Simultaneous determination of 74 pesticide residues in Panax notoginseng by QuEChERS coupled with gas chromatography tandem mass spectrometry
Huijun Li, Jishan Wu, Chao Chn, Wnfng Xin, Wnshng Zhang,*
a Engineering Research Center of Natural Medicine, Ministry of Education, Beijing Normal University at Zhuhai 519087, China
b Zhuhai Branch of State Key Laboratory of Earth Surface Processes and Resource Ecology, Beijing Normal University at Zhuhai 519087, China
c Gongbei customs of the People’s Republic of China, Zhuhai 519000, China
d Beijing Key Laboratory of Traditional Chinese Medicine Protection and Utilization, Faculty of Geographical Science, Beijing Normal University, Beijing 100875, China
e National and Local United Engineering Research Center for Panax Notoginseng Resources Protection and Utilization Technology, Kunming 650000, China
ABSTRACT
This study established a method for the simultaneous determination of 74 pesticide residues in Panax notoginseng by QuEChERS pretreatment method coupled with GC-MS/MS, and carried out pesticide residue analysis on 20 batches of market samples in China. The samples were extracted with acetonitrile, cleaned up with primary secondary amine (PSA) and octadecylsilane (C18) and determined by GC-MS/MS in multiple reaction monitoring (MRM) mode. Matrix-matched calibration was recommended to combat the matrix effect.A good linearity was observed in the range of 10-500 ng/mL with correlation coefficients ≥ 0.9950. The mean recoveries for most of the pesticides were in the range of 70%-120% with RSD < 20%. The limits of detection ranged 0.28-2.00 μg/kg, while the limits of quantification were 0.94-6.65 μg/kg. Following the application of“top-down” approach, the expanded measurement uncertainty for all the analytes was < 30%. The proposed method was successfully applied to determine pesticide residues in 20 market samples in China, where 9 pesticides were detected and quintozene exceeded the criteria domestically and abroad.
Keywords:
Panax notoginseng
QuEChERS
GC-MS/MS
Pesticide residues
1. Introduction
Panax notoginseng (Burk.) F.H. Chen is a species of the genus Panax, family Araliaceae. The root of P. notoginseng, known as Sanqi or Tianqi, is one of the important traditional Chinese medicine(TCMs) [1]. In Chinese pharmacopoeia, the functions of this herb include dispersing gore, stanching blood, relieving swelling and alleviating pain [2]. The stem, leaf and flower of P. notoginseng can also be medicinally used. As one of the earliest Chinese medicines with the same origin as food, P. notoginseng has been included in the list of items available for health food by the Ministry of Health in China. It can be added to cooking or wine due to its tonic action[3]. Some people consume Notoginseng powder daily as a health care product. It is reported that there are many active compounds in P.notoginseng, including saponins,flavonoid, saccharides, cyclopeptides and others [4]. So far, over 200 chemical constituents have been isolated from P. notoginseng, and Panax notoginseng saponins (PNS)are considered as the main constituents [5]. Scientific studies on P. notoginseng indicate that it has wide-reaching pharmacological activities, including the effects on the cardiovascular system and the immune system, as well as anti-inflammatory activity [6], antioxidant activity [7], hypoglycemic activity [8], antihypertension effect [9],neuroprotective effect [10] and so on.
The distribution of P. notoginseng is very narrow due to its sensitivity to sunlight. As a result of a long period of evolution, it grows primarily in mountain area with an altitude between 1 200 and 2 000 m of the Wenshan region, Yunnan province around N 23.5°and E 104° [3]. The species distributed in this region are all cultivated populations domesticated by local habitants, and its wild relatives have not been found until now [11]. Wenshan has been nominated as the hometown of P. notoginseng due to having the best quality. However, plant diseases and insect pests easily occur during its growth. This includes root rot, round spot and black spot[12]. So, a large amount of pesticides is used in the cultivation of P. notoginseng, leading to the problem of pesticide residues.This seriously affects its quality and safety, as well as industrial development.
Pesticide residues analysis in food usually involves extraction,clean-up and chromatographic determination combined with different detection systems. A number of different sample extraction procedures are applied, such as Soxhlet extraction, microwave-assisted extraction(MAE), accelerated solvent extraction (ASE) and ultrasonic extraction(UE) [13]. They are typically combined with solid-phase extraction(SPE) cleanup. Anastassiades et al. [14] developed the QuECHERS(Quick, Easy, Cheap, Effective, Rugged and Safe) technique for determination of pesticide residual in food. This technique involves liquid-liquid partitioning using acetonitrile and purifying the extract using dispersive solid-phase extraction (d-SPE). Compared to food[15,16], determination of residual pesticides in medicinal herbs is more difficult for the existence of secondary metabolites. These secondary metabolites may get extracted as a co-extract and thus make the analytical procedure highly complicated. Nowadays,QuECHERS has been the most effectual and efficient method in extracting traces of pesticides in a wide variety of matrices including TCMs [17-19]. Gas chromatography (GC) is an efficient tool for the separation of pesticides. During the assay of pesticide residues, GC is usually combined with different detection systems, such as electron capture detector (ECD), nitrogen phosphorus detector (NPD) and flame photometric detector (FPD) [20]. GC equipped with MS/MS is an advanced analytical method with high sensitivity and selectivity. It provides accurate qualitative and quantitative determination of several hundred possible single compounds or combinations in the presence of complex matrices [21].
The aim of this study was to develop a simple and effective method based on QuECHERS coupled with GC-MS/MS for the determination of multiclass pesticides in P. notoginseng. The extraction and cleanup steps of QuECHERS were optimized and validated in this study. This methodology was suitable for the determination of 74 pesticide residuals in P. notoginseng. which supplemented the existing knowledge. The modified QuEChERS followed by GC-MS/MS analysis was applied to analyzing multiple pesticide residues in 20 market samples to provide confidence of P.notoginseng safety.
2. Materials and methods
2.1 Instruments
The analytes were determined using an Agilent 7890 gas chromatograph coupled with an Agilent 7000 triple quadrupole mass spectrometer, equipped with an electron impact ion source(EI) (Agilent, USA). The laboratory shaker and vortex mixer were obtained from IKA, Germany. The high-speed centrifuge was bought from Anting Scientific Instrument Factory (Shanghai, China).Nitrogen blowing instrument was provided by Caliper, USA.
2.2 Chemicals and reagents
The 74 pesticide standards with purity > 98% were purchased from Dr. Ehrenstorfer Gmb (Augsburg, Germany). Acetonitrile was a chromatography grade solvent (Spectrum, USA). Analytical grade anhydrous magnesium sulfate (anhydrous MgSO4) and sodium chloride (NaCl) were obtained from Guangzhou Chemical Reagent Factory (Guangdong, China). Primary secondary amine (PSA) and octadecylsilane (C18) were bought from Agela Technologies Co., Ltd(Tianjin, China).
2.3 Samples
The market samples were procured from Anguo medicinal material market (Hebei, China, n = 10) and Bozhou medicinal material market (Anhui, China, n = 10). Roots of P. notoginseng were obtained from the field of Good Agricultural Practice (GAP),Wenshan, Yunnan, China, with no history of pesticide use and taken as alternative matrices for sample preparation investigation and method validation.
2.4 Preparation of standard solutions
Primary stock solution of each pesticide was made in acetone or methanol based on the solubility of individual pesticides. The stock standard mixture (each pesticide at the level of 10 μg/mL) of all analytes was obtained by diluting each primary solution with acetone.The stock mixture was applied for the preparation of working standard solutions and the spiked samples. All the stock and working standard solutions were stored at -18 °C when not in use.
Matrix-matched standards were prepared as follows: the blank sample was treated by the developed method, and the extract was then dried through nitrogen evaporation. After 1 mL of the working standard solutions with different concentrations was added, the resulting mixture was shaken and filtered through a 0.22 μm organic membrane to obtain matrix-matched standard solutions of the corresponding concentrations.
2.5 Sample preparation
All samples were crushed into fine powder. Two grams of each homogenized sample was accurately weighed into a 50 mL polypropylene (PP) centrifuge tube. Ten milliliter of water was added to the tube, which was then closed and left at room temperature for 30 min to make the mixtures fully soaked. After that, add 10 mL acetonitrile and shake the sample vigorously for 1 min by using the vortex mixer. Then, the sample was extracted on a laboratory shaker for 10 min. Subsequently, add 4 g anhydrous MgSO4and 1 g NaCl,and mix on a vortex mixer immediately for 1 min. The mixture was then centrifuged at 5 000 r/min for 2 min. A 7 mL aliquot of acetonitrile supernatant was transferred to a new clean 10 mL polypropylene centrifuge tube containing 100 mg PSA, 100 mg C18and 100 mg anhydrous MgSO4as sorbents. The extract was vortexmixed for 30 s and centrifuged at 6 000 r/min for 2 min. A 5 mL portion of the upper layer was transferred to a 50 mL round-bottom flask and concentrated to near dryness on a vacuum rotary evaporator at 40 °C. The resulting residue was redissolved with 1 mLn-hexane,filtrated through a 0.22 μm organic membrane, and then subjected to GC-MS/MS analysis.
2.6 GC-MS/MS conditions
GC separation was performed on a HP-5 MS (30 m × 250 mm ×0.25 μm) capillary column. Helium (purity > 99.999%) was selected as carrier gas with an identical flow rate of 0.8 mL/min. The inlet temperature was 280 °C. The injection volume was 1.0 μL in the splitless mode. The oven temperature was programmed from an initial value of 50 °C holding for 1 min, then raised at a rate of 40 °C/min up to 120 °C, then raised at a rate of 5 °C/min up to 310 °C.
The mass spectrometer was operated in electron impact ionization mode (EI). The electron energy and temperature of the EI source were set at 70 eV, 280 °C, respectively. Nitrogen (purity > 99.999%)worked as the collision gas for the multiple reaction monitoring(MRM) mode. Instrument control, data acquisition and evaluation were performed using the MassHunter software (The retention times and selective ions monitored for the different pesticides are given in Table 1). The total ion chromatograms (TICs) of 74 pesticides are shown in Fig. 1.

Fig. 1 The total ion chromatograms (TICs) obtained by GC-MS/MS analysis of (A) blank P. notoginseng extract, (B) matrix-matched standard solution at 100 μg/L and (C) standard solution in organic solvent at 100 μg/L.

Table 1Retention time (RT) and mass spectrometric conditions of 74 pesticides.
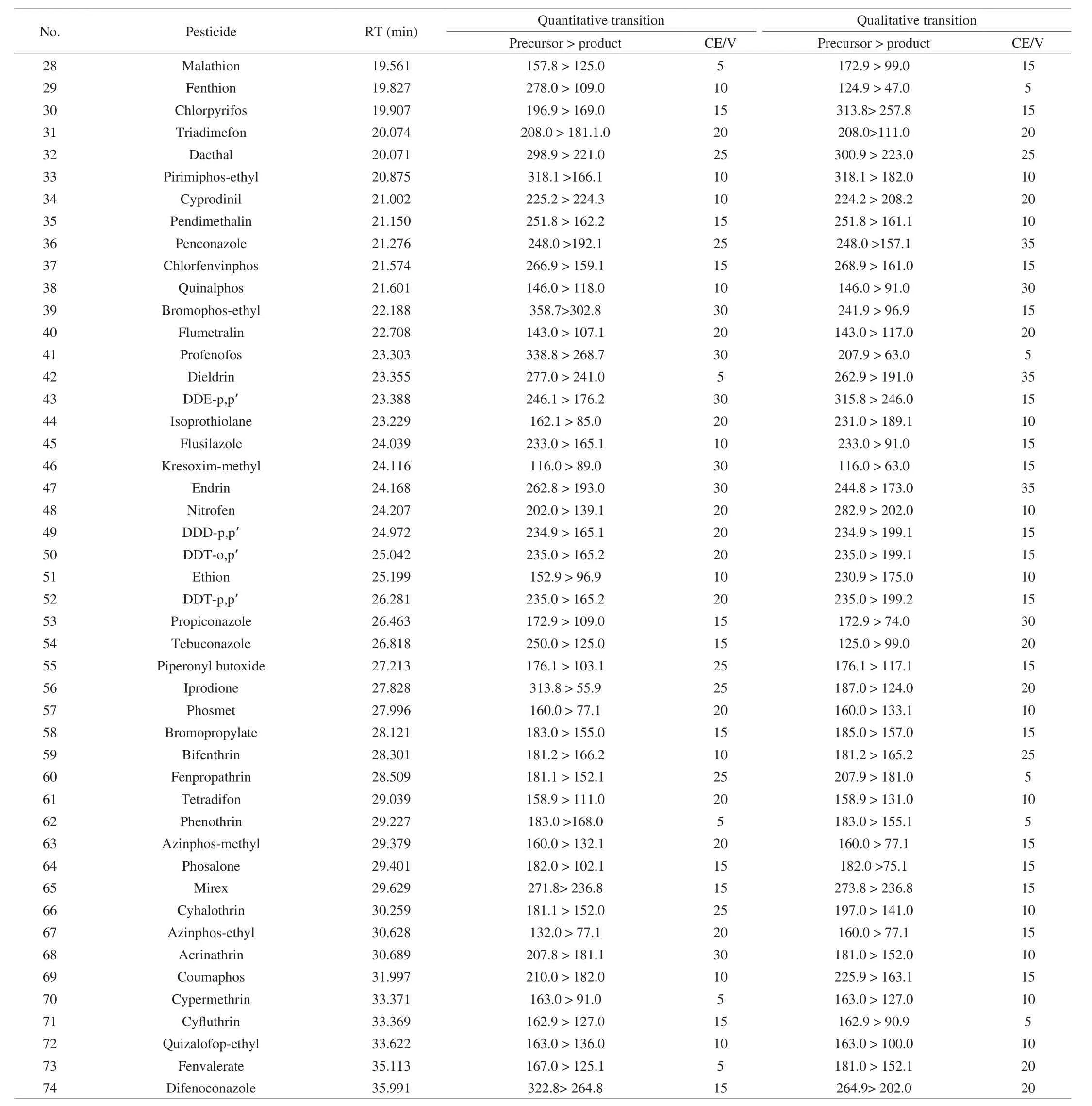
Table 1 (Continued)
2.7 Matrix effect
The matrix effect (ME) is a measurement of how much an analyte concentration or mass is influenced by one or more undetected components from the sample [22]. In order to estimate these potential impacts, the slopes obtained in the calibration curves by matrix-based standards were compared with those obtained by solvent-matched standards, and the ME of each pesticide was calculated by the following formula [23].

WhereAwas matrix-based standards,Bwas solvent-matched standards. The slope ratio of 1 denotes that the response of analyte is not suppressed or enhanced by matrix, otherwise meaning ionization enhancement (> 1) or suppression (< 1).
2.8 Method validation
The performance of the analytical method was evaluated by considering the following validation parameters viz., linearity,limit of detection (LOD), limit of quantification (LOQ), accuracy and precision. Linearity for all the target pesticides was evaluated by matrix-matched calibration. Calibration curves were drawn by plotting the relative peak area against the concentration of the corresponding calibration standards at calibration levels of 10,50, 100, 200 and 500 ng/mL. The LOD was determined as the concentration producing a signal-to-noise ratio of 3, and the LOQ was viewed as a signal-to-noise ratio of 10 at the lowest spiking level of the respective pesticides. The accuracy and precision were estimated at 25, 50 and 200 μg/kg for all the analytes in 6 replicates at each level. Mean recovery and relative standard deviation (RSD) were employed to measure the accuracy and precision. The samples were spiked with the pesticides before proceeding with the extraction and the results from the recovery study were assessed for compliance with the European SANTE/11813/2017 criteria [24], according to which the average recovery should fall in the range of 70%-120% with an associated RSD less or equal 20%. All the analyses were performed using the same blank samples.
The measurement uncertainty was estimated on the basis of the “top down” approach using the data derived from the validation experiments [25]. The uncertainty sources included in the uncertainty budget were repeatability of analysis of spiked samples (measured as RSD) and uncertainty of average recovery calculated from rectangular distribution. The expanded uncertainty was calculated as twice the value of the measurement uncertainty(k = 2, confidence level 95%) [26].
3. Results and discussion
3.1 Selection of extraction method
For sample preparation, the original QuEChERS method was adopted and modified in this study as it offers a very flexible approach and many options for analysis depending on the range of pesticides and matrices being tested. Considering that the target pesticides included a large cohort of different chemical families(such as organochlorine pesticides (OCPs), organophosphorus pesticides (OPPs) and pyrethroid pesticides (PYRs), etc.),acetonitrile was used as the extraction solvent because of its effectiveness for polar and non-polar pesticides from a diverse range of matrices and also give high recoveries of a wide polarity range of pesticides [27]. What’s more, acetonitrile has the potential of less co-extracted matrix components and less interference during GC analysis [20]. Besides, acetonitrile is miscible with water. When the crude drug powder is saturated with water,acetonitrile can penetrate the cell wall easier than other solvents like ethyl acetate and n-hexane [28].
The original QuEChERS method was a pretreatment technique developed for vegetables and fruits with water content greater than 80% [29]. For the low water content (≤ 25%) matrices like spices,cereals and dried fruits, sample amount is usually reduced and additional water is added to ensure optimum extraction [30]. For example, He et al. [31] used the technique of hydrating dry samples when analyzing cereal samples (ground rice, wheat flour and ground corn). A volume of 10 mL of purified water was added to each 5 g of cereal sample (rice, wheat flour and corn) that was subjected to the QuEChERS methodology. A similar hydrating strategy was successfully used by Lee et al. [32] during analysis of rice samples using the QuEChERS technique. During the QuEChERS procedure,2 g of rice sample was hydrated using 10 mL of water to ensure effective extraction of pesticides. The root of P. notoginseng is the kind of dry matrix. Therefore, we lowered the sample amount to 2 g and added 10 mL water before proceeding with the acetonitrile extraction [33].
Salting out effect with magnesium sulphate is one of the key elements in QuEChERS theory. Addition of salts induces phase separation and thus transition of analyte between two phases[34]. In this study, salting out of water from the sample is done using anhydrous MgSO4and NaCl [35]. The purpose of NaCl is to increase ionic strength of the water, and thereby increasing its polarity [34].
3.2 Optimization of cleanup procedure
Sample cleanup is necessary during QuEChERS to reduce interferences. The presence of interferences can damage analytical instrumentation and complicate pesticide identification and quantification. Thus, the option of suitable adsorbent is a key factor to obtain high enrichment and good recovery in QuEChERS procedure[36]. As a complex matrix, P. notoginseng contains a high amount of compounds, such as saponins, flavonoids, cyclopeptides, sterols,polyacetylenes, volatile oil, amino acids, and polysaccharides [37].To solve this problem, a cleanup step using some sorbents prior to the injection was proposed. PSA, C18and graphitized carbon black(GCB) are the most commonly used adsorbent in food matrices [38].PSA is mainly used to remove the organic acids and other polar matrix compounds [26], while C18can be used for the removal of lipids and non-polar interferences [39]. GCB can efficiently remove pigments, especially chlorophyll [40]. In addition, GCB is well known for adsorbing pesticides with a planar structure,such as chlorothalonil, hexachlorobenzene and quintozene, leading to unsatisfactory recoveries and poor precision [26,41]. In this study, we observed that the pigments contents were light in P.notoginseng extracts and no GCB was therefore used to avoid the potential loss of pesticides. Finally, PSA and C18were selected for the clean-up of samples. At the same time, anhydrous MgSO4was added to facilitate the partitioning process.
To acquire satisfactory recoveries and reduce MEs, the amount of PSA, C18and anhydrous MgSO4were optimized by the control variate method. The results are shown in Fig. 2. With the amount of PSA increasing from 50 mg to 100 mg, the number of pesticides with satisfied recoveries increased significantly. This was because some matrix components may be removed by PSA, which resulted in the increase of signal intensity of the analytes. There was no significant difference between the addition of 100 and 150 mg PSA. The recoveries of most pesticides decreased when the addition of PSA was more than 150 mg. The main reason for this may be that PSA sorbents result in the pH value of the final extract solutions being more than 8 [42]. Many pesticides become unstable and decompose easily in an alkaline environment. The degradation of chlorothalonil can occur at higher amount of PSA which has been confirmed by recovery loss and chromatogram appearances [34]. Therefore, 100 mg PSA was chosen.

Fig. 2 The relationship of the amounts of PSA, C18 and anhydrous MgSO4 and the pesticide number with satisfied recovery scopes (70%-120%) in 74 pesticides.
There are 3 primary QuEChERS methods including the original QuEChERS method, AOAC QuEChERS method and European (EN)QuEChERS method [43]. These methods are referred to as standard methods because international regulatory bodies such as the European Committee and the AOAC in the United States accept them. PSA is used alone as the sorbent in the original QuEChERS method,while a combination of PSA and C18is used as sorbent in the AOAC QuEChERS method and EN method [38]. Although previous study[44] showed that PSA alone is enough for cleaning the medicinal herb matrices during pesticide residues analysis. This study compared the effect of the addition of different amounts of C18on the recovery of target pesticides for its reduction of lipids and non-polar interference.As Fig. 2 showed, the recovery of most pesticides fell in the range of 70%-120%, when 25, 50 or 100 mg C18was used. A decline appeared when C18was over 100 mg. That is probably because that excessive C18could adsorb the pesticides to some extent. Finally, 100 mg C18was added because the adsorption of C18on nonpolar impurities was beneficial for the apparatus.
After 100 mg PSA and 100 mg C18were taken, the amount of anhydrous MgSO4was increased from 100 mg. Pelajić et al. [34]found that the amount of anhydrous MgSO4did not have significant influence at this step of sample preparation. In this study, when the amount of anhydrous MgSO4increased to 150 mg, no significant change was observed. However, when the amount of anhydrous MgSO4increased over 150 mg, the number of pesticides satisfied the requirement of recovery began to decline, as shown in Fig. 2.Similar to C18, this may be due to excess anhydrous MgSO4adsorbing part of the target compounds. For the removal of excess water and additional improvement of extraction, 100 mg anhydrous MgSO4has been added.
Therefore, the cleanup step was conducted using a combination of 100 mg PSA, 100 mg C18and 100 mg anhydrous MgSO4.
3.3 Matrix effects
In the case of GC-MS/MS, the matrix enhancement effect occurs predominantly [45]. P. notoginseng has complex components, which may affect the accurate quantification of target compounds when it enters GC-MSMS instrumental analysis. The MEs in P. notoginseng were analyzed. As a result, the analyte responses produced by matrixmatched standards were all substantially higher than the responses obtained for the equivalent standards in solvent (ME > 1). Most pesticides were medium signal enhancement(2 < ME <10)in P. notoginseng, while iprodione and azinphos-methyl exhibited strong matrix enhancement effects (ME > 10), as shown in Fig. 3. This might be related to the abundant active groups in these pesticides. It has reported that the pesticides with phosphate (-OP), hydroxyl (-OH),azoles (-N), amino groups (-R-NH-), imidazole, benzimidazole,carboxyl (-COOH), carbamate (-O-CO-NH-) and urea(-NH-CO-NH) are the most susceptible type of analytes to have strong matrix effect [46]. Therefore, the matrix-matched standards method was used to overcome the matrix effect.
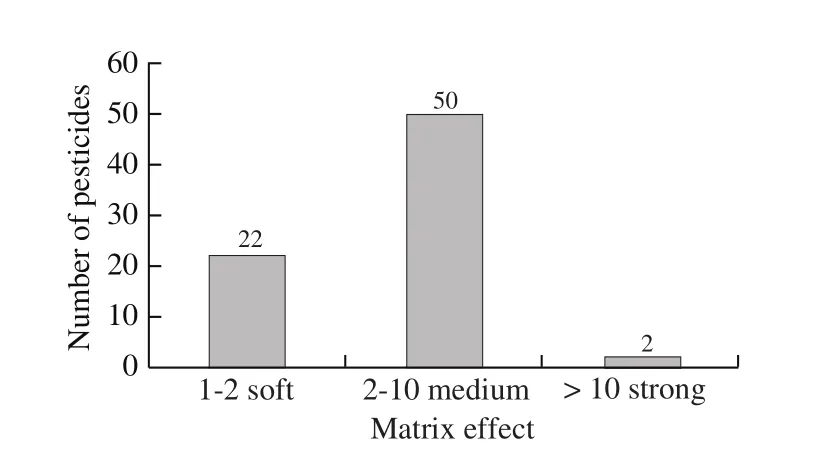
Fig. 3 Matrix effect distribution of 74 pesticides in P. notoginseng.
3.4 Method validation
3.4.1 Linearity
In this method, the calibration curves were linear within the range of 10-500 ng/mL for all analytes. Correlation coefficient (R2) for each pesticide was calculated by linear regression analysis of the signal responses (y-axis) and spiked concentrations (x-axis). The range of R2for 74 pesticides was between 0.995 0 and 0.999 9, indicating a good linearity for all target analytes. Table 2 demonstrated the calibration data for all the studied compounds.
3.4.2 LODs and LOQs
The terms of LODs and LOQs can be used to evaluate method sensitivity. The LODs and LOQs of all target analytes in P. notoginseng ranged 0.28-2.00 μg/kg and 0.94-6.65 μg/kg,respectively (Table 2). The LOQs of target pesticides were all lower than the maximum residue limits (MRLs) of herbs set by the EU(a general default limit of 0.01 mg/kg was adopted if there was no record) [47]. The results revealed that the proposed method was capable of providing competent sensitivity and satisfied for the quantitative analysis of all 74 target pesticides in P. notoginseng.
3.4.3 Accuracy and precision
Method accuracy and repeatability were estimated via recovery tests. Table 2 showed the mean recoveries and corresponding RSD values of 74 pesticides at all concentration levels. Mean recoveries of all target pesticides ranged from 71.37% to 121.18% with RSD values ranged from 1.28% to 9.21%. A majority (97.3%) of the target pesticides at the 3 spiking levels of 25, 50 and 200 μg/kg fell in the range between 70% and 120%. A tiny, but consistent,deviation from the range of 70%-120% for the mean recovery can be acceptable, especially for multiresidue analysis. Therefore, the results demonstrated fitness for the purpose of the developed and validatedmethod, according to its compliance with the requirements of the EU criteria of SANTE/11813/2017.
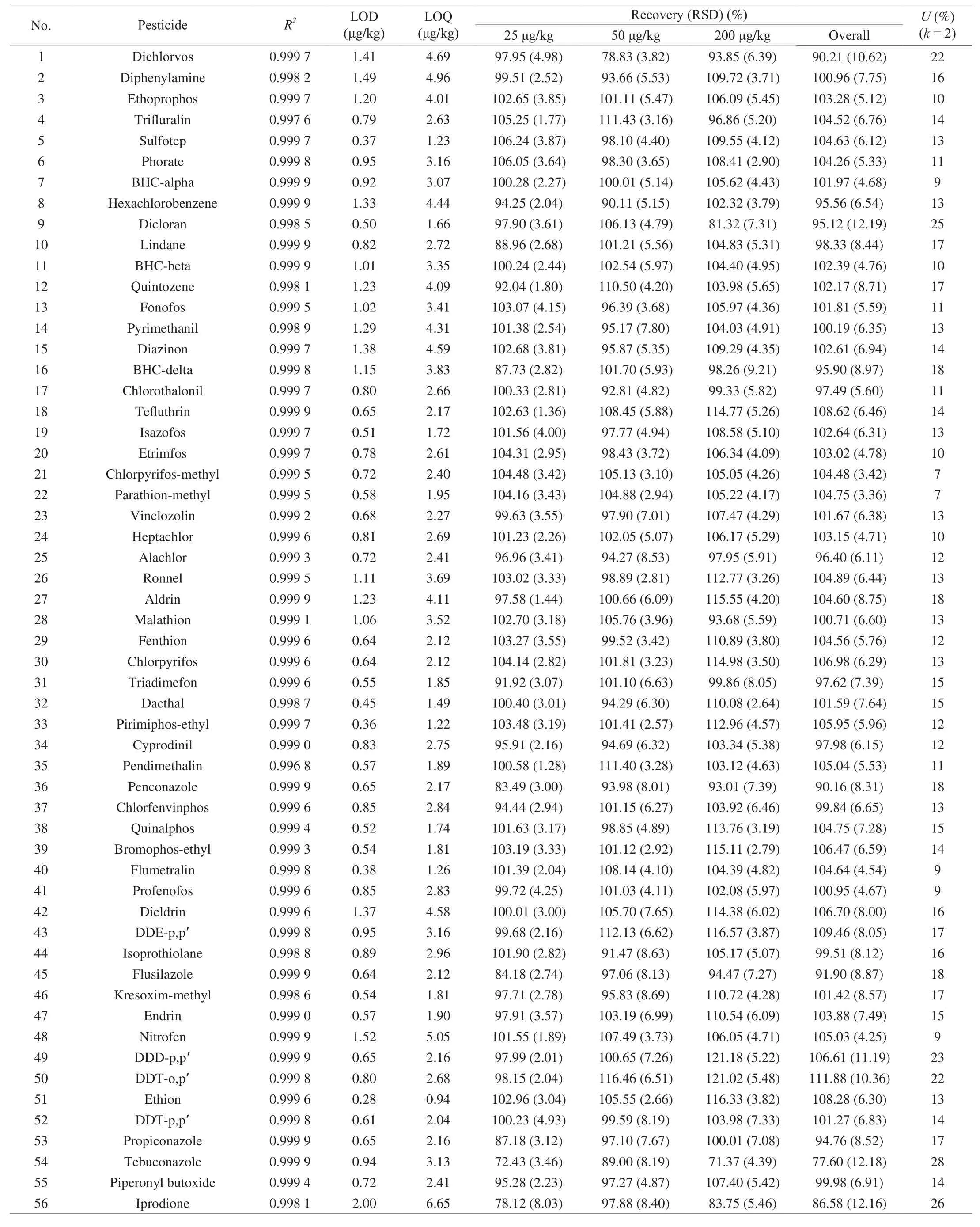
Table 2 Correlation coefficient, LODs, LOQs, recoveries, RSDs and expanded uncertainties U (%) (k = 2, confidence level 95%) in the GC-MS/MS analysis of 74 pesticides in P. notoginseng.

Table 2 (Continued)

Table 3 Comparison of the proposed method with other methods for the determination of pesticide residues in P. notoginseng.
3.4.4 Estimation of measurement uncertainty
In pesticide residue analysis, uncertainty is the most important parameter describing the quality of measurement. Although precision is identified as the main contribution to the uncertainty.The uncertainty associated with the recovery was also included in the uncertainty budget of the method to avoid underestimation of the total uncertainty. The measurement uncertainty was estimated on the basis of “top-down” experimental model using overall recovery and precision data [48]. Table 3 showed that the expanded uncertainty values ranged from 7% to 29%. All of the expanded uncertainty values were < 30%. These values were lower than a default value of 50% recommended by the SANTE/11813/2017 document, demonstrating fitness for purpose of the developed and validated method.
3.5 Comparison of proposed method with other methods
At present, there are some reports on the detection of pesticide residues inP. notoginseng. Table 3 presents a comparison of the current analytical method with the published methods for the multiresidue analysis of pesticides inP. notoginseng. There are some considerable superiorities over other works. First, the method established in this study involves 74 pesticides (including OCPs,OPPs and PYRs etc.), while the number of pesticides tested in other methods is small and the types are relatively single. Second, the pretreatment method adopted in the current analytical method is QuEChERS. Compared with other techniques such as SPE, it costs less time for sample preparation and less solvent consumption. Third, the recovery rate is comparable with those of other methods. Besides, the LOQ and LOD values are slightly better than some of other methods.
3.6 Application to real samples
The developed and verified method was applied to the analysis of 20 batches of samples collected from two different medicinal material markets. According to the European guideline document of SANTE/11813/2017, the positive results were confirmed by the retention time and ion ratio. Totally, 9 pesticides including quintozene, pyrimethanil, chlorpyrifos, quinalphos, flusilazole,propiconazole, tebuconazole, iprodione and cyhalothrin were detected in all the samples. Among them, quintozene, pyrimethanil,chlorpyrifos and cyhalothrin were detected in all of the batches.The concentrations of positive target pesticides were all lower than the MRLs of herbs regulated by the EU [47], except for quintozene. Fig. 4 showed that the residue levels of quintozene inP. notoginsengranged 0.03-0.18 mg/kg (0.66 mg/kg on average).The residue of quintozene in 8 batches of samples exceeded the limit of EU (0.05 mg/kg). Both the Chinese pharmacopoeia (2015 edition) [2] and Chinese Standard Green Standards of Medicinal Plants and Preparations for Foreign Trade and Economy (WMT2-2004) [53] set a limit of 0.1 mg/kg for quintozene residual in TCMs. Based on this criteria, 20% of 20 samples was out of the limit for quintozene.

Fig. 4 Quintozene contents of different samples and different MRLs of quintozene in P. notoginseng (A, Anguo; B, Bozhou).
These results demonstrate that pesticide residues are common in P. notoginseng, which may lead to some safety risks. On one hand, it is susceptible to various insect pests and diseases for P. notoginseng due to the strict requirements on environmental conditions during its growth. On the other hand, the types of pesticides registered in China for the prevention and control of diseases and insect pests are very limited. Therefore, growers tend to apply a large amount of pesticides to P. notoginseng based on their experience, even use pesticides indiscriminately, abuse pesticides, misuse pesticides. Quintozene is a soil treatment organochlorine fungicide for controlling fungal diseases on P. notoginseng with contact action and works as a lipid peroxidation inhibitor [54]. Quintozene can be enriched in soil and plants due to its stable chemical property and long degradation time. Its high efficacy and low cost facilitate its widespread use, although the government has banned the use during P. notoginseng plantation. Therefore, more attention should be paid to the detection and management of pesticide residues in P. notoginseng especially for quintozene.
4. Conclusions
Determination of the pesticides in P. notoginseng is essential to assess the safety to the consumers and avoid the chronic toxicity related to its long-term use. In this study, a modified QuEChERS method coupled with GC-MS/MS for the simultaneous determination of 74 pesticide residues in P. notoginseng was developed. By comparing the standards prepared in simple solvent with that prepared in blank matrix extracts, we found that many compounds were susceptible to the matrix effect. The matrix-matched standards method was adopted to compensate for the matrix effect. Based on the original QuEChERS pretreatment method, the extraction method and cleanup procedure were optimized. A comprehensive methodology validation was carried out, which provided good linearity, sensitivity, accuracy and precision in P. notoginseng. Finally, the validated methodology was applied for 20 actual samples from the medicinal material market in China. In conclusion, pesticide residues in P. notoginseng were common, and more attention should be paid to the management and usage of P. notoginseng.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgment
This work was supported by the National Key Research and Development Plan of China (2017YFC1702500).
- 食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- Approaches to evaluate nutrition of minerals in food
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
- Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
- Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments

