Eff ect of polystyrene microplastics and temperature on growth, intestinal histology and immune responses of brine shrimp Artemia franciscana*
Xuekai HAN, Yuyu ZHENG , Chaoling DAI, Hu DUAN, Meirong GAO, Md Rayhan ALI,Liying SUI
Asian Regional Artemia Reference Center, Tianjin Key Laboratory of Marine Resources and Chemistry, College of Marine and Environmental Sciences, Tianjin University of Science and Technology, Tianjin 300457, China
Abstract Microplastics pollution and seawater temperature rise have been the major environmental issues, threatening the survival and biodiversity of marine organisms. This study evaluated the combined eff ect of temperature and polystyrene microplastics (MP) on Artemia, a filter-feeding crustacean that is widely used for environmental toxicology studies. Brine shrimp Artemia franciscana were exposed to three MP concentrations (0, 0.2, and 2.0 mg/L) and three temperatures (22, 26, and 30 °C) for 14 d. In general,higher MP concentration and temperature led to a decreased survival rate and growth. Two-way ANOVA analysis indicated that the survival rate of Artemia was significantly impacted by both MP concentration and temperature ( P <0.05), but there was no significant interaction between two factors ( P >0.05). Growth of Artemia was significantly impacted by temperature ( P <0.05), and with a significant interaction between two factors ( P <0.05). Furthermore, the enzymatic activity, intestinal histological analyses, and immune gene expression were determined for Artemia reared at 30 °C with three MP concentrations (0, 0.2, and 2.0 mg/L). The results showed that 2.0-mg/L MP resulted in reduced Artemia intestinal microvilli and exfoliated epithelia cells, significantly increased acid phosphatase (ACP) activity ( P <0.05) and immunerelated gene ADRA1B and CREB3 expression, revealing that higher MP concentration could induce oxidative and immunological stress on Artemia at 30 °C. Overall, our study suggests that MP and temperature have combined adverse eff ect on Artemia, especially at relatively high temperature and polystyrene MP concentration. These findings are important to understand the potential ecological risks posed by these two factors on the organisms in marine environment.
Keyword: Artemia franciscana; combined eff ect; microplastics; temperature
1 INTRODUCTION
Nowadays, plastic pollution has become one of the serious environmental problems worldwide(Andrady, 2011; PlasticsEurope, 2017). It has been reported that 10% of the world’s plastic waste eventually enters the ocean (Secretariat of the Convention on Biological Diversity and Scientific and Technical Advisory Panel GEF, 2012), and that up to 90% of the waste in ocean are small fragments(Eriksen et al., 2014). Microplastics (MP), usually referring particles less than 5 mm, has become an emerging threat to marine environment and biodiversity due to its toxic and bio-accumulative eff ects (Jambeck et al., 2015; Bakir et al., 2016). MP can easily get into aquatic food web and result negative impact on marine organisms, and thus threaten the food safety of fisheries and aquaculture products (Wright et al., 2013; Lusher, 2015; Jabeen et al., 2017; Barboza et al., 2018b; Li et al., 2018).Being as a main planktonic consumer of the marine food web, zooplankton plays an important role in energy transfer and greatly contributes to the diversity of marine ecosystem. Excess intake of MP causes a series of adverse impacts on zooplankton, including intake disorders, acute oxidative stress, reduced reproduction, and survivals (Lee et al., 2013; Cole et al., 2015; Jeong et al., 2016). In addition, the retention of MP in the body may be transferred and accumulated along marine trophic webs, further aff ecting the stability of the entire marine ecosystem (Wang et al.,2016; Martins and Guilhermino, 2018).
Although MP has proven many negative impacts on zooplankton, few studies have investigated the combined eff ects of MP and other environmental factors on marine species. The interaction of environmental factors are not only the sum of the independent responses but mostly be synergistic or antagonistic (Crain et al., 2008). Temperature is one of the key drivers of biological response, which determine the physiological fitness and distribution range of a species by influencing its growth, feeding,metabolism, reproduction, and behavior (Place et al.,2008). In recent years anthropogenic environmental changes likely cause global warming and result in the rise of ocean temperatures (Brierley and Kingsford,2009). Therefore, marine organisms are facing threats posed by the simultaneous presence of MP and elevated temperature.
Brine shrimpArtemiais a filter-feeding zooplankton that can adapt to a wide salinity range. It has some virtues such as shorter life cycle, bigger off spring number per brood, and easy to handle in the laboratory(Lavens and Sorgeloos, 1996), and thus has been widely used as a test organism for ecotoxicology study (Manfra et al., 2015; Vannuccini et al., 2015;Ekonomou et al., 2019). Nowadays,Artemiaare increasingly used as test organisms for marine ecotoxicology study, with special focus on the toxicity of micro- and nano-particles onArtemiagrowth,morphology and physiology properties (Rodd et al.,2014; Bergami et al., 2017; Minetto et al., 2017;Rotini et al., 2018; Sarkheil et al., 2018; Varó et al.,2019). These studies mostly focused on short-term toxicity test withArtemianauplii. In fact, the increase of MP concentration may lead to increased mortality of some marine species after a long-term exposure.Thereby, it is necessary to provide evidence of the chronic eff ects by exposingArtemiato MP for long time course.
In this study, the 14-d culture was conducted to evaluate the eff ects of MP and temperature onArtemiain the laboratory condition. Our hypothesis is that MP and temperature have combined eff ects onArtemiafor a long-term exposure, and cause a series of responses at physiological, biochemical, molecular and histological level. To test this hypothesis, the survival rate was determined in time course. After 14-d exposure,Artemiagrowth was determined(Bergami et al., 2017). The immune-related oxidative enzymes acid phosphatase (ACP) and catalase (CAT)were used as indicators of biochemical responses(Hou et al., 2000; Bhuvaneshwari et al., 2018). Two immune genes adrenergic receptor alpha-1B(ADRA1B) and cyclic AMP-responsive elementbinding protein 3 (CREB3) were used as indicators of apoptosis pathways (Zhang et al., 2018). Moreover,intestinal histology was further examined to assess possible damage of epithelial cells lining the digestive tract (Wang et al., 2019). The outcome of this study may provide insight into the combined eff ect of MP and temperature on marine organism.
2 MATERIAL AND METHOD
2.1 Experimental design
The cysts ofArtemiafranciscanafrom the Great Salt Lake, USA were hatched following the general procedure. Exact 0.100-g cysts were placed in a glass cone containing 200-mL artificial seawater (30 salinity, ASW, Instant Ocean, USA), and with continuous aeration and illumination (1 000 lx) at 28 °C. Polystyrene microplastic beads with particle size of 4–6 μm were purchased from Tianjin BaseLine ChromTech Research Centre (Tianjin, China). The MP suspension (25 mg/mL) were prepared using deionized water and were sonicated prior to use.
Experiment was conducted in combination of three temperature (22, 26, and 30 °C) and three MP concentrations (0, 0.2, and 2.0 mg/L) with nine treatments. Each treatment was run in three replicates.The temperature degrees were selected according to surface temperature variability (20–30 °C) of the ocean (Shen et al., 2007). For the MP levels, 0.2 mg/L was considered ecologically relevant MP concentration in marine environments (Barboza et al.,2018a), and 2.0 mg/L was selected considering a severe scenario where MP pollution occurs.
One hundred newly hatched nauplii were randomly transferred into a 250-mL glass bottle containing200-mL artificial seawater (ASW) (salinity 30).Artemiawere fed twice a day with microalgaeDunaliellasalinaat a final density of 100 000 cells/mL. To evaluate the eff ect of MP onArtemiaduring long-term exposure, the survival rate was determined after 1-, 3-, 6-, 10-, and 14-d culture.Artemiawere put back in the bottles after counting the survivals.The survival rate was calculated as follows:
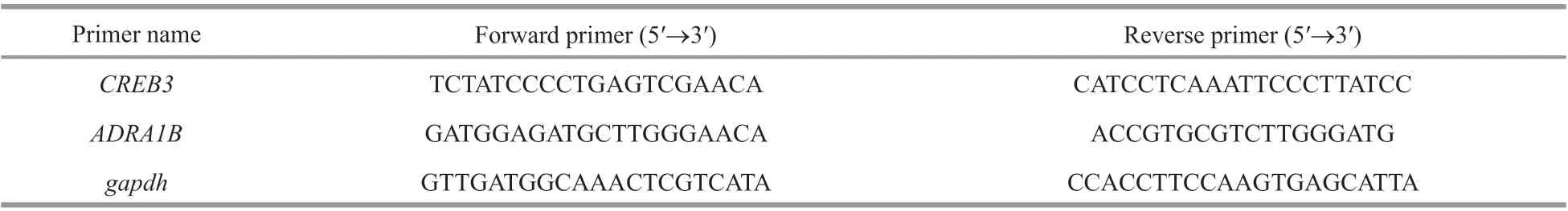
Table 1 Primers used for real time-PCR
Survival rate (SR, %)=N/N0×100,
whereNis the survivedArtemianumber, andN0is the initialArtemianumber.
On day 14, thirtyArtemiafrom each replicate were randomly collected, the average individual body length was measured under a stereomicroscope.
The eff ect of three MP concentrations (0, 0.2, and 2.0 mg/L) combined with 30 °C were studied to evaluate the immune-related enzyme activity, immune gene expression and intestine histology ofArtemiain bigger scale setup. Newly hatchedArtemianauplii were randomly distributed into 9 plastic cones containing 5-L ASW. The initial density ofArtemiawas 100 ind./L. Each treatment was run in three replicates.Artemiawere reared at 30 °C with 14 h L:10 h D photoperiod for 14 d, and were covered to prevent water evaporation. The nauplii were fedD.salinatwice a day at a final density of 100 000 cells/mL.
2.2 Enzyme activity assays
On day 14,Artemiawere collected and rinsed with deionized water. The excess water was blotted using tissue paper. Exact 0.100-gArtemiawere weighed and homogenized in physiological saline (1:9, w:v).The suspension was centrifuged at 2 500 r/min for 10 min at 4 °C, and supernatant was collected. The enzyme activity of acid phosphatase (ACP) and catalase (CAT) assays were performed using analytical kits (Nanjing Jiancheng, China) and expressed as unit per milligram of soluble protein (U/mg protein). The soluble protein content was determined using the Coomassie Brilliant Blue (G-250) method.
2.3 Intestinal histology assays
On day 14, the intestine ofArtemiain the group of 0 and 2.0-mg/L MP were dissected, and fixed in 4%paraformaldehyde for 48 h. After rinsed with phosphate buff er and dehydrated subsequently with ethanol dilutions (70%, 80%, 90%, and 100%), the tissues were made transparent with xylene, embedded in paraffi n and cut in a microtome at 4-μm thickness.After hematoxylin-eosin staining, pictures were taken under Inverted Phase Contrast Microscope (Nikon-TiE, Japan).
2.4 RNA extraction, cDNA synthesis and real time-PCR
Gene expression analysis was performed according to Vannuccini et al. (2015). RNA concentrations were measured using a traycell spectrophotometer(Eppendorf, Germany) and RNA quality was detected on 1.2% agarose gel. Two milligram of total RNA was transcribed to cDNA using PrimeScript™ RT reagent Kit (Code No. RR037A). Two genes of adrenergic receptor alpha-1B (ADRA1B) and cyclic AMPresponsive element-binding protein 3 (CREB3) were chosen to perform real time-PCR, and glyceraldehyde 3-phosphatase dehydrogenase (gapdh) was selected as housekeeping gene (Chen et al., 2009).
Real time-PCR was performed using a CFX Connect thermal cycler. Amplification was performed in triplicate in a total volume of 25 μL containing 2-μL cDNA, 100 nmol/L of each primer and 12.5 μL of TB Green®Premix Ex Taq (RR820A, TaKaRa).The cycling conditions were 95 °C for 30 s for polymerase activation, followed by 40 PCR cycles of 95 °C of 10 s, at 50–60 °C of 30. The calculated relative expression ratio of each gene was based on the PCR effi ciency (E) andCtof sample compared with control, and expressed in comparison to the reference genes. Primers of these genes are listed in Table 1.
2.5 Statistical analysis
Diff erences in enzymatic activity and gene expression between treatments were analyzed using one-way ANOVA. The eff ects of diff erent temperatures and MP concentrations on survival and growth ofArtemiawere analyzed by two-way ANOVA. All data were tested for normality and homogeneity of variance before ANOVA was done.Tukey’s honest significant diff erence test was performed to identify diff erences atP<0.05.
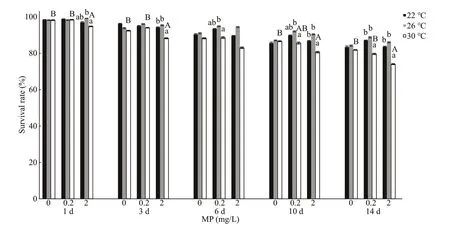
Fig.1 One-way ANOVA analysis on survival rate of Artemia exposed to diff erent temperatures and MP concentrations for 1, 3, 6, 10, and 14 d

Table 2 Two-way ANOVA analysis on the eff ects of temperature (22, 26, and 30 °C) and MP concentration (0, 0.2, and 2.0 mg/L) on Artemia survival rate
3 RESULT
3.1 Survival and growth
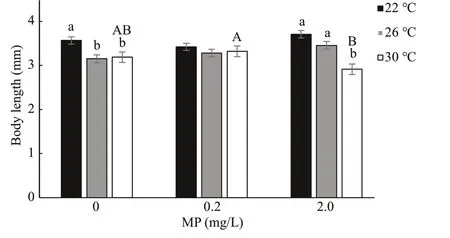
Fig.2 One-way ANOVA analysis on body length of Artemia exposed to diff erent temperatures and MP concentrations for 14 d
One-way ANOVA analysis showed higher MP concentration and temperature led to a decrease in survival rate ofArtemia(Fig.1). Throughout the experiment,Artemiaexposed to 30 °C and 2.0 mg/L showed the lowest survival rate compared to other groups. By using two-way ANOVA analysis,temperature showed significantly influence on the survival rate ofArtemiathroughout the experiment(P<0.05), with the exception after 1-d exposure (Table 2). In addition, survival rate was significantly aff ected by MP after 1-d exposure and day 14 (P<0.05). There was no interaction between MP and temperature with the exception after 1-day exposure.
After 14-d exposure,Artemialength increased from 0.35–0.40 mm to 3–4 mm. High temperature and MP concentration significantly decreasedArtemiagrowth in a long-term exposure, which is consistent with the survival rate (Fig.2). Two-way ANOVA analysis showed thatArtemialength was significantly aff ected by temperature (P<0.05), and there was a significant interaction between MP and temperature(P<0.05) (Table 3).

Fig.3 CAT and ACP activity of Artemia exposed to diff erent MP concentrations at 30 °C for 14 d
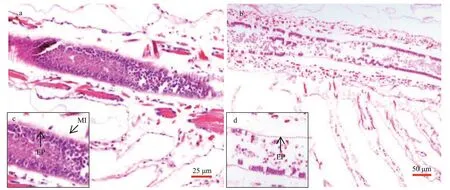
Fig.4 Intestinal tissue of Artemia exposure to 0 (a) and 2.0-mg/L (b) MP at 30 °C for 14 d
3.2 Enzymatic activity
Both CAT and ACP activity increased with the increasing MP concentrations (Fig.3). There was no significant diff erence in CAT activity in diff erent groups (P>0.05), while ACP activity showed significant diff erence between 0 and 2.0-mg/L MP(P<0.05).
3.3 Intestinal histopathological analyses
Longitudinal sections ofArtemiaintestines in the group of 0 and 2.0-mg/L MP were shown in Fig.4.After 14-d exposure, the intestinal epithelial cells were closely connected and microvilli wereregularly-ordered in control group. By contrast, the number ofintestinal microvilli decreased and epithelia cells exfoliated in 2.0-mg/L MP treatment,which implied that 2.0-mg/L MP could damage the intestinal tissue ofArtemia.

Table 3 Two-way ANOVA analysis on the eff ects of temperatures (22, 26, and 30 °C) and MP (0, 0.2,and 2.0 mg/L) on Artemia growth on day 14
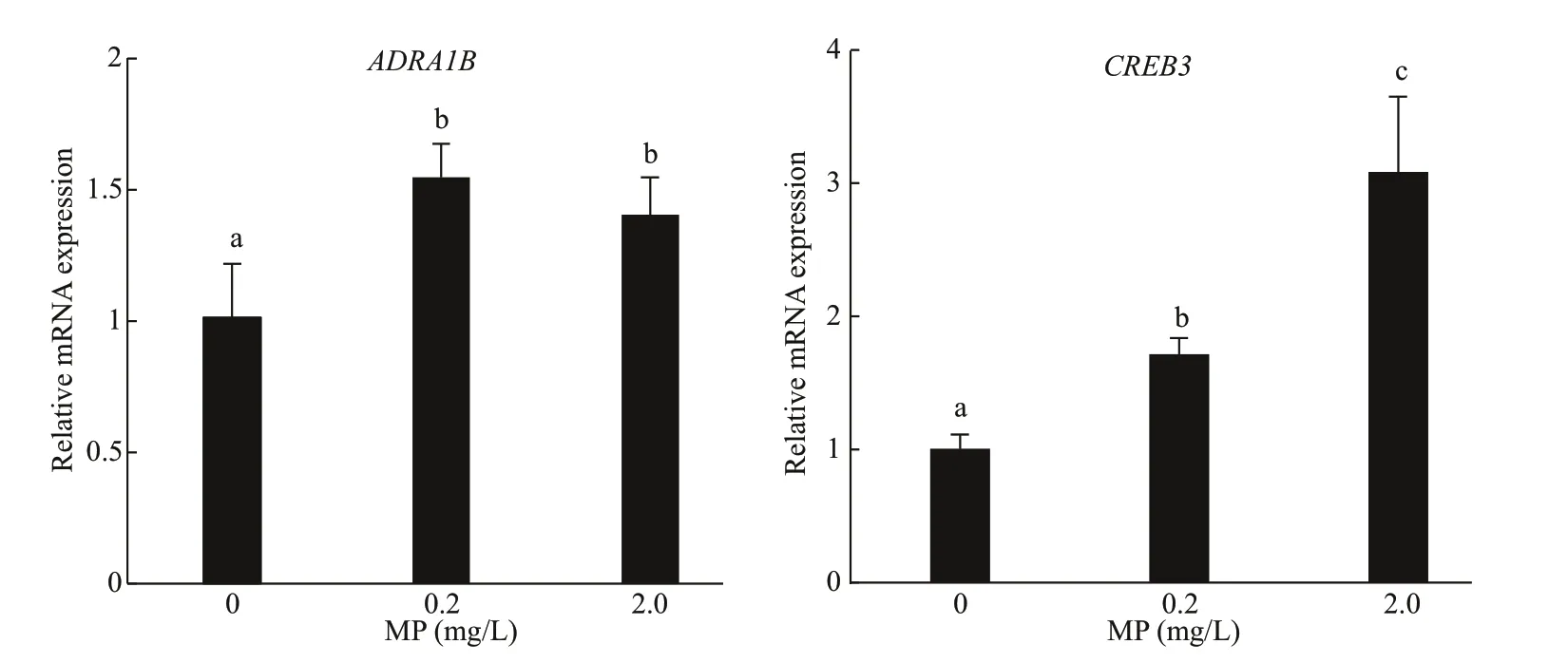
Fig.5 Genes expression of ADRA1B and CREB3 in Artemia after 14-d exposure to diff erent MP concentrations at 30 °C
3.4 Immune gene expression
A further confirmation of the defense mechanism is given by the gene expression of bothADRA1BandCREB3, which was significantly upregulated inArtemiaexposed to 0.2- and 2.0-mg/L MP for 14 d.After long-term exposure, a dose-dependent modulation ofCREB3was observed, and the gene expression increased with the increase of MP concentration (P<0.05). Compared to the control,0.2- and 2.0-mg/L MP exposure resulted in significant up-regulation ofADRA1B(P<0.05) (Fig.5).
4 DISCUSSION
To the best of our knowledge, the present study is the first to investigate the long-term eff ects of both MP and temperature on juvenile and adultA.franciscana, using multi-eff ect criteria, such as survival rate, growth, intestinal histology, and immune responses. Researchers believed that increases in temperature beyond the marine organism's suitable temperature range directly aff ect the metabolism and cause growth and physiological stress (Barber and Blake, 2006). Our results showed that temperature had significant eff ects on survival and growth ofArtemiaafter a long-term exposure, this is consistent with the funding of Browne and Wanigasekera (2000).Given that the temperature was the main factor aff ecting oxygen consumption (Irwin et al., 2007), it is a reasonable inference that survival rate and growth relate to the fact that rising temperatures impose additional developmental stress by speeding metabolism.
Artemianauplii is capable ofingesting MP beads ranging from 1 to 20 μm, and even bigger (Batel et al.,2016; Cole et al., 2016). A recent report suggested that there were no significant detrimental eff ects on growth and survival after a 14-d exposure to MP with 0.4, 0.8, and 1.6 mg/L at 25 °C, andArtemiacan ingest and egest MPs when continuously exposed to these concentrations (Peixoto et al., 2019). However,our study found that MP had significant eff ects on the survival rate ofArtemiaafter 14-d exposure, and survival rate and body length were significantly decreased at 30 °C and 2.0-mg/L MP concentration.Bergami et al. (2017) reported that naonoplastics PSNH2were able to disrupt the physiology and the energy flow in developingA.franciscana, and we can certainly deduce that the ingestion and accumulation of polystyrene MP particles (4–6 μ m) in the digestive tract might limit food intake and significantly aff ect growth and development ofArtemia(Besseling et al.,2014; Cole et al., 2016; Wang et al., 2019). Moreover,although temperature had significant eff ects on survival and growth ofArtemiaafter a long-term exposure, MP and temperature showed a combined stress onArtemia, especially at relatively higher level.Prior research has suggested that temperature was the dominant factor aff ecting oxygen consumption rates inArtemia, which showed a significant increase with increasing temperature from 0 to 30 °C (Irwin et al.,2007). Therefore, it can be hypothesized that the higher temperature could increase MP ingestion,metabolism and energy consumption ofArtemia, and thus aggravating the toxic eff ect of MP.
Oxidative stress occurs when the antioxidant defenses are overwhelmed by the production of reactive oxygen species (ROS). ACP is often used as a marker for intracellular lysosomal detection and as a reliable tool for environmental pollution assessment(Rajalakshmi and Mohandas, 2005). CAT is well known as an antioxidant enzyme that catalyzes hydrogen peroxide to water and oxygen, and plays an important role against hydroxyl radical toxicity(Bagnyukova et al., 2005; Ighodaro and Akinloye,2018). Our data showed that the enzyme activity of CAT and ACP increased with the increasing of MP concentration after 14 d. In particular, a significant increase in ACP activity was observed inArtemiawhen exposed to 30 °C and 2-mg/L MP concentration,revealing that high temperature and MP concentration could induced oxidative stress onArtemiamore easily. This may be due to environmental stimuli that lead to lysosomal membrane instability and therefore to increased ACP activity (Pampanin et al.,2002).
For most of marine organisms, especially zooplankton, their alimentary canal (gut) is the most important target organ where MP accumulates(Browne et al., 2008; Jemec et al., 2016). Wang et al.(2019) reported that after 24 h of exposure to 10-μm polystyrene at 10 and 100 particles/mL, the decreased and disordered microvilli, increased mitochondria and the presence of autophagosomes were observed inArtemiaintestine tissue. Similarly, we found that the intestinal microvilli and epithelia cells ofArtemiadecreased significantly and arranged disorderly when exposed to 2.0-mg/L MP at 30 °C, suggesting the relatively high MP concentration and temperature can cause an adverse impact on intestinal tissue. Since intestinal microvilli and epithelia are involved in a series of physiological processes, we can speculate that the damage ofintestinal tissue could aff ect the nutrient absorption and energy metabolism, and ultimately pose a threat toArtemiagrowth and health(Gunasekara et al., 2011).
Apoptosis is a programmed cell death that is regulated by genes, which is essential for normal development and homeostasis in plants and throughout the animal kingdom (Kiss, 2010).CREB3andADRA1Bhave been identified as apoptosis-related genes inArtemia(Zhang et al., 2018). Our study showed thatADRA1BandCREB3genes had high expressions in response to high MP concentration and temperature, indicating that the two genes may play an important role in regulating apoptosis, development and metabolism (Ping et al., 2011; Tressel et al.,2011). The strong induction ofCREB3andADRA1Bgenes indicates that high MP concentration and temperature may cause immunological stress, and aff ect the energy flow as well as the normal physiological processes inArtemiaover prolonged exposure.
5 CONCLUSION
MP and temperature have a combined adverse eff ect on survival and growth ofArtemia, especially at relatively high temperature and MP concentration.Temperature had significant eff ect onArtemiagrowth,and had significant interaction with MP concentrations on growth at 14 d. Exposure ofArtemiaat 30 °C and 2.0-mg/L MP significantly decreased the growth and survival rate ofArtemia, reduced the number of intestinal microvilli and epithelia cell, as well as induced oxidative stress and immunological stress.Our research provides a scientific basis for the risk assessment of MP pollution and elevated temperature on zooplankton in the marine environment. Further research is required to evaluate the combined eff ects of MP and temperature on intestinal absorptive function and intestinal microflora, and their possible impacts on the immune function ofArtemia.
6 CONFLICT OF INTEREST
The authors confirm that this article content has no conflict ofinterest.
7 DATA AVAILABILITY STATEMENT
The research data used in this study can be shared upon request.
8 ETHICS STATEMENT
The study protocol was approved by the Committee on the Ethics of Animal Experiments of Tianjin University of Science and Technology.
 Journal of Oceanology and Limnology2021年3期
Journal of Oceanology and Limnology2021年3期
- Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
