高能量密度锂离子电芯设计开发策略*
殷志刚,王 静,曹敏花
高能量密度锂离子电芯设计开发策略*
殷志刚1,2†,王 静1,曹敏花2
(1. 北京智行鸿远汽车有限公司,北京 102202;2. 北京理工大学,北京 100081)
提高单体电芯能量密度是锂离子电池重要的发展方向。提高锂离子电芯能量密度的主要途径包括开发高比容量和高放电电压平台正极材料、高比容量负极材料、高适用性电解液、选择合适的电芯类型、开发具有高黏结性的黏结剂及优良的导电剂等。另外也可通过适当地改善正负极配方来提高活性材料的有效占比以达到提高电芯能量密度的目的。本文概括总结了高能量密度锂离子电芯正负极材料的研究方向,电解液的研究思路,以及导电剂、黏结剂、结构和工艺路线的选择。
高能量密度;锂离子电芯;开发策略
0 引 言
电动汽车及其他可再生能源发电技术的广泛应用为新能源推广带来了巨大的商业机遇,同时也面临能源储存领域的挑战,特别是可充电二次电池[1-7]。电动汽车取代传统化石燃料汽车进入了发展快轨道。电动汽车产业的发展壮大,要求电池包的续航里程达到500 km以上,具有更长的循环寿命,更高的安全性等[8-9]。目前制约电动汽车发展的关键因素是动力锂离子电池不能满足电动汽车发展所需的高能量密度、高功率密度和快充要求[10-12]。解决电动汽车里程焦虑问题最为有效的方法之一是采用具有更高能量密度的电芯。本文将分别从高容量和高电压正极材料的开发、石墨及其他高容量负极材料开发、适合不同体系要求的电解液、正负极配比的优化、结构及工艺设计等方面对高能量密度锂离子电芯开发进行简要概述。
1 锂离子电芯能量密度的估算
当前锂离子电芯采用负极过量的设计原则。设计过程中以负/正电极容量比(negative/positive capacity ratio, NP比)作为负极过量系数。因此考虑最终电芯的容量或能量时要根据正极的实际容量作为基准。假设正极质量为p,正极的克容量发挥是p,负极的克容量发挥为n,设定所需的负极质量为n。依据锂离子产生等于锂离子消耗即锂离子守恒规则可得式(1):
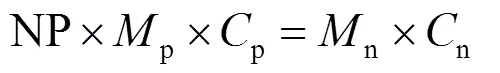

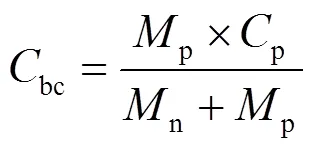

上式为理想化公式,锂离子电芯必然包括铜箔、铝箔、隔膜、电解液、导电剂、黏结剂、铝壳钢壳或铝塑膜等密封保护部件以及辅助配件。因此实际电芯的克容量要在理论基础上考虑相应的质量系数。以国内某动力三元−石墨电池为例,其电芯参数如表1所示。
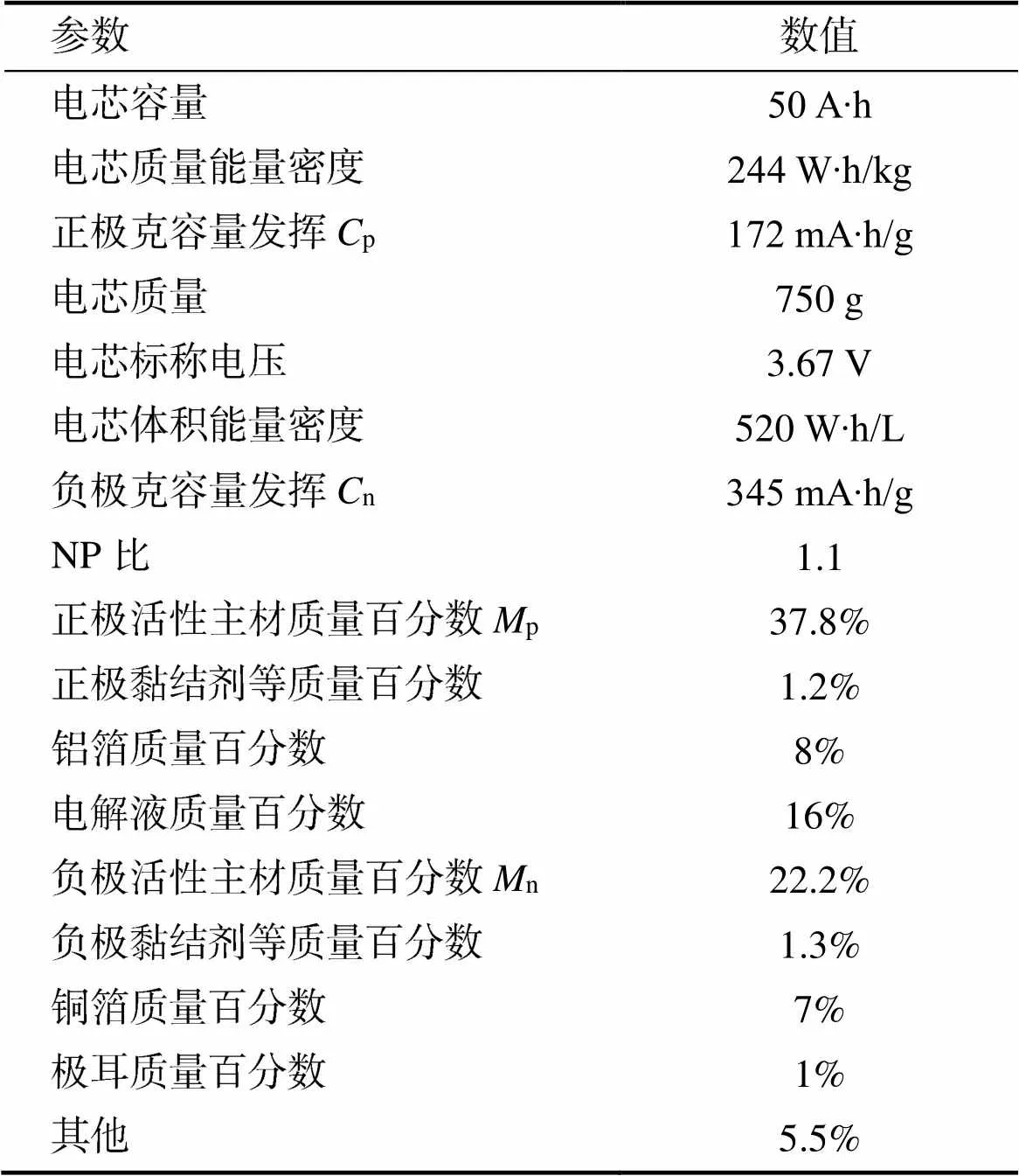
表1 电芯参数表
正负极活性主材质量分数为= 60%,bc= 111.08 A∙h/kg,则电芯质量能量密度=bc××= 111.08 × 60% × 3.67 = 244.6 W∙h/kg。计算结果与电芯测算结果十分吻合。
2 正极材料体系开发
通过上述锂离子电芯能量密度的计算公式可知,锂离子电芯正负极材料的比容量直接与电芯能量密度相关,当正极材料比容量逐渐增加时,电池的能量密度也逐渐增大[13-14]。当前电池负极材料以石墨占比最高,负极石墨材料的比容量在360 mA∙h/g左右,而正极材料的比容量要明显低于负极的比容量[15-16],因此提高正极材料比容量是提高电芯能量密度比较理想的选择。
2.1 高镍正极材料
当前已经成熟且应用于车载动力电芯上的正极材料有LiNi0.6Co0.2Al0.2O2(NCA622)及LiNi0.6Co0.2Mn0.2O2(NCM 622),该类材料配合石墨负极可以实现240 ~ 250 W∙h/kg的能量密度,上述表1计算实例即为NCM 622正极材料。材料厂商和电池企业协同正在开发的另一类正极材料为LiNi0.8Co0.1Al0.1O2(NCA811)及LiNi0.8Co0.1Mn0.1O2(NCM 811),该类材料搭配石墨负极,以上述参数为参考,正极有效克容量发挥为197 mA∙h/g,可以实现265 ~ 275 W∙h/kg的电芯能量密度。当前高镍低钴四元正极材料也引起了人们的广泛关注,有进一步工业化的趋势。这类材料中钴的含量通常在6%左右,而镍的含量要不低于88%,因此这类材料具有更高的正极容量,电芯的能量密度也能进一步提高[17-21]。然而高镍正极材料有其本身的制约因素,材料容易发生相转变、氧气及相关气体产生、阳离子混排、离子表面致密层的形成、过渡金属溶出、微裂纹等[22-26]。因此需要对材料进行修饰改性。高镍材料通过材料表面包覆、掺杂和合成稳定的单晶材料[27-32],是正极材料开发的主要方向。合理调控材料包覆层能够有效缓解正极材料的微裂纹、产氧、锂镍混排等危害材料性能的问题。NCM(A)811材料由于镍含量增加导致材料在更低的充电截止电压发生危害材料性能的问题,采用单晶类包覆材料能够有效缓解上述问题,提高设计电芯的常温和高温循环性能。
2.2 高镍正极材料上限电压提高
对于当前已经工业化的稳定正极材料,提高正极材料的脱嵌锂电位也能够实现电芯更高的能量密度[33-36]。同一电芯,充电截止电压为4.3 V电芯与充电截止电压4.2 V比较,电芯的能量密度可以提升5% ~ 10%;同样的,充电截止电压为4.4 V电芯的能量密度比充电截止电压为4.35 V的电芯能量密度提升5% ~ 10%。当前较高电压电芯还处于研究阶段,主要原因在于锂镍钴猛(LiNiCoMn1−−O2)三元体系和钴酸锂(LiCoO2)体系电池的稳定电压极限值在4.5 V左右,过高电压将导致材料稳定结构的破坏进而导致电芯在循环过程中快速衰减。本公司开发270 W∙h/kg能量密度电芯另一个方案是提高NCM622与人造石墨负极电芯体系的上限充电截止电压,然而上限截止电压的提高需开发与之匹配的电解液。
2.3 新型高电压正极材料
正极材料Li[Ni0.5Mn1.5]O4、Li[Co,Mn]O4、Li[V,Ni]O4(尖晶石结构)和LiCoPO4虽然电压高达5 V[37-41],但其成熟量产还需要克服许多问题。而且电压上限值已经接近或超过了现今商品化电解液的电压窗口范围[42]。因此需要同步开发高电压电解液。
2.4 小结
根据正极材料厂商的技术成熟性,当前高能量密度电芯设计主要是对高镍正极NCM(622和811)体系开展设计工作。其中比较成熟的正极材料为单晶NCM622,材料具有较高的热稳定性和安全性。现有的单晶NCM811正极材料也取得技术上的突破,其镍含量已经超过83%,并且实现了大批量的生产供货。对于300 W∙h/kg及以上电芯的设计开发,主要是采用上述NCM(622和811)材料配合具有更高克容量的负极材料。
3 负极材料体系开发
3.1 碳基材料
当前车载动力锂离子电芯通常采用石墨作为负极材料,包括人造石墨、天然石墨、硬碳、软碳以及中间相碳球等[43-45]。石墨负极材料的容量已提升至360 mA∙h/g左右,接近其理论容量372 mA∙h/g,因此靠提高石墨实际容量来提升电芯能量密度的策略实际意义不是很大。表2是国内某一负极材料厂商生产的人造石墨物理化学性能。

表2 人造石墨材料参数
当前的改进主要是从提高材料的压实密度、高低温性能及改善材料的加工特性等方面着手。人造石墨又分为一次颗粒石墨和二次颗粒石墨。通常一次颗粒石墨循环性能不佳,因此要进行二次造粒成二次颗粒石墨,二次颗粒石墨又难于加工。因此一次颗粒石墨和二次颗粒石墨混合使用有利于提高材料的循环性能和加工性能。人造石墨以其可靠性和安全性成为了负极材料的首选。天然石墨负极材料具有高的各向异性特点,且吸液和循环性能不佳。天然石墨虽然克容量高、压实密度高、价格低廉,但是由于颗粒大小不一,表面缺陷较多,在与人造石墨的竞争中处于劣势。改善天然石墨性能思路是实现颗粒表面均匀包覆,降低包覆层厚度和电化学阻抗,但材料的优化需要一些理论和工程上的技术突破。中间相碳球具有良好的倍率性能,主要用作对能量密度要求不高而对倍率性能严格要求的电芯负极。软碳的压实密度和克容量低,主要用作包覆材料来改善人造石墨和天然石墨的某些性能。硬碳由于放电电压高,与其他材料的吻合度不高,因此很少用作动力电芯负极。
3.2 硅基材料
单质硅、硅的氧化物、硅的金属化合物及硅碳复合材料[46-49]等是目前研究最多的高容量负极材料。在获得材料高克容量的同时如何提高硅基材料的长期循环性能,是这些材料需要解决的主要问题。目前措施是对材料结构以及复合方式进行优化,例如将单质硅或硅的氧化物与具有较小体积效应的材料进行复合,达到缓冲硅在脱嵌锂过程中的巨大体积效应,从而实现高容量材料的可应用性[50-53]。如硅−碳复合负极材料中,结合硅主要作为活性物质,用于脱嵌锂,而商用石墨类负极在脱嵌锂过程中具有较小的体积变化和优良的长期循环性能,主要用作缓冲层,因此制备与碳类负极材料复合的硅/碳(Si/C)材料具有现实的意义。以硅碳负极(550 mA∙h/g)与NCM811正极(197 mA∙h/g)做成电芯后的能量密度能够达到307 W∙h/kg。当前多家电芯企业开发的300 W∙h/kg能量密度的电芯均是采用富镍正极匹配硅碳负极。然而电芯距离达到设定的电化学性能还需进行技术及配方的改进。
3.3 锡基和金属氧化物材料
锡氧化物、锡盐、锡基合金等均可以作为负极材料。锡材料理论容量(990 mA∙h/g)较高、导电率较高、对环境敏感程度较低,但也具有明显的体积效应问题。同样,锡基材料也要与其他材料复合来应用[54-56]。但锡基材料的实际应用还需要科研工作者努力改进其缺陷。具有氧化还原反应特性的金属氧化物在Li储存和循环过程中显示出不同于插入−脱嵌和合金化过程的特点。金属氧化物的可逆容量比石墨高约2 ~ 3倍,然而首次库伦效率低、形成的固体电解质界面(solid electrolyte interphase, SEI)膜不稳定、存在电压滞后现象、循环稳定性差等是该类材料需要重点解决的问题。当前,许多研究者选择过渡金属(Mo、Fe、Cu等)氧化物作为锂离子电池的负极材料,主要是利用材料的低维度、多孔性结构特性,达到实现锂离子快速迁移的目的[57-60]。但是过高的比表面积也会导致电极材料与电解液过高的副反应以及较低的材料压实密度。这些缺点也会制约相关材料进一步在产品中应用。
3.4 小结
高能量密度电芯负极材料的选择主要集中在硅碳复合负极材料,较成熟的含硅材料的克容量达到600 mA∙h/g左右。该硅碳复合材料和富镍NCM正极材料构成的体系是研究的热点。
4 电解液体系开发
在电芯的性能和稳定性方面,电解液一直居于主要位置。目前电芯能量密度的提高要求对新型锂盐和溶剂等进行持续深入地研究,科研人员提出了许多改善电芯性能和安全性的方法。向电解液中加入添加剂能够弥补电解液某些方面的不足,特别是在正极和负极表面形成保护膜(SEI膜)领域,已经取得了显著成果[61-65]。电解液需要与电芯体系相适应,因此电解液配方的设计和研究必须围绕不同的电芯体系展开。
4.1 热稳定性电解液
目前动力锂离子电芯的电解液一般包括有机溶剂、锂盐和添加剂。其中甲基乙基碳酸酯(ethyl methyl carbonate, EMC)、碳酸乙烯酯(ethylene carbonate, EC)、碳酸丙烯酯(propylene carbonate, PC)、二甲基碳酸酯(dimethyl carbonate, DMC)、二乙基碳酸酯(diethyl carbonate, DEC)为常见的几种有机溶剂,锂盐是LiPF6。研究表明,电解液中的EMC能和H2O共同作用,降低LiPF6的热稳定性[66]。当电芯应用于高温条件,或者应用于热安全性较高环境时,要尽可能采用具有较低EMC含量的电解液。EC具有高的双电常数和良好的导电性,能形成稳定高质量的SEI膜,提高电池寿命,但是常温下EC为固态,因此高EC含量适合作为高温电解液。PC具有好的双电常数和低的熔点,是低温电解液溶剂的首选。其缺点是形成的SEI膜质量不佳,另外PC也容易在嵌锂过程中与锂一起发生共嵌入,导致石墨剥离和石墨颗粒的破裂[67-71]。因此电芯设计开发要考虑溶剂的选择及配比以达到电解液的热稳定性。研究指出电解液中添加碳酸亚乙烯酯(vinylene carbonate, VC)能在正负极表面形成致密的保护膜,改善高温性能。另外有机硅化合物R4Si添加剂会与电解液中的无机小分子H2O和HF发生反应,阻止H2O和HF与SEI膜发生危害负极性能的副反应,从而改善电芯的热稳定性。电解液厂家根据客户需要可以通过添加1,3-丙烷磺内酯(1,3-propane sultone, PS)、硫酸乙烯酯(1,3,2-dioxathiolane 2,2-dioxide, DTD)、双氟代磺酰亚胺锂[lithium bis(fluorosulfonyl)imide, LiFSI]等不同添加剂[72-81],改善电解液性能以满足相关产品需要。
4.2 高压电解液
高压电解液应具有较高的电化学窗口,通常在4.5 V以上。砜类电解液电化学窗口超过5 V,是锂离子电芯潜在的高电压电解液之一。这类电解液通常是碳酸酯类与砜类一起作为共溶剂,目的是优化砜类与正极材料的兼容性[82-84]。另一类电解液是腈类电解液,其优点是电化学窗口宽,如单腈类抗氧化稳定性可达到7 V,应用于5 V高电压锂离子电芯中不易发生分解。腈类电解液与碳酸酯类电解液相比,在高电压下更加稳定,在低温下具有更出色的性能[85-87]。氟原子具有大的电负性,弱的极性,氟代溶剂的化学稳定性也较好,在高电压电解液应用方面具有很大的潜力[88-90]。具有低挥发性、优异阻燃性、较宽电化学窗口的离子液体是最近的研究热点[91]。但这些高电压电解液还没有大量应用于工业生产中。
4.3 小结
当前商业化电解液开发以常规EC、DEC、EMC溶剂进行适当比例混合,主要研究方向是向电解液中添加电解液添加剂以满足不同体系的需要。高容量体系电解液添加剂有VC、LiPO2F2、FEC、PS等。电解液厂商根据客户需求设计并由客户进行验证。
5 正负极辅材及配比选择
锂离子电芯正极混料主要采用油性N-甲基吡咯烷酮(N-methylpyrrolidone, NMP)体系,负极主要采用水性体系。因此,对正负极要分别选择合适的导电剂、黏结剂以最大化提高正负极配比中活性物质含量,进而提高电芯整体容量,是当前高能量密度电芯研究的方向。
5.1 导电剂的选择
导电剂的作用是在活性物质之间、活性物质与集流体之间起到收集微电流,减小电极的接触电阻,加快电子的移动速率,同时导电剂也应该能够提高锂离子在电极材料中的迁移速率,达到提高电极充放电效率的目的[92-93]。目前导电剂主要分为纤维状和颗粒状两种。纤维状导电剂的典型代表是碳纳米纤维和碳纳米管,这两种材料普遍具有较大的长径比,有利于形成导电网格,也具有一定的黏结作用,这有利于提高活性物质与导电剂之间的黏结性。纤维状导电剂应用于三元材料体系,既可以减少导电剂添加量又能减少黏结剂的使用量,能够进一步提高电池的能量密度[94-97]。颗粒状导电剂的代表是乙炔黑、炭黑及石墨等,这些材料的成本低并且易分散,是锂离子电池正负极材料导电剂的不错选择。然而颗粒状导电剂与活性物质之间是点对点接触,导电性不如链状导电剂,因此添加量要在2.5% ~ 4%之间,使得活性物质含量相对较低[92-93]。可以通过添加纤维状和颗粒状混合导电剂改变电极的导电网络来改善电极的性能[94,98-101]。负极材料通常采用炭黑作为导电剂,正极材料通常采用碳纳米管及炭黑为导电剂。在导电剂选择时可以对极片通过极片电阻仪测试分析,仪器测量的体电阻用于分析极片导电剂配比和导电剂添加类型是否为最佳选择。
5.2 黏结剂的选择
黏结剂通常是一类高分子化合物,是电极片中的非活性组分,是制备锂离子电芯必备的重要材料之一。锂离子电芯专用黏结剂需要具备黏结性和导电性特点。通常状况下,对锂离子电芯专用黏结剂的要求体现在黏结力、柔韧性、耐碱性、亲水性、电导率等方面[102-104]。当前广泛应用的锂离子电芯黏结剂主要有三大类:聚偏氟乙烯(polyvinylidene fluoride,PVDF)、丁苯橡胶(styrene butadiene rubber,SBR)乳液和羧甲基纤维素(carboxymethyl cellulose,CMC)[105-107]。另外,聚丙烯腈(polyacrylonitrile,PAN)、聚丙烯酸(polyacrylic acid,PAA)等作为主要成分的黏结剂也占有一定市场份额[108-110]。好的黏结剂不仅有利于锂离子电芯能量密度的提高,对锂离子电芯内阻也有明显的降低作用,对电芯的电化学性能也具有重要的影响。石墨负极材料通常采用CMC+SBR组合水性黏结剂,其中CMC的主要作用是增稠剂,SBR起黏结剂作用。正极材料通常采用PVDF黏结剂。应用研究发现,水溶性黏结剂的添加量在2%左右为宜,油性黏结剂的添加量在1.5% ~ 3%之间。在黏结剂选择时会通过极片电阻仪对极片进行测试分析,仪器测量的面电阻用于分析极片黏结剂配比是否为最佳选择。
5.3 小结
高能量密度电芯的设计过程中导电剂的选择主要在碳纳米管和超导炭黑(super P, SP)领域。碳纳米管的使用需要注意提高其分散性以避免在浆料制备过程中再次团聚。当前碳纳米管需要生产厂家事先分散到水性或油性溶剂中并且需要加入分散剂保持纳米管的分散性。在保证分散效果的前提下,少加入或不加入分散剂能够提高电芯能量密度。而石墨类黏结剂主要以SBR配合CMC使用,硅碳类可选用PAA作为黏结剂。
6 结构及工艺设计
电芯的外观结构设计直接关系到电芯的空间有效利用率,进而影响到电芯的能量密度。从结构的角度要提高电芯的能量密度,应该选择薄而轻的软包铝塑膜作为电芯外壳。从电芯内部的制作方式考虑,隔膜往复Z字折叠式比卷绕多极耳方式要好。在不改变混料配方的前提下(即降低电芯无效重量)可以从以下几点提高电芯的能量密度:①提高正、负极极片的面密度,减少裸电芯的叠片层数或卷绕层数,避免因层数过多导致隔膜和导电箔材使用量增加;②使用更薄的铜铝箔材、隔膜和胶纸等;③减小软包电芯的顶侧封边宽或缩短其他电芯盖帽和裸电芯间的距离;④减小正负极片宽度差和隔膜与极片间的宽度差;⑤尽量缩短极片的非敷料区长度;⑥设计电芯外壳几何尺寸,使得充分利用电芯外壳空间,提高电芯外壳的空间利用率等[111-112]。本公司开发的电芯采用Z字折叠式软包方式,最大程度提高电芯能量密度。
7 总 结
开发具有高容量的正负极材料已成为提高电芯能量密度的最有效方法,研究开发高镍正极材料及新型宽工作电压窗口的电极材料是提高电芯能量密度的有效途径。碳基材料主要是在提高材料压实和改善加工性等方面入手。具有大克容量的硅系、锡系、过渡金属氧化物负极材料,需要寻找能有效缓解体积膨胀的方法和策略。寻找稳定可靠的新型高电压电解液是锂离子电芯材料领域的重要发展方向。正负极材料配比的进一步改进也能适当提高电芯的容量或性能。设计研究更加合理的结构及工艺也是提高电芯能量密度可选方法。根据以上设计思想,本公司已经成功完成能量密度为240 W∙h/kg电芯开发工作,产品已经在电动汽车上使用。能量密度为270 W∙h/kg的电芯也已经到中试阶段,样品也已经在相关企业进行测试,结果表明电芯能量密度处于领先水平。对于300 W∙h/kg电芯开发应选择高镍(811)正极材料配合碳纳米管导电剂、PVDF黏结剂作为正极体系,面密度控制在390 g/m2左右。选择硅碳复合负极材料配合SP导电剂和PAA黏结剂作为负极体系,面密度控制在155 g/m2左右。采用软包叠片电芯设计思路。采用EC + EMC溶剂含有LiPO2F2、FEC添加剂的电解液。电芯长×宽×高尺寸应该不小于320mm×100 mm×10mm。应选择具有陶瓷涂层的隔膜;考虑加工性,铝箔材应该在12 μm左右;铜箔材在6 μm左右。
[1] YOON C S, RYU H H, PARK G T, et al. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2cathodes for high-energy-density lithium-ion batteries[J]. Journal of materials chemistry A, 2018, 6(9): 4126-4132. DOI: 10.1039/c7ta11346c.
[2] 殷志刚, 王静, 曹敏花. 层状富镍过渡金属氧化物正极材料衰减机理研究进展[J]. 新能源进展, 2020, 8(3): 216-226. DOI: 10.3969/j.issn.2095-560X.2020.03.008.
[3] LU Y, HOU X S, MIAO L C, et al. Cyclohexanehexone with ultrahigh capacity as cathode materials for lithium-ion batteries[J]. Angewandte chemie international edition, 2019, 58(21): 7020-7024. DOI: 10.1002/anie.201902185.
[4] LIU T C, LIN L P, BI X X, et al. In situ quantification of interphasial chemistry in Li-ion battery[J]. Nature nanotechnology, 2019, 14(1): 50-56. DOI: 10.1038/s41565-018-0284-y.
[5] DU Z J, WOOD III D L, DANIEL C, et al. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries[J]. Journal of applied electrochemistry, 2017, 47(3): 405-415. DOI: 10.1007/s10800-017-1047-4.
[6] YANG C Y, CHEN J, JI X, et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite[J]. Nature, 2019, 569(7755): 245-250. DOI: 10.1038/s41586-019-1175-6.
[7] ZENG X Q, LI M, EI-HADY D A, et al. Commercialization of lithium battery technologies for electric vehicles[J]. Advanced energy materials, 2019, 9(27): 1900161. DOI:10.1002/aenm.201900161.
[8] HARPER G, SOMMERVILLE R, KENDRICK E, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575(7781): 75-86. DOI: 10.1038/s41586-019-1682-5.
[9] 殷志刚, 王静, 郝彦忠. 富镍三元层状过渡金属氧化物正材料的改性方法研究综述[J].河北科技大学学报, 2020, 41(2): 181-190. DOI: 10.7535/hbkd.2020yx02008.
[10] EFTEKHARI A. Lithium batteries for electric vehicles: from economy to research strategy[J]. ACS sustainable chemistry & engineering. 2019, 7(6): 5602-5613. DOI:10.1021/acssuschemeng.8b01494.
[11] MIAO Y, HYNAN P, VON JOUANNE A, et al. Current Li-ion battery technologies in electric vehicles and opportunities for advancements[J]. Energies, 2019, 12(6): 1074. DOI:10.3390/en12061074.
[12] BALALI Y, STEGEN S. Review of energy storage systems for vehicles based on technology, environmental impacts, and costs[J]. Renewable and sustainable energy reviews, 2021, 135: 110185. DOI: 10.1016/j.rser.2020.110185.
[13] NITTA N, WU F X, LEE J T, et al. Li-ion battery materials: present and future[J]. Materials today, 2015, 18(5): 252-264. DOI: 10.1016/j.mattod.2014.10.040.
[14] WINTER M, BARNETT B, XU K. Before Li ion batteries[J]. Chemical reviews, 2018, 118(23): 11433- 11456. DOI: 10.1021/acs.chemrev.8b00422.
[15] ENDO M, KIM C, NISHIMURA K, et al. Recent development of carbon materials for Li ion batteries[J]. Carbon, 2000, 38(2): 183-197. DOI: 10.1016/s0008-6223(99)00141-4.
[16] CHOU N H, PIERCE N, LEI Y, et al. Carbon-rich shungite as a natural resource for efficient Li-ion battery electrodes[J]. Carbon, 2018, 130: 105-111. DOI: 10.1016/j.carbon.2017.12.109.
[17] ZHANG C F, WAN J J, LI Y X, et al. Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping[J]. Journal of materials chemistry A, 2020, 8(14): 6893-6901. DOI: 10.1039/D0TA00260G.
[18] WU Y Q, XIE L Q, MING H, et al. An empirical model for the design of batteries with high energy density[J]. ACS energy letters, 2020, 5(3): 807-816. DOI: 10.1021/ acsenergylett.0c00211.
[19] KIM U H, KUO L Y, KAGHAZCHI P, et al. Quaternary layered Ni-rich NCMA cathode for lithium-ion batteries[J]. ACS energy letters, 2019, 4(2): 576-582. DOI: 10.1021/acsenergylett.8b02499.
[20] SONG X, LIU G X, YUE H F, et al. A novel low-cobalt long-life LiNi0.88Co0.06Mn0.03Al0.03O2cathode material for lithium ion batteries[J]. Chemical engineering journal, 2021, 407: 126301. DOI: 10.1016/j.cej.2020.126301.
[21] KIM U H, KIM J H, HWANG J Y, et al. Compositionally and structurally redesigned high-energy Ni-rich layered cathode for next-generation lithium batteries[J]. Materialstoday, 2019, 23: 26-36. DOI: 10.1016/j.mattod.2018.12.004.
[22] DING Y, MU D B, WU B R, et al. Recent progresses on nickel-rich layered oxide positive electrode materials used in lithium-ion batteries for electric vehicles[J]. Applied energy, 2017, 195: 586-599. DOI: 10.1016/j.apenergy.2017.03.074.
[23] XIONG D J, ELLIS L D, NELSON K J, et al. Rapid impedance growth and gas production at the Li-ion cell positive electrode in the absence of a negative electrode[J]. Journal of the electrochemical society, 2016, 163(14): A3069-A3077. DOI: 10.1149/2.1031614jes.
[24] YANG P H, LI H L, WEI X, et al. Structure tuned Li1.2Mn0.6Ni0.2O2with low cation mixing and Ni segregation as high performance cathode materials for Li-ion batteries[J]. Electrochimica acta, 2018, 271: 276-283. DOI: 10.1016/j.electacta.2018.01.104.
[25] GU M, BELHAROUAK I, ZHENG J M, et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries[J]. ACS nano, 2013, 7(1): 760-767. DOI: 10.1021/nn305065u.
[26] GILBERT J A, SHKROB I A, ABRAHAM D P. Transition metal dissolution, ion migration, electrocatalytic reduction and capacity loss in lithium-ion full cells[J]. Journal of the electrochemical society, 2017, 164(2): A389-A399. DOI: 10.1149/2.1111702jes.
[27] KIM H, KIM M G, JEONG H Y, et al. A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2cathode material: nanoscale surface treatment of primary particles[J]. Nano letters, 2015, 15(3): 2111-2119. DOI: 10.1021/acs.nanolett.5b00045.
[28] WEIGEL T, SCHIPPER F, ERICKSON E M, et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2cathode materials doped by various cations[J]. ACS energy letters, 2019, 4(2): 508-516. DOI: 10.1021/acsenergylett. 8b02302.
[29] CHEN Z, WANG J, CHAO D L, et al. Hierarchical porous LiNi1/3Co1/3Mn1/3O2Nano-/micro spherical cathode material: minimized cation mixing and improved Li+mobility for enhanced electrochemical performance[J]. Scientific reports, 2016, 6(1): 25771. DOI: 10.1038/srep25771.
[30] BECKER D, BÖRNER M, NÖLLE R, et al. Surface modification of Ni-rich LiNi0.8Co0.1Mn0.1O2cathode material by tungsten oxide coating for improved electrochemical performance in lithium-ion batteries[J]. ACS applied materials & interfaces, 2019, 11(20): 18404-18414. DOI: 10.1021/acsami.9b02889.
[31] WATANABE S, KINOSHITA M, HOSOKAWA T, et al. Capacity fading of LiAlNi1−−CoO2cathode for lithium-ion batteries during accelerated calendar and cycle life tests (effect of depth of discharge in charge–discharge cycling on the suppression of the micro-crack generation of LiAlNi1−−CoO2particle)[J]. Journal of power sources, 2014, 260: 50-56. DOI: 10.1016/j.jpowsour.2014.02.103.
[32] RAMASAMY H V, SINHA S, PARK J, et al. Enhancement of electrochemical activity of Ni-rich LiNi0.8Mn0.1Co0.1O2by precisely controlled Al2O3nanocoatings via atomic layer deposition[J]. Journal of electrochemical science and technology, 2019, 10(2): 196-205. DOI: 10.5229/JECST.2019.10.2.196.
[33] ZHANG S, MA J, HU Z L, et al. Identifying and addressing critical challenges of high-voltage layered ternary oxide cathode materials[J]. Chemistry of materials, 2019, 31(16): 6033-6065. DOI: 10.1021/acs.chemmater. 9b01557.
[34] CHEN Y X, LI Y J, TANG S Y, et al. Enhanced electrochemical properties of the Cd-modified LiNi0.6Co0.2Mn0.2O2cathode materials at high cut-off voltage[J]. Journal of power sources, 2018, 395: 403-413. DOI: 10.1016/j.jpowsour.2018.05.088.
[35] DONG P, WANG D, YAO Y, et al. Stabilizing interface layer of LiNi0.5Co0.2Mn0.3O2cathode materials under high voltage usingtoluenesulfonyl isocyanate as film forming additive[J]. Journal of power sources, 2017, 344: 111-118. DOI: 10.1016/j.jpowsour.2017.01.116.
[36] ZHANG F, LOU S F, LI S, et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage[J]. Nature communications, 2020, 11(1): 3050. DOI: 10.1038/s41467-020-16824-2.
[37] XU J T, DOU S X, LIU H K, et al. Cathode materials for next generation lithium ion batteries[J]. Nano energy, 2013, 2(4): 439-442. DOI: 10.1016/j.nanoen.2013.05.013.
[38] DRÄGER C, SIGEL F, INDRIS S, et al. Delithiation/ relithiation process of LiCoMnO4spinel as 5 V electrode material[J]. Journal of power sources, 2017, 371: 55-64. DOI: 10.1016/j.jpowsour.2017.10.039.
[39] ZHENG X, LIAO Y, Zhang Z R, et al. Exploring high- voltage fluorinated carbonate electrolytes for LiNi0.5Mn1.5O4cathode in Li-ion batteries[J]. Journal of energy chemistry, 2020, 42: 62-70. DOI: 10.1016/j.jechem.2019.05.023.
[40] BHATTACHARYA J, WOLVERTON C. Prediction of quaternary spinel oxides as li-battery cathodes: cation site preference, metal mixing, voltage and phase stability[J]. Journal of the electrochemical society, 2014, 161(9): A1440-A1446. DOI: 10.1149/2.0961409jes.
[41] LIU D Q, KIM C, PEREA A, et al. High-voltage lithium-ion battery using substituted LiCoPO4: electrochemical and safety performance of 1.2 Ah pouch cell[J]. Materials, 2020, 13(19): 4450. DOI: 10.3390/ma13194450.
[42] WANG Y W, YU L, LOU X W D. Formation of triple-shelled molybdenum-polydopamine hollow spheres and their conversion into MoO2/carbon composite hollow spheres for lithium-ion batteries[J]. Angewandte chemie international edition, 2016, 55(47): 14668-14672. DOI: 10.1002/anie.201608410.
[43] JALKANEN K, KARPPINEN J, SKOGSTROM L, et al. Cycle aging of commercial NMC/graphite pouch cells at different temperatures[J]. Applied energy, 2015, 154: 160-172. DOI: 10.1016/j.apenergy.2015.04.110.
[44] ITURRONDOBEITIA A, AGUESSE F, GENIES S, et al. Post-mortem analysis of calendar-aged 16 Ah NMC/graphite pouch cells for EV application[J]. The journal of physical chemistry C, 2017, 121(40): 21865-21876. DOI: 10.1021/acs.jpcc.7b05416.
[45] SCHMID A U, RIDDER A, HAHN M, et al. Aging of extracted and reassembled Li-ion electrode material in coin cells-capabilities and limitations[J]. Batteries, 2020, 6(2): 33. DOI: 10.3390/batteries6020033.
[46] LI X M, COLCLASURE A M, FINEGAN D P, et al. Degradation mechanisms of high capacity 18650 cells containing Si-graphite anode and nickel-rich NMC cathode[J]. Electrochimica acta, 2019, 297: 1109-1120. DOI: 10.1016/j.electacta.2018.11.194.
[47] SHOBUKAWA H, ALVARADO J, YANG Y Y C, et al. Electrochemical performance and interfacial investigation on Si composite anode for lithium ion batteries in full cell[J]. Journal of power sources, 2017, 359: 173-181. DOI: 10.1016/j.jpowsour.2017.05.044.
[48] ZHANG L H, QIN Y, LIU Y Z, et al. Capacity fading mechanism and improvement of cycling stability of the SiO anode for lithium-ion batteries[J]. Journal of the electrochemical society, 2018, 165(10): A2102-A2107. DOI: 10.1149/2.0431810jes.
[49] KIM S H, KIM Y S, BAEK W J, et al. Nanoscale electrical degradation of silicon–carbon composite anode materials for lithium-ion batteries[J]. ACS applied materials & interfaces, 2018, 10(29): 24549-24553. DOI: 10.1021/acsami.8b07012.
[50] AUPPERLE F, ESHETU G G, EBERMAN K W, et al. Realizing a high-performance LiNi0.6Mn0.2Co0.2O2/silicon- graphite full lithium ion battery cella designer electrolyte additive[J]. Journal of materials chemistry A, 2020, 8(37): 19573-19587. DOI: 10.1039/d0ta05827k.
[51] LIU B H, JIA Y K, LI J N, et al. Multiphysics coupled computational model for commercialized Si/graphite composite anode[J]. Journal of power sources, 2020, 450: 227667. DOI: 10.1016/j.jpowsour.2019.227667.
[52] HEENAN T M M, JNAWALI A, KOK M D R, et al. An advanced microstructural and electrochemical datasheet on 18650 li-ion batteries with nickel-rich NMC811 cathodes and graphite-silicon anodes[J]. Journal of the electrochemical society, 2020, 167(14): 140530. DOI: 10.1149/1945-7111/abc4c1.
[53] JIAO M L, WANG Y F, YE C L, et al. High-capacity SiOx(0≤x≤2) as promising anode materials for next-generation lithium-ion batteries[J]. Journal of alloys and compounds, 2020, 842: 155774. DOI: 10.1016/j. jallcom.2020.155774.
[54] BRUTTI S, HASSOUN J, SCROSATI B, et al. A high power Sn–C/C–LiFePO4lithium ion battery[J]. Journal of power sources, 2012, 217: 72-76. DOI: 10.1016/j. jpowsour.2012.05.102.
[55] TIAN H J, XIN F X, WANG X L, et al. High capacity group-IV elements (Si, Ge, Sn) based anodes for lithium-ion batteries[J]. Journal of materiomics, 2015, 1(3): 153-169. DOI: 10.1016/j.jmat.2015.06.002.
[56] LIU D Q, LIU Z J, LI X W, et al. Group IVA element (Si, Ge, Sn)-based alloying/dealloying anodes as negative electrodes for full-cell lithium-ion batteries[J]. Small, 2017, 13(45): 1702000. DOI: 10.1002/smll.201702000.
[57] JIANG H, HU Y J, GUO S J, et al. Rational design of MnO/carbon nanopeapods with internal void space for high-rate and long-life Li-ion batteries[J]. ACS Nano, 2014, 8(6): 6038-6046. DOI: 10.1021/nn501310n.
[58] XIAO S N, PAN D L, WANG L J, et al. Porous CuO nanotubes/graphene with sandwich architecture as high- performance anodes for lithium-ion batteries[J]. Nanoscale, 2016, 8(46): 19343-19351. DOI: 10.1039/c6nr07802h.
[59] OH S H, KIM J K, KANG Y C, et al. Three- dimensionally ordered mesoporous multicomponent (Ni, Mo) metal oxide/N-doped carbon composite with superior Li-ion storage performance[J]. Nanoscale, 2018, 10(39): 18734-18741. DOI: 10.1039/C8NR06727A.
[60] ZHAO X J, JIA W, WU X Y, et al. Ultrafine MoO3anchored in coal-based carbon nanofibers as anode for advanced lithium-ion batteries[J]. Carbon, 2020, 156: 445-452. DOI: 10.1016/j.carbon.2019.09.065.
[61] CHOOBAR B G, MODARRESS H, HALLADJ R, et al. Multiscale investigation on electrolyte systems of [(solvent + additive) + LiPF6] for application in lithium-ion batteries[J]. The journal of physical chemistry C, 2019, 123(36): 21913-21930. DOI: 10.1021/acs.jpcc.9b04786.
[62] LIU Q, WU F, MU D B, et al. Comparative calculation on Li+ solvation in common organic electrolyte solvents for lithium ion batteries[J]. Chinese physics B, 2020, 29(4): 048202. DOI: 10.1088/1674-1056/ab75cc.
[63] DING M S, LI Q Y, LI X, et al. Effects of solvent composition on liquid range, glass transition, and conductivity of electrolytes of a (Li, Cs) PF6salt in EC-PC-EMC solvents[J]. The journal of physical chemistry C, 2017, 121(21): 11178-11183. DOI: 10.1021/acs.jpcc. 7b03306.
[64] WU X Y, LIU T Y, BAI Y C, et al. Effects of solvent formulations in electrolytes on fast charging of Li-ion cells[J]. Electrochimica acta, 2020, 353: 136453. DOI: 10.1016/j.electacta.2020.136453.
[65] DAVOODABADI A, LI J L, ZHOU H, et al. Effect of calendering and temperature on electrolyte wetting in lithium-ion battery electrodes[J]. Journal of energy storage, 2019, 26: 101034. DOI: 10.1016/j.est.2019.101034.
[66] YANG C R, WANG Y Y, WAN C C. Composition analysis of the passive film on the carbon electrode of a lithium-ion battery with an EC-based electrolyte[J]. Journal of power sources, 1998, 72(1): 66-70. DOI: 10.1016/s0378-7753(97)02655-4.
[67] USHIROGATA K, SODEYAMA K, OKUNO Y, et al. Additive effect on reductive decomposition and binding of carbonate-based solvent toward solid electrolyte interphase formation in lithium-ion battery[J]. Journal of the American chemical society, 2013, 135(32): 11967- 11974. DOI: 10.1021/ja405079s.
[68] XU M Q, LI W S, ZUO X X, et al. Performance improvement of lithium ion battery using PC as a solvent component and BS as an SEI forming additive[J]. Journal of power sources, 2007, 174(2): 705-710. DOI: 10.1016/j.jpowsour.2007.06.112.
[69] WANG Q S, SUN J H, YAO X L, et al. Thermal stability of LiPF6/EC+DEC electrolyte with charged electrodes for lithium ion batteries[J]. Thermochimica acta, 2005, 437(1/2): 12-16. DOI: 10.1016/j.tca.2005.06.010.
[70] GUO F, OZAKI Y, NISHIMURA K, et al. Experimental study on flame stability limits of lithium ion battery electrolyte solvents with organophosphorus compounds addition using a candle-like wick combustion system[J]. Combustion and flame, 2019, 207: 63-70. DOI: 10.1016/j.combustflame.2019.05.019.
[71] NYMAN A, BEHM M, LINDBERGH G. Electrochemical characterisation and modelling of the mass transport phenomena in LiPF6-EC-EMC electrolyte[J]. Electrochimica acta, 2008, 53(22): 6356. DOI: 10.1016/j.electacta.2008. 04.023.
[72] LI B, WANG Y Q, LIN H B, et al. Performance improvement of phenyl acetate as propylene carbonate- based electrolyte additive for lithium ion battery by fluorine-substituting[J]. Journal of power sources, 2014, 267: 182-187. DOI: 10.1016/j.jpowsour.2014.05.073.
[73] AURBACH D. A review on new solutions, new measurements procedures and new materials for rechargeable Li batteries[J]. Journal of power sources, 2005, 146(1/2): 71-78. DOI: 10.1016/j.jpowsour.2005.03.151.
[74] WRODNIGG G H, WRODNIGG T M, BESENHARD J O, et al. Propylene sulfite as film-forming electrolyte additive in lithium ion batteries[J]. Electrochemistry communications, 1999, 1(3/4): 148-150. DOI: 10.1016/ s1388-2481(99)00023-5.
[75] SASAKI Y. Organic electrolytes of secondary lithium batteries[J]. Electrochemistry, 2008, 76(1):2-15. DOI: org/10.5796/electrochemistry.76.2.
[76] LI X C, YIN Z L, LI X H, et al. Ethylene sulfate as film formation additive to improve the compatibility of graphite electrode for lithium-ion battery[J]. Ionics, 2014, 20(6): 795-801. DOI: 10.1007/s11581-013-1036-5.
[77] YANG T X, WANG W L, LI S, et al. Sulfur-containing C2H2O8S2molecules as an overall-functional electrolyte additive for high-voltage LiNi0.5Co0.2Mn0.3O2/graphite batteries with enhanced performance[J]. Journal of power sources, 2020, 470: 228462. DOI: 10.1016/j.jpowsour. 2020.228462.
[78] LIU Q Q, MA L, DU C Y, et al. Effects of the LiPO2F2additive on unwanted lithium plating in lithium-ion cells[J]. Electrochimica acta, 2018, 263: 237-248. DOI: 10.1016/j.electacta.2018.01.058.
[79] ZHANG L F, CHAI L L, ZHANG L, et al. Synergistic effect between lithium BIS (fluorosulfonyl) imide (LiFSI) and lithium BIS-Oxalato borate (LiBOB) salts in LiPF6-based electrolyte for high-performance Li-ion batteries[J]. Electrochimica acta, 2014, 127: 39-44. DOI: 10.1016/j.electacta.2014.02.008.
[80] AN F Q, ZHAO H L, ZHOU W N, et al. S-containing and Si-containing compounds as highly effective electrolyte additives for SiOx-based anodes/NCM 811 cathodes in lithium ion cells[J]. Scientific reports, 2019, 9(1): 14108. DOI: 10.1038/s41598-019-49568-1.
[81] DONG N, YANG G H, LUO H, et al. A LiPO2F2/LiFSI dual-salt electrolyte enabled stable cycling of lithium metal batteries[J]. Journal of power sources, 2018, 400: 449-456. DOI: 10.1016/j.jpowsour.2018.08.059.
[82] ZHENG X Z, HUANG T, PAN Y, et al. High-voltage performance of LiNi1/3Co1/3Mn1/3O2/graphite batteries with di (methylsulfonyl) methane as a new sulfone-based electrolyte additive[J]. Journal of power sources, 2015, 293: 196-202. DOI: 10.1016/j.jpowsour.2015.05.061.
[83] XUE L G, UENO K, LEE S Y, et al. Enhanced performance of sulfone-based electrolytes at lithium ion battery electrodes, including the LiNi0.5Mn1.5O4high voltage cathode[J]. Journal of power sources, 2014, 262: 123-128. DOI: 10.1016/j.jpowsour.2014.03.099.
[84] HILBIG P, IBING L, WAGNER R, et al. Ethyl methyl sulfone-based electrolytes for lithium ion battery applications[J]. Energies, 2017, 10(9): 1312. DOI: 10.3390/en10091312.
[85] ZHI H Z, XING L D, ZHENG X W, et al. Understanding how nitriles stabilize electrolyte/electrode interface at high voltage[J]. The journal of physical chemistry letters, 2017, 8(24): 6048-6052. DOI: 10.1021/acs.jpclett.7b02734.
[86] KIM G Y, DAHN J R. The effect of some nitriles as electrolyte additives in Li-ion batteries[J]. Journal of the electrochemical society, 2015, 162(3): A437-A447. DOI: 10.1149/2.0651503jes.
[87] XIAN F, LI J D, HU Z L, et al. Investigation of the cathodic interfacial stability of a nitrile electrolyte and its performance with a high-voltage LiCoO2cathode[J]. Chemical communications, 2020, 56(37): 4998-5001. DOI: 10.1039/D0CC00049C.
[88] ZHANG Z C, HU L B, WU H M, et al. Fluorinated electrolytes for 5 V lithium-ion battery chemistry[J]. Energy & environmental science, 2013, 6(6): 1806-1810. DOI: 10.1039/c3ee24414h.
[89] IM J, LEE J, RYOU M H, et al. Fluorinated carbonate-based electrolyte for high-voltage Li (Ni0.5Mn0.3Co0.2) O2/graphite lithium-ion battery[J]. Journal of the electrochemical society, 2017, 164(1): A6381-A6385. DOI: 10.1149/2.0591701jes.
[90] HU L B, XUE Z, AMINE K, et al. Fluorinated electrolytes for 5-V Li-ion chemistry: synthesis and evaluation of an additive for high-voltage LiNi0.5Mn1.5O4/ graphite cell[J]. Journal of the electrochemical society, 2014, 161(12): A1777-A1781. DOI: 10.1149/2.0141412jes.
[91] FRANCIS C F J, KYRATZIS I L, BEST A S. Lithium-ion battery separators for ionic-liquid electrolytes: a review[J]. Advanced materials, 2020, 32(18): 1904205. DOI: 10.1002/adma.201904205.
[92] 刘露, 戴永年, 姚耀春. 导电粘结剂对锂离子电池性能的影响[J]. 材料导报, 2007, 21(S1): 267-269. DOI: 10.3321/j.issn:1005-023X.2007.z1.081.
[93] 陈志金, 张一鸣, 田爽, 等. 锂离子电池导电剂研究进展[J]. 电源技术, 2019, 43(2): 333-338. DOI: 10.3969/ j.issn.1002-087X.2019.02.046.
[94] SONG H, OH Y, ÇAKMAKC N, et al. Effects of the aspect ratio of the conductive agent on the kinetic properties of lithium ion batteries[J]. RSC advances, 2019, 9(70): 40883-40886. DOI: 10.1039/c9ra09609d.
[95] WANG K, WU Y, LUO S, et al. Hybrid super-aligned carbon nanotube/carbon black conductive networks: A strategy to improve both electrical conductivity and capacity for lithium ion batteries[J]. Journal of power sources, 2013, 233: 209-215. DOI: 10.1016/j.jpowsour.2013.01.102.
[96] ZHU J H, WU Y P, HUANG X K, et al. Self-healing liquid metal nanoparticles encapsulated in hollow carbon fibers as a free-standing anode for lithium-ion batteries[J]. Nano energy, 2019, 62: 883-889. DOI: 10.1016/j.nanoen.2019.06.023.
[97] 邓凌峰, 陈洪. 纳米碳纤维导电剂改善锂离子电池性能的研究[J]. 电池工业, 2009, 14(2): 79-83. DOI: 10.3969/j.issn.1008-7923.2009.02.002.
[98] WANG J S, SHEN Z G, YI M. Liquid-exfoliated graphene as highly efficient conductive additives for cathodes in lithium ion batteries[J]. Carbon, 2019, 153: 156-163. DOI: 10.1016/j.carbon.2019.07.008.
[99] 孟仙雅, 王睿, 钟小华, 等. 导电剂对LiFePO4锂离子电池性能的影响[J]. 电池工业, 2012, 17(5): 296-298, 305. DOI:10.3969/j.issn.1008-7923.2012.05.010.
[100] CHEON S E, KWON C W, KIM D B, et al. Effect of binary conductive agents in LiCoO2cathode on performances of lithium ion polymer battery[J]. Electrochimica acta, 2000, 46(4): 599-605. DOI: 10.1016/s0013-4686(00)00626-5.
[101] YANG H Q, LIU S W, CAO L H, et al. Superlithiation of non-conductive polyimide toward high-performance lithium-ion batteries[J]. Journal of materials chemistry A, 2018, 6(42): 21216-21224. DOI: 10.1039/c8ta05109g.
[102] 王斌, 吴婷, 刘恋, 等. 镍钴锰体系锂离子电池粘结剂性能研究[J]. 电池工业, 2019, 43(1): 45-47. DOI: 10.3969/j.issn.1002-087X.2019.01.012.
[103] LANDESFEIND J, ELDIVEN A, GASTEIGER H A. Influence of the binder on lithium ion battery electrode tortuosity and performance[J]. Journal of the electrochemical society, 2018, 165(5): A1122-A1128. DOI: 10.1149/2.0971805jes.
[104] YEN J P, CHANG C C, LIN Y R, et al. Effects of styrene- butadiene rubber/carboxymethylcellulose (SBR/CMC) and polyvinylidene difluoride (PVDF) binders on low temperature lithium ion batteries[J]. Journal of the electrochemical society, 2013, 160(10): A1811-A1820. DOI: 10.1149/2.083310jes.
[105] EOM J Y, CAO L. Effect of anode binders on low-temperature performance of automotive lithium-ion batteries[J]. Journal of power sources, 2019, 441: 227178. DOI: 10.1016/j.jpowsour.2019.227178.
[106] MÜLLER M, PFAFFMANN L, JAISER S, et al. Investigation of binder distribution in graphite anodes for lithium-ion batteries[J]. Journal of power sources, 2017, 340: 1-5. DOI: 10.1016/j.jpowsour.2016.11.051.
[107] WANG R, FENG L L, YANG W R, et al. Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries[J]. Nanoscale research letters, 2017, 12(1): 575. DOI: 10.1186/s11671- 017-2348-6.
[108] HU B, SHKROB I A, ZHANG S, et al. The existence of optimal molecular weight for poly (acrylic acid) binders in silicon/graphite composite anode for lithium-ion batteries[J]. Journal of power sources, 2018, 378: 671-676. DOI: 10.1016/j.jpowsour.2017.12.068.
[109] KOO B, KIM H, CHO Y, et al. A highly cross-linked polymeric binder for high-performance silicon negative electrodes in lithium ion batteries[J]. Angewandte chemie, 2012, 124(35): 8892-8897. DOI: 10.1002/ange.201201568.
[110] GONG L Y, NGUYEN M H T, OH E S. High polar polyacrylonitrile as a potential binder for negative electrodesin lithium ion batteries[J]. Electrochemistry communications, 2013, 29: 45-47. DOI: 10.1016/j.elecom.2013.01.010.
[111] QIAN G Y, LIAO X B, ZHU Y X, et al. Designing flexible lithium-ion batteries by structural engineering[J]. ACS energy letters, 2019, 4(3): 690-701. DOI: 10.1021/ acsenergylett.8b02496.
[112] LI Y, SONG J, YANG J. A review on structure model and energy system design of lithium-ion battery in renewable energy vehicle[J]. Renewable and sustainable energy reviews, 2014, 37: 627-633. DOI: 10.1016/j.rser.2014.05.059.
Design and Development Strategy of High Energy Density Lithium-Ion Cell
YIN Zhi-gang1,2, WANG Jing1, CAO Min-hua2
(1. Beijing Idrive Automotive Co, Ltd., Beijing 102202, China; 2. Beijing Institute of Technology, Beijing 100081, China)
Increasing the energy density of single cell is an important development direction of lithium-ion battery. In summary, the main ways to improve the energy density of lithium-ion cell are development of high specific capacity and high discharge voltage platform cathode materials, high specific capacity anode materials, high applicability electrolyte, selection of appropriate cell types, development of binders with high adhesive properties and excellent conductive agents. In addition, the effective proportion of active materials can be increased by appropriately improving the formula of positive and negative electrodes to achieve the purpose of increasing the energy density of the cell. In this paper, the research directions of cathode materials and anode materials for high energy density lithium-ion cell, the research ideas of electrolyte, the reasonable selection of conductive agent and binder agent, the selection of cell structure and process route, were briefly summarized.
energy density; lithium-ion cell; development strategy
TK02;TM912
A
10.3969/j.issn.2095-560X.2021.03.010
2095-560X(2021)03-0248-10
2021-01-25
2021-03-23
国家自然科学基金面上项目(21872008)
殷志刚,E-mail:hbtsyzg@163.com
殷志刚(1978-),男,博士,高级工程师,主要从事高能量密度电池的开发和相关机理研究。
曹敏花(1969-),女,教授,博士生导师,主要从事锂离子电池和燃料电池中材料制备与电化学问题研究。

