Investigation of the decline of Ulva prolifera in the Subei Shoal and Qingdao based on physiological changes*
Ya HAO, Chen GUAN, Xinyu ZHAO , Tongfei QU, Xuexi TANG ,2,**, Ying WANG,2,**
1 College of Marine Life Sciences, Ocean University of China, Qingdao 266100, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology,Qingdao 266071, China
Abstract It has been a scientific consensus that Ulva prolifera green tide that break out annually between May and August in the recent decade off eastern coast of Yellow Sea. To investigate the ecological mechanism of the decline of U. prolifera green tide, we compared the physiological characteristics of U.prolifera samples collected in three stages of the green tide in Subei Shoal north Jiangsu, the initiation point,on May 10 (early, the initiation), July 10 (middle, the outbreak), and in Taipingjiao, Qingdao on August 10(late, the decline). Compared to the early samples, the middle, and late samples showed a lower chlorophyll content and photosynthetic effi ciency at a higher lipid peroxidation level. The middle and later samples had fewer chloroplasts in the cells of U. prolifera than those in the early samples. In addition, the cells of U.prolifera sampled in the late stage showed ruptured and deformed cell walls. Diff erences in physiological function were reflected in the growth rate. Both the middle and late U. prolifera samples presented negative growth rates. Correlation analysis results show that the rising temperature was mainly responsible for the local decline of U. prolifera green tide. The high light-intensity that could induce the photoinhibition was also an important factor for the decline. Therefore, U. prolifera population that remained in the Subei Shoal and those that northward drifted to Qingdao experienced the same decline processes: rising temperature and continuous high light conditions in the late phase of green tide reduced the photosynthetic capacity and destroyed the antioxidant system of the thallus, which lead to decrease of biomass. The only diff erence is that the temperature in the Subei Shoal rose earlier than that in Qingdao; thus, U. prolifera remaining in the Subei Shoal went disappeared first.
Keyword: Ulva prolifera; green tide; decline process; photoinhibition; lipid peroxidation
1 INTRODUCTION
Since 2007, Yellow Sea green tide occurred in coastal China almost every year, and large-scale green tides caused considerable negative impact on off shore aquaculture, shipping, and tourism, resulting in many economic losses (Liu et al., 2009; Hu et al., 2010;Pang et al., 2010; Zhang et al., 2014, 2019).Ulvaproliferahas been identified as the causative macroalgal species based on morphological taxonomy and molecular biology techniques (Shen et al., 2012;Han et al., 2013; Zhao et al., 2013). Combining the results of a number of previous studies and satellite remote-sensing data (Zhang et al., 2014; Bao et al.,2015), it can be found that the green tide ofU.proliferawas originated fromPyropiaaquaculture rafts in the Subei Shoal prior to the formation oflarge-scale green tides in the southern Yellow Sea (Liu et al.,2010, 2013; Fan et al., 2012; Huo et al., 2016).

Fig.1 The occurrence and decline of the Yellow Sea green tide
In recent years, thePyropiaaquaculture rafts,which have continuously expanded to over 20 000 ha,were the adherent base for the growth of floating green macroalgae (Zhu and Chang, 2001; Song et al.,2015). Under the influence of natural and human factors,U.proliferafalling off the rafts has become an important source of floating green macroalgae.The green tide ofU.proliferausually initiated in the Subei Shoal about 2 weeks after the harvest ofPyropia, and a large amount ofU.proliferawas gradually exported from the Subei Shoal to the southern Yellow Sea. The algal mass drifted northward under the driving forces of seasonal winds and winddriven surface currents (Keesing et al., 2011; Fu et al.,2019). The green tide ofU.proliferacontinued to expand in the course of drifting north, and eventually developed into a large-scale green tide ecological disaster (Cui et al., 2012; Liu and Zhou, 2018). Because of the particular geographic location of the Shandong Peninsula, massive amounts ofU.proliferawere dominated the coast of the peninsula (Qiao et al.,2011). In the Subei Shoal, which is the area of origin of the green tide, decline of theU.proliferapopulation generally occurs in July, and the population ofU.proliferathat migrated to the nearshore of Qingdao generally disappears in August (Fig.1).
A considerable amount of research has been carried out on the occurrence and development of the green tide, but relatively little attention has been paid to the decline of the green tide. In this study, we focused on the relationship between environmental factors and the decline of theU.proliferapopulation by comparing the growth rate, photosynthetic capacity, lipid membrane peroxidation, and tissue structure ofU.proliferasamples in the outbreak and declinedecline stages. The environmental factors in each stage were measured,followed by correlation analysis between the environmental factors and the physiological indicators of theU.proliferasamples. The correlation analysis results were used to determine the key environment factors leading to the decline of theU.proliferapopulation. We aimed to explore the ecological reasons for the decline of theU.proliferagreen tide and provide ecological data for the prevention of the green tide in the South Yellow Sea of China.
2 MATERIAL AND METHOD
2.1 Sample collection
Free-floatingU.proliferawere collected in Xiaoyangkou, Rudong, Jiangsu Province on May 10 and July 10, and Taipingjiao, Qingdao, Shandong Province on August 10 (Fig.2). Samples collected in July and August were used as the research object for the decline of the green tide, and the samples collected during the early stage of the green tide outbreak in May were used as the comparison. Complete algalU.proliferawas collected at the sampling site and rinsed with sterile seawater to remove surface impurities.Field experiments were performed at the sampling site, which included the measurement of relative growth rate (RGR) and photosynthetic fluorescence parameters, and the preliminary fixation of transmission electronic microscopy (TEM) tissue sections. The remaining portion ofU.proliferasamples was stored in a -80-°C refrigerator for subsequent chlorophyll and lipid membrane peroxidation analysis.
Various environmental parameters were measured during the sample collection. Photosynthetically active radiation (PAR) was measured using a dual radiation meter (Zhuochuan, China). A standard thermometer(Fuyang Instrument Co., China) was used to measure temperature. Salinity was measured using a salinity meter (Lichen, China), and pH was measured using a portable pH meter (Mettler Toledo, Switzerland).
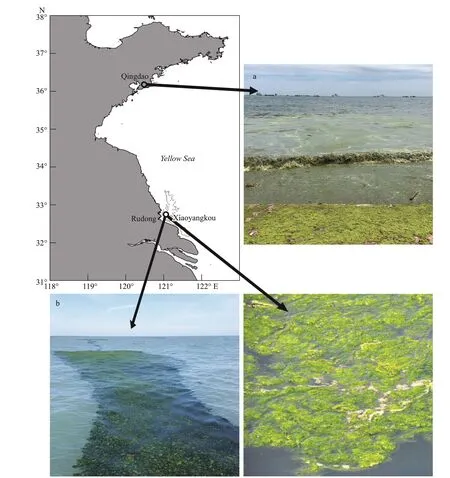
Fig.2 Sites of Ulva prolifera samples collection in the Subei Shoal off Rudong, Jiangsu, and the coast of Qingdao, Shandong
2.2 Growth rate
Relative growth rates (RGRs) ofU.proliferasamples were tested at the sampling site in a sevenday period. TheU.proliferasamples with initial biomass of 20 g were cultured in mesocosm (Fig.3).The culture conditions were consistent with the environmental factors at the corresponding times(Table 1). Algal material was reweighed after seven days, and relative growth rates were calculated as follows (Wu et al., 2018):
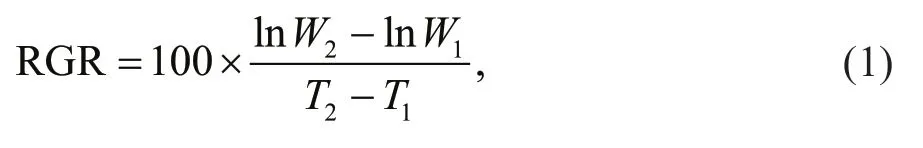
whereW1is the initial fresh weight andW2is the fresh weight after Δt(T2–T1) days.
2.3 Pigments extraction
Samples were ground to powder in liquid nitrogen and placed in a centrifuge tube. A precooling extraction buff er of 90% (v/v) acetone buff er (5 mL)was used to extract the pigments at 4 °C in darkness.The mixture was then centrifuged at 10 000 r/min and 4 °C, and the supernatant was filtered and analyzed spectrophotometrically (TU-1800, PERSEE, China).The chlorophylla(Chla), chlorophyllb(Chlb), and total chlorophyll (Chl) contents were calculated asfollows (Arnon, 1949):

Table 1 Environmental parameters of sampling site in diff erent months

2.4 Measurement of chlorophyll fluorescence
A Dual-PAM-100 fluorometer (Walz, Germany)was used to measure the photosynthetic performance ofU.proliferasamples. Samples were acclimated in the dark for 20 min prior to measurements. The settings of the Dual-PAM-F fluorometer followed previous research in this laboratory (Zhao et al., 2016). The maximum quantum yield (Fv/Fm) of photosystem II(PSII) can be calculated by the equation:

whereFmis the maximum fluorescence for darkadapted tissue,F0is the minimum fluorescence for dark adapted tissue, andFvis the maximum fluorescence. YII is the eff ective quantum yield of PSII, and can be used to evaluate photosynthetic activity in the thalli.
The rapid light response curves (RLCs) were measured after the induction curve experiment. When the RLCs were measured, the samples were exposed to a light intensity gradient (PAR: 0, 25, 40, 56, 73,129, 169, 276, 342, 534, 828, and 1 597 μmol photons/(m2·s)). The RLCs were fitted using Platt’s empirical equation to obtain the following parameters: rETRmax(maximum relative electron transport rate),α(photosynthetic rate in the light-limited region of the RLCs),β(photoinhibition parameter), andEk(minimum saturating irradiance) (Platt et al., 1980).
2.5 Analysis oflipid peroxidation
The level oflipid peroxidation was studied as malondialdehyde (MDA) formation (Buege and Aust,1978). Total levels of soluble protein of the crude extract for antioxidant enzyme activities((g protein)/mL or (g pro)/mL) were determined using a commercial protein assay kit (Nanjing Jiancheng,China). The MDA content was determined using a commercial assay kit (Nanjing Jiancheng, China) and results are expressed as n·mol MDA per mg of total soluble protein (nmol MDA/(mg pro)).
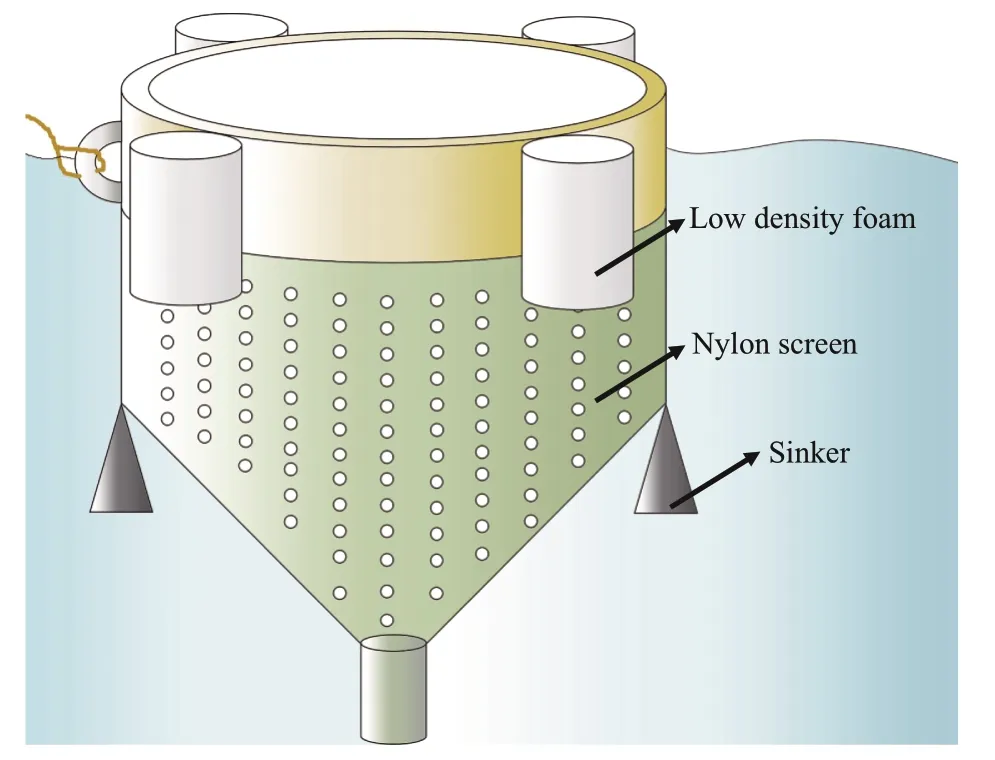
Fig.3 Schematic diagram of the mesocosm experiment for measurement of relative growth rate
2.6 Transmission electron microscope observation
For TEM analysis, samples of 1-mm length were fixed in 5-mL 2.5% glutaraldehyde and 2%paraformaldehyde in 0.01-mol/L phosphate buff er saline (PBS) (pH 7.2) for 24 h at 4 °C (Farias et al.,2017). After initial fixation, samples were sent to Servicebio technology company (Wuhan, China) for subsequent preparation, block cutting and transmission electron microscopy analysis. The specimens were observed with a transmission electronic microscopy(JEOL JEM-1011, Japan).
2.7 Data analysis
All data were obtained by analyzing three biological replicates and displayed as mean±standard deviation(SD). The environmental parameters and laboratory data were tested using one-way ANOVA and a Fisher LSD mean comparison. Bivariate Pearson’s correlation analysis was performed to assess the relationships of the physiological indices ofU.proliferaand environmental factors. The data were analyzed using IBM SPSS Statistics 22 (SPSS Inc.,USA), and P-values ofless than 0.05 were considered statistically significant.
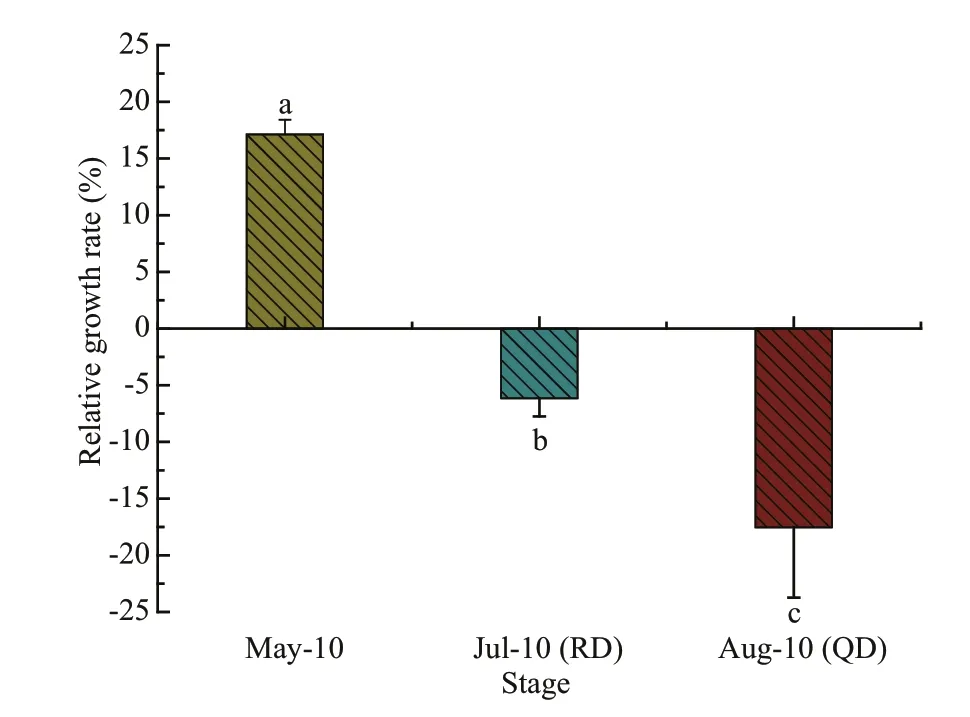
Fig.4 Relative growth rate (RGR) of U. prolifera samples collected in three stages
3 RESULT
3.1 Environmental factor
The environmental parameters of the sampling locations are shown in Table 1. The survey data indicate that no significant diff erence was detected in the parameters of salinity or pH between the three stages (one-way ANOVA;P<0.05). The temperature of Rudong in July and that of Qingdao in August were significantly higher than that of Rudong in May (oneway ANOVA;P<0.05). The PAR value of Qingdao in August was significantly higher than those of Rudong in May and July (one-way ANOVA;P<0.05).
3.2 Growth rate
The RGR ofU.proliferain three stages are shown in Fig.4. The culture conditions of the samples collected in each phase were consistent with the onsite environmental conditions of the corresponding month (Table 1). The growth rate ofU.proliferain May was the highest, reaching 17.13%±1.29%. The samples ofU.proliferain July and August showed negative growth, and the negative growth rate ofU.proliferain August reached -17.54%±6.2%, which was significantly higher than that in July (one-way ANOVA;P<0.05).
3.3 Pigment content

Fig.5 Photosynthetic pigment content of U. prolifera samples in three stages
The results for pigment content ofU.proliferasamples in three stages are shown in Fig.5. The Chla, Chl-b, and total Chl concentrations ofU.proliferasamples collected in Rudong in May were significantly higher than those of Rudong in July and Qingdao in August (one-way ANOVA;P<0.05). The Chl-a, Chl-b, and total Chl concentrations of theU.proliferasamples from Qingdao in August were all clearly the lowest of the three stages (one-way ANOVA;P<0.05).
3.4 Chlorophyll fluorescence parameters
Figure 6a shows the diff erence ofFv/Fmin the thalli ofU.proliferain the three phases. TheFv/Fmvalue ofU.proliferasamples from Rudong in May was significantly higher than those from Rudong in July and Qingdao in August (Fig.6a). Similar results can be seen in the eff ective quantum yields of PSII (YII)(Fig.6b), and the YII value forU.proliferasamples from Rudong in May was noticeably higher than those from Rudong in July and Qingdao in August.
The results of the RLCs are shown in Fig.7. The RLCs of the samples from Rudong in May show a linear rise at the beginning followed by stabilization as the intensity of the light increases. The convexity of the curves was noticeably lower for both samples from Rudong in July and from Qingdao in August compared with that from Rudong in May (one-way ANOVA;P<0.05).
A further conclusion can be drawn based on the results of the RLCs. Table 2 shows that the values of the parameters rETRmaxandEkofU.proliferasamples collected in Rudong in May were significantly higher than those of Rudong in July and Qingdao in August(one-way ANOVA;P<0.05). Theβvalues of samples collected in Rudong in May were noticeably lowerthan those from both Rudong in July and Qingdao in August (one-way ANOVA;P<0.05). We observed that the value ofαfrom samples collected in Rudong in July was significantly lower than the other two stages (one-way ANOVA;P<0.05).
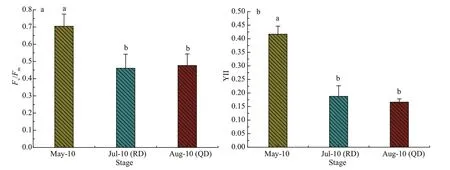
Fig.6 Results ofinduction curve parameters measurement

Table 2 Mean parameters of the RLCs of Ulva prolifera samples in three stages
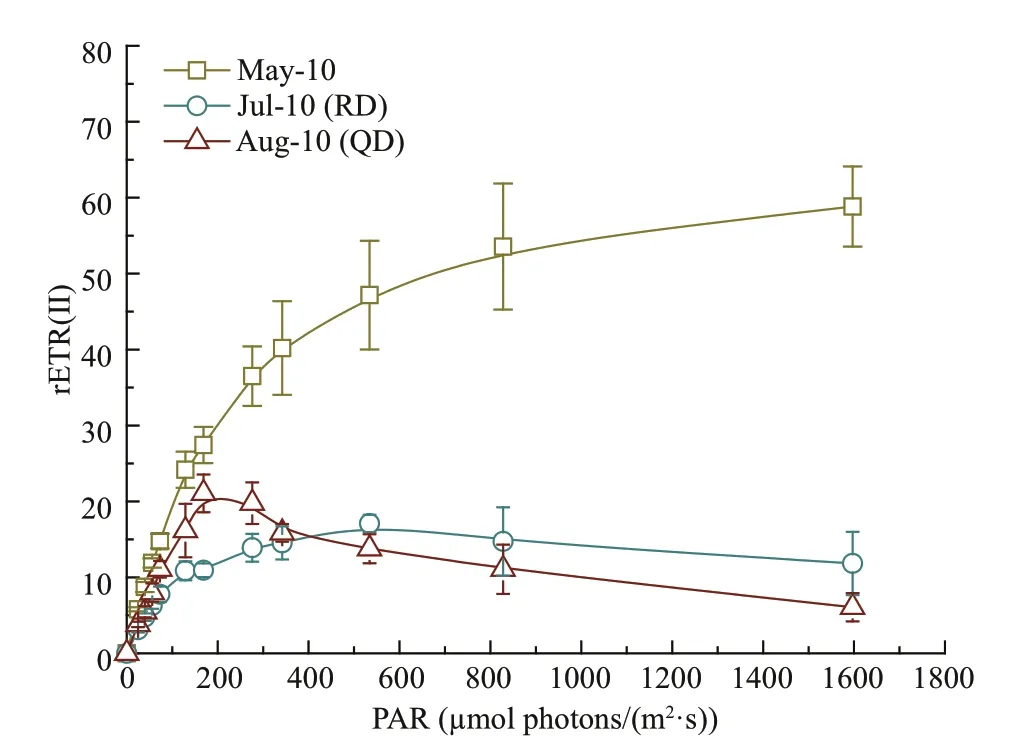
Fig.7 Mean relative electron transport rate (rETR) of rapid light response curves (RLCs) of Ulva prolifera in three stages
3.5 Oxidative stress
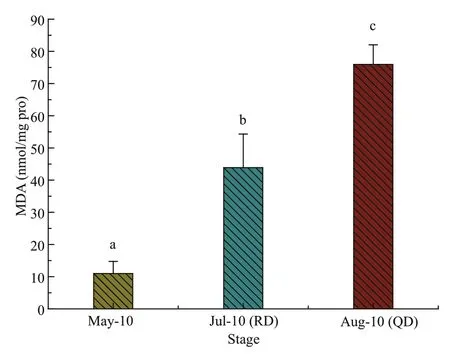
Fig.8 The content of MDA from Ulva prolifera samples collected in three stages
Figure 8 shows the contents of MDA. The MDA contents of the samples were noticeably higher for samples both from Rudong in July and from Qingdao in August compared with those from Rudong in May,and the MDA contents of the samples from Qingdao in August were significantly higher than those from Rudong in July (one-way ANOVA;P<0.05).
3.6 Ultrastructure

Fig.9 Ultrastructure of Ulva prolifera samples in the three stages revealed in transmission electronic microscopy
The transmission electronic microscopy results are shown in Fig.9. Transmission electronic micrographs ofU.proliferasamples from the outbreak of the green tide in May showed that the cell structure was intact, and several protein cores and starch grains were surrounded by chloroplasts inside and the inner edge of the cell wall was smooth(Fig.9a). Compared with theU.proliferasamples from the green tide outbreak phase, transmission electronic micrographs ofU.proliferasamples from the decline phase of the green tide, which included both the samples from Rudong in July and those from Qingdao in August, showed that the number of chloroplasts in the cells was significantly reduced;the nucleus, protein nucleus, and starch granules disappeared; the inner edge of the cell wall was ruptured and deformed; and lipid droplets increased(Fig.9b & c). Overall, there was significant cellular structure damage in theU.proliferasamples of the decline period of the green tide.
3.7 Correlation analysis
Table 3 shows that the RGR ofU.proliferawas significantly positively correlated with the values ofFv/Fm, YII, rETRmax,Ek, and chlorophyll content, and significantly negatively correlated with the photoinhibition parameterβ, MDA content, ambient temperature, and PAR intensity (one-way ANOVA;P<0.05). MDA content was significantly positively correlated with ambient temperature and PAR intensity (one-way ANOVA;P<0.05). The photoinhibition parameterβand PAR intensity showed a significant positive correlation (one-way ANOVA;P<0.05). The correlation between salinity and each physiological index ofU.proliferawas not significant (one-way ANOVA;P>0.05). In addition,there was no significant correlation between the pH of seawater in the survey area and each physiological index ofU.prolifera(one-way ANOVA;P>0.05).
4 DISCUSSION
4.1 The intrinsic factor of U. prolifera population decline
We collected samples ofU.proliferafrom the outbreak of the green tide in the Subei Shoal in May and samples ofU.proliferafrom the decline phase of the green tide in the Subei Shoal in July, as well as from Qingdao in August. The growth rate,photosynthetic capacity, lipid membrane peroxidation,and tissue ultrastructure ofU.proliferasamples in the decline phase were studied.
Through experiments, we could conclude that the photosynthetic ability and chlorophyll content ofU.proliferain the decline period were significantly lower than those in the outbreak phase. These physiological changes adversely aff ected the material synthesis process ofU.prolifera, and the growth of theU.proliferapopulation slowed compared with the rapid population growth in the outbreak stage. In the decline phase, the MDA content ofU.proliferawas significantly higher than that in the outbreak phase,and the lipid membrane peroxidation was severely aggravated, which led to the loss of structural integrity of the tissue cells. Therefore, the intensification of lipid membrane peroxidation and further cell necrosis were the main internal causes of the decline of theU.proliferapopulation.
4.2 External drivers of U. prolifera population decline
Previous research confirmed that algal growth rates are regulated by environmental factors such as temperature, irradiance, and nutrient level (Taylor et al., 2001; Kim et al., 2016; Wu et al., 2018). High temperatures would inhibit the growth ofU.prolifera(Wu et al., 2018). Through the correlation analysis between the physiological indicators ofU.proliferasamples and environmental factors of the three phases,we could conclude that high temperature was the most important external environmental factor leading to ineffi cient photosynthesis, increased lipid peroxidation,and structural damage ofU.prolifera. Therefore, we conclude that the long-term and sustained high temperature conditions were the main cause of the decline of theU.proliferapopulation. The PAR intensity in Qingdao in August was higher compared with that in Rudong in July, which further deepened the lipid peroxidation level ofU.proliferaand led to higher photoinhibition (Table 2), and eventually presented as a higher negative growth rate than that in Rudong in July.
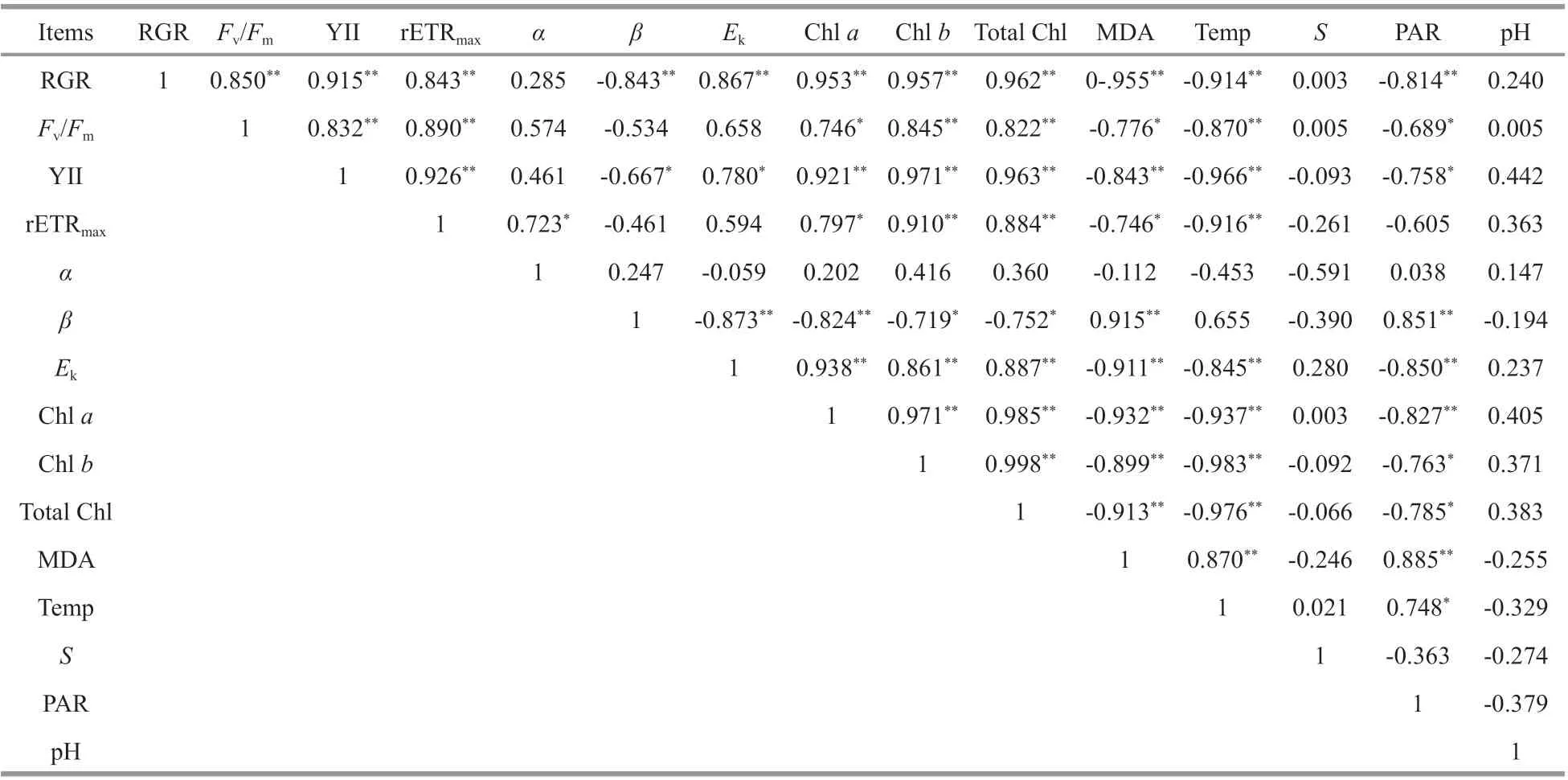
Table 3 Correlation analysis between physiological parameters of Ulva prolifera samples and environmental factors
Given the relatively stable salinity and pH conditions in the space-time span of the Yellow Sea,their correlation with the changes of physiological process parameters ofU.proliferawas not significant(Table 3). The spatio-temporal variation of nutrient content, seawater transparency and other marine physicochemical indicators in the survey area and their eff ects on the structure of theU.proliferapopulation need further field investigation and statistical verification.
4.3 Analysis of U. prolifera population decline in the Subei Shoal and off shore of Qingdao
As the origin area of the South Yellow Sea green tide, there was a large amount of biomass of the primordial population ofU.proliferain the Subei Shoal from April to June. A large amount ofU.proliferawas exported from the Subei Shoal to the South Yellow Sea under the dual eff ects of seasonal winds and wind-driven surface currents (Keesing et al., 2011; Liu and Zhou, 2018; Fu et al., 2019). The temperature of seawater increased in mid-July (Fan et al., 2015), and the environmental stress on theU.proliferapopulation caused by persistent high temperature increased. The chlorophyll content and photosynthetic effi ciency decreased significantly(Figs.5–7), and the tissue cells ruptured (Fig.9), which ultimately led to the decline of theU.proliferapopulation.
Because of the higher latitude, the ambient temperature rising in Qingdao off shore occurred later than that in the Subei Shoal, and theU.proliferapopulation that constituted the green tide drifted over a long distance before reaching off shore of Qingdao.The harsh environment had a negative impact on the physiological activities and organizational structure ofU.proliferaduring the drift course (Mou et al.,2013; Zhao et al., 2016). The ambient temperature and the light intensity off shore of Qingdao increased in August, and as a result, the external environmental stress experienced by theU.proliferapopulation blocked on the coast of Qingdao was intensified.
TheU.proliferasamples of this phase showed low photosynthetic effi ciency and photoinhibition, and exacerbated lipid membrane peroxidation, cell necrosis. The seawater temperature in the Subei Shoal increased earlier than that off shore of the Shandong Peninsula because of the diff erence in latitude.Therefore, the decline of theU.proliferapopulation in the Subei Shoal was earlier than that off Qingdao.Macroscopically, theU.proliferapopulation that constitutes the green tide of the South Yellow Sea had lost the supplementary source of biomass after the decline of theU.proliferapopulation remaining in the Subei Shoal in mid-July. Aff ected by topography,theU.proliferapopulation was stranded on the coast of the Shandong Peninsula during northward drifting and eventually died out because of the high temperature and high intensity radiation.
5 CONCLUSION
Through determination of the physiological parameters ofU.proliferasamples in the outbreak phase and the decline phase, and combined multiparameter correlation analysis of environmental factors, we conclude that the most important environmental factor leading to the decline of theU.proliferapopulation was temperature. High ambient temperature could lead to intensification of the lipid peroxidation ofU.proliferaand reduce photosynthesis effi ciency and chlorophyll content, which ultimately led to negative growth and decline of theU.proliferapopulation. In addition, the PAR intensity was significantly positively correlated with the photoinhibition parameter. Excessive PAR intensity could cause photoinhibition during the photosynthetic process, which was also an important environmental factor leading to the decline of theU.proliferapopulation. The salinity and pH of the seawater were not inducing factors for the decline of theU.proliferapopulation. In conclusion, it can be inferred from this experiment that the decline of theU.proliferapopulation remaining in the Subei Shoal was mainly attributed to the increase of temperature, and that this local decline in the Subei Shoal was earlier than that of theU.proliferapopulation blocked off shore of Qingdao because of the diff erence in latitude between the two places. In addition, the impact of this longdistance drift process on the physiological activity and population dynamics ofU.proliferarequires further research and demonstration.
6 DATA AVAILABILITY STATEMENT
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2021年3期
Journal of Oceanology and Limnology2021年3期
- Journal of Oceanology and Limnology的其它文章
- Steady increase in water clarity in Jiaozhou Bay in the Yellow Sea from 2000 to 2018: Observations from MODIS*
- Phylogenetic diversity and bioactivity of culturable deepsea-derived fungi from Okinawa Trough*
- Allelopathic eff ects of mixotrophic dinoflagellate Akashiwo sanguinea on co-occurring phytoplankton: the significance of nutritional ecology*
- Effi ciency of phosphorus accumulation by plankton,periphyton developed on submerged artificial substrata and metaphyton: in-situ observation in two shallow ponds*
- Petroleum exploitation enriches the sulfonamide resistance gene sul2 in off shore sediments
- Mesoscale wind stress-SST coupling induced feedback to the ocean in the western coast of South America*
