469.
[9]LIU H Y, WU H, ZHU N, et al. Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-me-thyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced Parkinsons disease in mice[J]. Journal of Neurochemistry, 2020,152(3):397-415.
[10]WANG Z, ZENG Y N, YANG P, et al. Axonal iron transport in the brain modulates anxiety-related behaviors[J]. Nature Chemical Biology, 2019,15(12):1214-1222.
[11]HAGENAH J M, BECKER B, BRGGEMANN N, et al. Transcranial sonography findings in a large family with homozygous and heterozygous PINK1 mutations[J]. Journal of Neurology, Neurosurgery, and Psychiatry, 2008,79(9):1071-1074.
[12]BRGGEMANN N, HAGENAH J, STANLEY K, et al. Substantia nigra hyperechogenicity with LRRK2 G2019S mutations[J]. Movement Disorders, 2011,26(5):885-888.
[13]WARD R J, ZUCCA F A, DUYN J H, et al. The role of iron in brain ageing and neurodegenerative disorders[J]. The Lancet Neurology, 2014,13(10):1045-1060.
[14]WANG J, JIANG H, XIE J X. Ferroportin1 and hephaestin are involved in the nigral iron accumulation of 6-OHDA-lesioned rats[J]. The European Journal of Neuroscience, 2007,25(9):2766-2772.
[15]MA Z G, WANG J, JIANG H, et al. Myricetin reduces 6-hydroxydopamine-induced dopamine neuron degeneration in rats[J]. Neuroreport, 2007,18(11):1181-1185.
[16]WANG J, XU H M, YANG H D, et al. Rg1 reduces nigral iron levels of MPTP-treated C57BL6 mice by regulating certain iron transport proteins[J]. Neurochemistry International, 2009,54(1):43-48.
[17]YOUDIM M B. What have we learnt from CDNA microarray gene expression studies about the role of iron in MPTP induced neurodegeneration and Parkinsons disease[J]? Journal of Neural Transmission Supplementum, 2003(65):73-88.
[18]HARE D J, ADLARD P A, DOBLE P A, et al. Metallobiology of 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine neurotoxicity[J]. Metallomics, 2013,5(2):91-109.
[19]RHODES S L, BUCHANAN D D, AHMED I, et al. Pooled analysis of iron-related genes in Parkinsons disease: association with transferrin[J]. Neurobiology of Disease, 2014,62:172-178.
[20]KALIVENDI S V, KOTAMRAJU S, CUNNINGHAM S, et al. 1-Methyl-4-phenylpyridinium (MPP+)-induced apoptosis and mitochondrial oxidant generation: role of transferrin-receptor-dependent iron and hydrogen peroxide[J]. The Biochemical Journal, 2003,371(pt 1):151-164.
[21]KAUR D, LEE D, RAGAPOLAN S, et al. Glutathione depletion in immortalized midbrain-derived dopaminergic neurons results in increases in the labile iron pool: implications for Parkinsons disease[J]. Free Radical Biology & Medicine, 2009,46(5):593-598.
[22]JIANG H, SONG N, XU H M, et al. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent[J]. Cell Research, 2010,20(3):345-356.
[23]SONG N, WANG J, JIANG H, et al. Ferroportin 1 but not hephaestin contributes to iron accumulation in a cell model of Parkinsons disease[J]. Free Radical Biology & Medicine, 2010,48(2):332-341.
[24]SALAZAR J, MENA N, HUNOT S, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinsons disease[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008,105(47):18578-18583.
[25]BURDO J R, MENZIES S L, SIMPSON I A, et al. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat[J]. Journal of Neuroscience Research, 2001,66(6):1198-1207.
[26]HUANG E, ONG W Y, CONNOR J R. Distribution of divalent metal transporter-1 in the monkey basal Ganglia[J]. Neuroscience, 2004,128(3):487-496.
[27]GUNSHIN H, ALLERSON C R, POLYCARPOU-SCH-WARZ M, et al. Iron-dependent regulation of the divalent metal ion transporter[J]. FEBS Letters, 2001,509(2):309-316.
[28]LEE D W, RAJAGOPALAN S, SIDDIQ A, et al. Inhibition of prolyl hydroxylase protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity: model for the potential involvement of the hypoxia-inducible factor pathway in Parkinson disease[J]. The Journal of Biological Chemistry, 2009,284(42):29065-29076.
[29]JIANG H, QIAN Z M, XIE J X. Increased DMT1 expression and iron content in MPTP-treated C57BL/6 mice[J]. Sheng Li Xue Bao, 2003,55(5):571-576.
[30]CRIPPA J A S, HALLAK J E C, ZUARDI A W, et al. Is ca nnabidiol the ideal drug to treat non-motor Parkinsons disease symptoms[J]? European Archives of Psychiatry and Clinical Neuroscience, 2019,269(1):121-133.
(本文編辑 马伟平)

