Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
Maojun Yao, Hui Teng, Qiyan Lv, Huifang Gao, Tengming Guo, Yiwen Lin, Sihai Gao*, Meihu Ma*, Lei Chen,e,*
a College of Chemistry and Chemical Engineering, Jishou University, Jishou, 416000,China
b College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China
c National Research and Development Center for Egg Processing, College of Food Science and Technology, Huazhong Agricultural University, Wuhan, Hubei, 430070. China
d Department of Cardiothoracic and Vascular Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430070, China
e College of Food Science and Technology,Guangdong Ocean University,Zhanjiang 524025, China
ABSTRACT
Dihydromyricetin (DHM), as a bioactive flavanonol compound, is mainly found in “Tengcha” (Ampelopsis grossedentata) cultivated in south of China. This study aimed to investigate the anti-hyperglycemic and antidyslipidemic activities of DHM using type 2 diabetes mellitus (T2D) rats, which was induced by feeding with high fat and fructose diet for 42 days and intraperitoneal administration of streptozocin. Forty-eight freshlyweaned rats were randomly assigned into the negative control (Blank), low dose (100 mg/kg), medium dose(200 mg/kg), high dose (400 mg/kg), and positive (40 mg/kg, met) groups. Fasting blood glucose and body weight were measured at weekly interval. Oral glucose tolerance tests were performed on days 42. The results revealed that DHM possessed significant antihyperglycaemic and antihyperinsulinemic effects. Moreover,after the DHM treatment, p-Akt and p-AMPK expression was upregulated, and glycogen synthase kinase-3β(GSK-3β) expression was downregulated, indicating that the potential anti-diabetic mechanism of DHM might be due to the regulation of the AMPK/Akt/ GSK-3β signaling pathway.
Keywords:
Dihy dromyricetin
Type 2 diabetes
Hypolipidemic
Hypoglycemic
AMPK/Akt/ GSK-3β signaling pathway
1. Introduction
According to updated statistical results, nearly 415 million people suffered from diabetes in 2016, among which type 2 diabetic(T2D) was responsible for over 90% [1]. As we know, the T2D is characterized by progressive β cell failure and insulin resistance,which can cause insulin deficiency and irregular increase in glucose level, leading to many chronic diseases [2]. Thus, good control of blood glucose is an effective method to prevent the progression of T2D and its complications [3]. Currently, hypoglycemic drugs are primarily divided into oral administered insulin secretagogues,metformin, α-glucosidase inhibitors and thiazolidinedione derivatives,and subcutaneous insulin infusion [4]. However, the toxic and adverse effects of these drugs are indeed concerned [5]. Therefore, it is urgent to search for natural displacement resources with fewer side effects for diabetes treatment.
Tengcha, with the botanical name of Ampelopsis grossedentata,is a traditional Chinese herb cultivated in Hunan province which has been primarily used by the Yao nationality as a health tea for centuries. It was also found effective for treating sexual conjunctivitis, fever, hepatitis and other diseases (Chinese Herbal Manual, 1578 by Shih-Chen Li, registered as a UNESCO World Heritage Site), and its appli cable usages were summarized by Zhou et al. [6]. Dihydromyricetin (DHM), also called ampelopsin, is the main bioactive compound of Tengcha, exhibiting a series of biopharmaceutical activities, such as anti-inflammatory, anti-oxidation,antibacterial and anti-tumor, and is also effective in hypoglycemic and hyperlipidemia treatments [7-9]. Qin and co-authors found that the blood glucose levels in alloxan-induced diabetic mice were significantly reduced after the oral administration of 0.25 and 0.125 g/kg/d of DHM. Another early study [10] has proved that the DHM could improve insulin resistance in skeletal muscle by inducing autophagy via the activation of adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) signaling pathway. A newly published work by Chen et al. [11] confirmed that the DHM was able to prevent or postpone diabetes development via the regulation of glycolipid metabolism. However, few literatures have reported that DHM reduces hypolipidemic and hypoglycemic through the liver signaling pathway and describe its mechanism of action. This study investigated the antihyperglycaemic and antihyperinsulinemic effects of DHM in the management of diabetes in a diet and low dose streptozocin animal model of type 2 diabetes.
2. Materials and methods
2.1 Chemicals and Reagents
Dihydromyricetin (purity over 98%) and cholesterol were purchased from Sigma (St. Louis, MO) and HuiSheng Biochemical Reagent Co.Ltd. (Shanghai, China), respectively; cholesterol (TC), triglyceride(TG), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) test kits were all obtained from Biosino Biotechnology Co. Ltd. (Beijing, China); superoxide dismutase(SOD), glutathione peroxidase (GSH-Px), and malondialdehyde(MDA) test kits were produced by Jian-Cheng Bioengineering Institute(Nanjing, China); blood glucose test paper and HGM-114 blood glucometer purchased from Omron Automation co. (Japan). Antibodies of phosphorylated AKT (P-AKT) (Ser473), phosphorylated AMPK(P-AMPK) (Thr172) and glycogen synthase kinase-3β (GSK-3β)(Ser21 + Ser29) were purchased from BIOSS co. (Beijing, China).
2.2 Construction of animal models
Adult male Sprague Dawley (SD) rats (180-200 g) were supplied by Fuzhou General Hospital of Nanjing Military Command Animal Center (Fuzhou, China), were kept under standardized conditions: room temperature, (22 ± 2) °C; relative humidity 45% to 55%; 12-hour light/dark cycle in the animal facility with free access to food and water.
All rats were kept free access to water and food during the experiment, which was approved by The Animal Ethics Committee of Fuzhou General Hospital of Nanjing Military Command.
Two weeks after the adaptive feeding of the rats, the normal group continued to give a normal diet. The remaining rats were fed with high-fat, high-sugar diet (15% lard, 30% sucrose, 2% cholesterol,1% sodium cholate, 5% protein powder, and 47% regular diet). After 8 weeks of feeding (with body weight around 350 g and FBG around 5 mmol/L), rats were injected with 35 mg/kg streptozocin (STZ) (in ice-cold citrate buffer (0.1 mol/L, pH 4.35) intraperitoneally to induce the model of type 2 diabetes, and then treated with normal diet until the end of the experiment. Animals whose fasting blood sugar level was higher than 16.7 mmol/L three days after STZ injection were successfully molded. After seven days of the injection of STZ, oral administration of DHM was treated the rats at the dose of 100 mg/kg/day(low dose, LD), 200 mg/kg/day (medium dose, MD), or 400 mg/kg/day(high dose, HD) and 40 mg/kg/d Metformin during 6 weeks.
2.3 Biochemical measurements
2.3.1 Fasting blood glucose determination (FBG)
The FBG level of blood samples which were obtained from lateral tail of the diabetes rats was measured with a glucometer (StatStrip Xpress(R) Nova Biomedical, Waltham MA, USA).
2.3.2 Oral glucose tolerance tests
Oral glucose tolerance tests were performed on days 21 and 42 based on the protocol reported by Barret [12]. In brief, the experimental animals were fasted for 6-8 h before the test. The baseline of blood glucose was then evaluated and blood glucose levels were measured at 30, 60, 90 and 120 min after the administration of a loading glucose dose of 2 g/kg to each rat by oral gavage. The areas under the curve (AUC) were then calculated.
2.3.3 Fasting serum insulin levels
Enzyme-linked immunosorbent assay (ELISA) method was employed for the determination of insulin level in serum using rat insulin kits (Hangzhou Sunlong Biotech co, Ltd, China).The homeostasis model of assessment of insulin resistance (HOMA-IR)was calculated according to the equation below:
HOMA-IR= Insulin (in mU/L) × glucose (mg/dL)/405
2.3.4 Serum lipid profile
The levels of triglycerides (TG), cholesterol (TC), lowdensity lipoprotein (LDL), high-density lipoprotein (HDL), alanine transaminase (ALT) were measured by automatic biochemical analyzer following the instructions of manufacturer.
2.4 Immunohistochemistry
Taken a small piece of tissue and fixed it in 10% paraformaldehyde.After washing with tap water, it was subjected to a series of operations such as dehydration, transparent, paraffin embedding and sectioning for HE staining. For immunohistochemical detection, paraffin sections were repaired with citric acid, antibody was added to each section respectively at 4 °C incubation. Secondary antibody was added and incubated at room temperature for 15 min. After staining with hematoxylin for 10 min,it was differentiated with ethanolic hydrochloric acid for 20 s and rinsed with water for 10 min. After dehydration with ethanol, it was made transparent with xylene, sealed with a neutral gel. For TUNEL staining,after dewaxing the liver paraffin section, the section was added to the proteinase K for 30 min, and the mixture solution of TdT enzyme and Biontin-dUTP (TdT:Biontin-dUTP = 1:9) was incubated in 37 °C for 1 h,then the Converter-AP solution was added in 37 °C incubate for 20 min;The BCIP/NBT solution and the nucleus red were subjected to a color reaction, and the AEC aqueous sealing tablets were used for sealing.
2.5 Western blotting
Total protein was extracted with radio-immunoprecipitation assay lysate added with PMSF, then quantified by bicinchoninic acid (BCA)and finally the protein concentration was pulled to 5 μg/μL. The protein was loaded at 40 μg, and the protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) 8% gel and transferred to a polyvinylidene fluoride (PVDF) membrane and blocked with 5% milk for 120 min at room temperature [13,14].Then, the membrane was incubated with CBS polyclonal antibody(1:1 000), CSE polyclonal antibody (1:4 000), MTR polyclonal antibody, MTHFR polyclonal antibody (1:2 000), cleave-caspase 3 polyclonal antibody (1:1 000), procaspase 3 polyclonal antibody (1:1 000) and β-actin polyclonal antibody (1:4 000) overnight at 4 °C.
2.6 Statistical analysis
All analyzes were conducted in triplicate and statistical analyzes were performed using the Statistical Package for Data Processing Station (DPS) 16.05 system (Zhejiang University, Hangzhou, China).ANOVA was performed at the 5% level of significance.
3. Result
3.1 Effect of DHM on fasting blood glucose
The results on acute oral toxicity suggested that the DHM had no mortality when the administrational concentration up to 2 000 mg/kg.The anti-hyperglycemic effect of DHM on the fasting blood glucose(FBG) levels of diabetic rats was displayed in Fig. 2. Six weeks of daily administration of the DHM dramatically improved the quality of life for diabetic rat (Fig. 2A). Fig. 2B showed that the STZ injection led to a 5-fold elevation of FBG levels, which was maintained for 6 weeks throughout the experimental period. On the other hand, 6 weeks of daily DHM treatment resulted in a significant decline of blood glucose levels by 40%-60%. Additionally, it was found that the body weight of normal group animals slightly increased but diabetic rats showed predominant reduction during 6 weeks (Fig. 2A).However, the body weight reduction caused by STZ was reversed when treated by DHM at 400 mg/kg, which was even more effective than metformin (40 mg/kg) after 6 weeks of treatment.

Fig. 1 Experimental protocol. Group I: Normal control (vehicle treated); Group II: Diabetic control (vehicle treated); Group III: Diabetic rats supplemented with metformin; Group IV, V & VI: STZ hyperglycemic rats supplemented with DHM (100, 200, 400 mg/kg b.w).

Fig. 2 Effect of DHM on appearance (A) and fasting blood glucose (B) of rats. Data are expressed as the mean ± SD for 8 rats in each group. #. P < 0.05,##. P < 0.01; compare with model group, *. P < 0.05, **. P < 0.01.

Fig. 3 Photomicrographs of histological sections of liver tissue of normal and diabetic treated rats (A). Non-diabetic section of liver showing normal histological structure, liver of diabetic rat showing loss of the normal architecture with the distended portal vein,fibrosis, leucocytic inflammation; diabetic liver treated with metfoemin shows portal inflammation. Diabetic rats treated with DHM at 400 mg/kg/day for 6 weeks show portal tract and mildfibrous expansion without sep inflammation formed of lymphocutes (B). Diabetic rats treated with DHM at 200 mg/kg/day for 6 weeks shows tiny residual foci inflammation. Diabetic rats treated with lentil with DHM at 100 mg/kg/day for 6 weeks show near normal hepatocytes, portal tract and focal inflammation (C). DHM effect on ACC, p-ACC, PPARα and PPARγ expressions. Data were shown as mean ± SD; *** P < 0.001, **P < 0.01, *P < 0.05, compared to the vehicle group (diabetic vs. normal group). n = 8 in each group.
3.2 Blood lipids
The contents of TG, TC, LDL-C, and HDL-C in blood serum were summarized in Table 1. As compared to the blank, significant increases of TG, TC, and LDL-C levels and a dramatically decrease of HDL-C level (P < 0.01) were found in T2D group. Surprisingly,these biochemical parameters of the rats’ serum of the HD group were similar with those in the metformin group. For blood lipid levels of TC and LDL-C, the HD group decreased by 34.9% and 19.5% as compared to the T2D group, and the HDL-C level increased by 47.2%. Notably, after DHM administration for 6 weeks, the AI(atherosclerosis index) values of HD and MD group significantly decreased (P < 0.01).

Table 1 iochemical parameters of serum of T2D treated with DHM.
3.3 DHM ameliorated the diabetes-induced liver histological alterations
Histopathological examination of the normal liver tissue shown in Fig. 3A verified the healthy hepatic structure in which lobule is made up of radiating plates, strands of cells forming a network around a central vein. Liver biopsy of diabetic rats showed moderate fibrosis,leucocytic infiltration around central vein inflammation, dilation in central vein, as well as the loss of normal architecture indicating hepatocellular injury of the liver (Fig. 3A). However, less difference of such structural characteristics was observed in diabetic animals administrated with either metformin or DHM at high dose. Jayaraman and co-authors [15] have previously confirmed that liver cells of diabetic rats were irrevocably damaged after STZ injection, which may cause the hepatic microsomal cells to release ALT and AST enzymes. Other similar results were also reported that flavonoids could alleviate hepatic injury, which may through scavenging the free radicals and enhancing the enzymatic antioxidant activity as demonstrated in diethylnitrosamine-induced hepatocellular carcinoma Wistar rats [16,17].
3.4 DHM improved OGTT of diabetic rats
After oral administration with DHM for 6 weeks, all animals were fasted for 12 h and subjected to an OGTT test. It was known that the diabetic can cause severe liver disease and lead to an impaired glucose tolerance (Fig. 4A). As contrasted with the normal animals, the blood glucose level of T2D group animals quickly increased after the oral supplementation of glucose(2 g/kg) within 30 min, and kept a high level for the next 90 min.Fig. 4B represents OGTT areas under the glucose curve (AUC)over 120 min, in which the AUG of DHM groups significantly increased as compared to T2D (P < 0.01), suggesting that the high or middle dose of DHM may be effective in relieving liver disease and improving glucose tolerance.
3.5 Effects of DHM on serum insulin and HOMA-IR
Fig. 4C showed that serum insulin level in diabetic rats was significantly higher than that in blank rats (P < 0.01). Compared with T2D group, HD and MD of DHM had a significant effect on insulin content (P < 0.01), while LD showed 9.39% reduction of serum insulin level, indicating a dose-depend manner between DHM and the ability of down-regulation of serum insulin. Moreover, the insulin level in HD and MD groups were significantly lower (P < 0.01) than diabetic animals (T2D), which indicated that the higher dose of DHM could relieve the state of insulin resistance and enhance the insulin sensitivity. On the other hand, HOMA-IR dramatically decreased(P < 0.01) after DHM intervention for 6 weeks (Fig. 4D), revealing that the DHM may also effective in controlling plasma insulin through increasing insulin sensitivity.
3.6 DHM modulated AMPK/Akt/GSK-3β signaling pathway
In order to figure out the underline mechanism for anti-diabetic effect of the DHM, the modulation activity of DHM on AMPL/Akt/GSK-3β were investigated. As compared to normal animals, Fig. 5B and 5C showed significant decreases in relative expressions of both p-Akt ((0.46 ± 0.01)-fold, P < 0.01) and p-AMPK ((0.21 ± 0.01)-fold,P < 0.01). AMPK/Akt activities were suppressed by DHM treatments since the decrease of AMPK were ameliorated. These reductions in p-Akt and its ratio were not attenuated by the DHM because of a remarkable increase in the protein levels. In contrast, Fig. 5D exhibited a predominant decrease in protein level of p-GSK-3β((0.22 ± 0.01)-fold induction, P < 0.01), which suggested that the activation of GSK-3β in the liver was enhanced in diabetic induced animals (T2D). Whereas, the enhanced activation of GSK-3β was attenuated by DHM, since the cardiac protein levels of p-GSK-3β((0.59 ± 0.01)-fold, P < 0.01 for LD) remarkably increased after the DHM treatment.
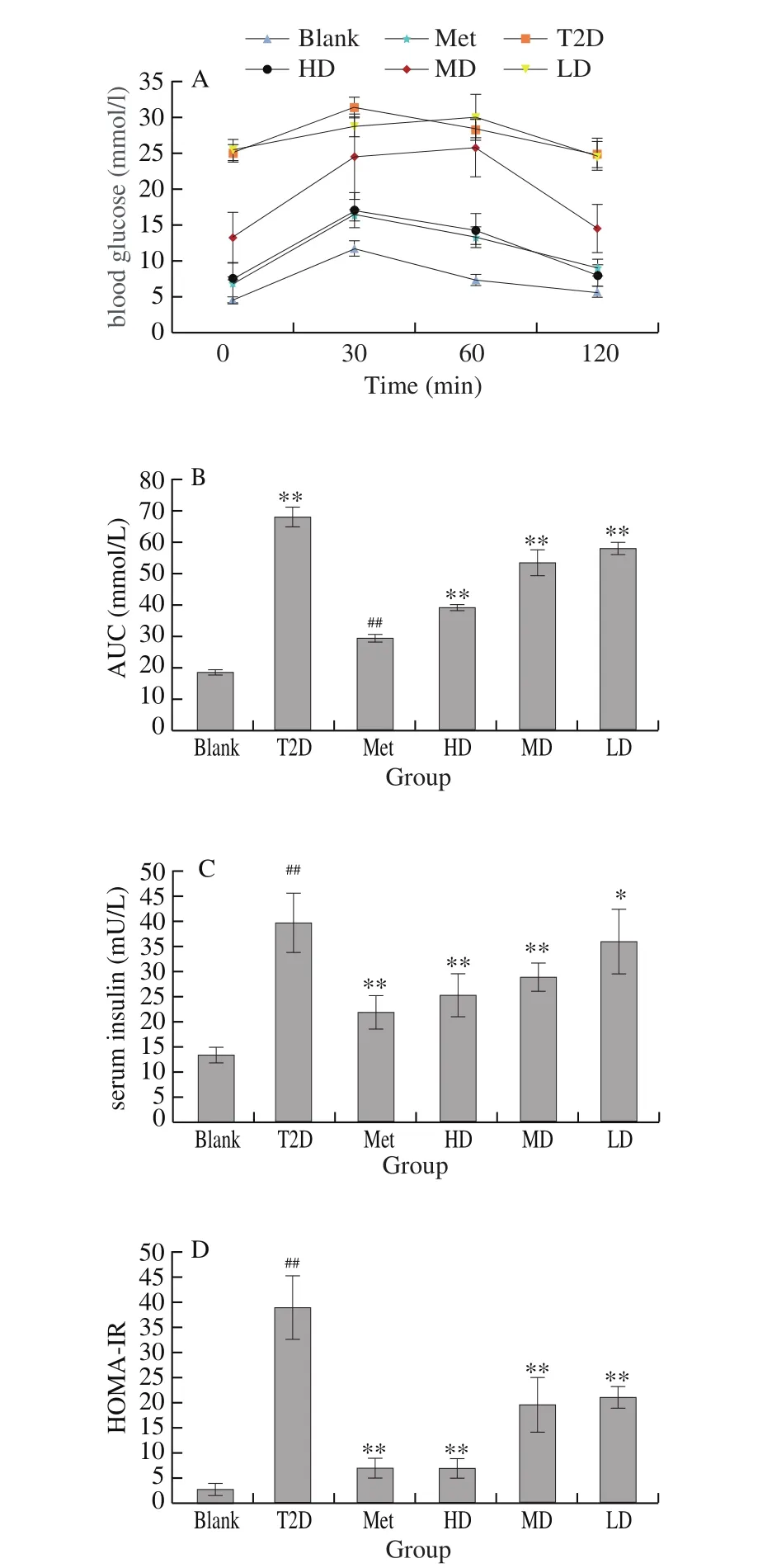
Fig. 4 Effects of DHM on OGTT (A), AUC (B), insulin level (C) and HOMAIR (D) in rats. Data were shown as mean ± SD; ###P < 0.001, ##P < 0.01, #P < 0.05,compared to normal control ***P < 0.001, **P < 0.01, *P < 0.05, compared to the vehicle group (diabetic vs. normal group). n = 8 in each group.
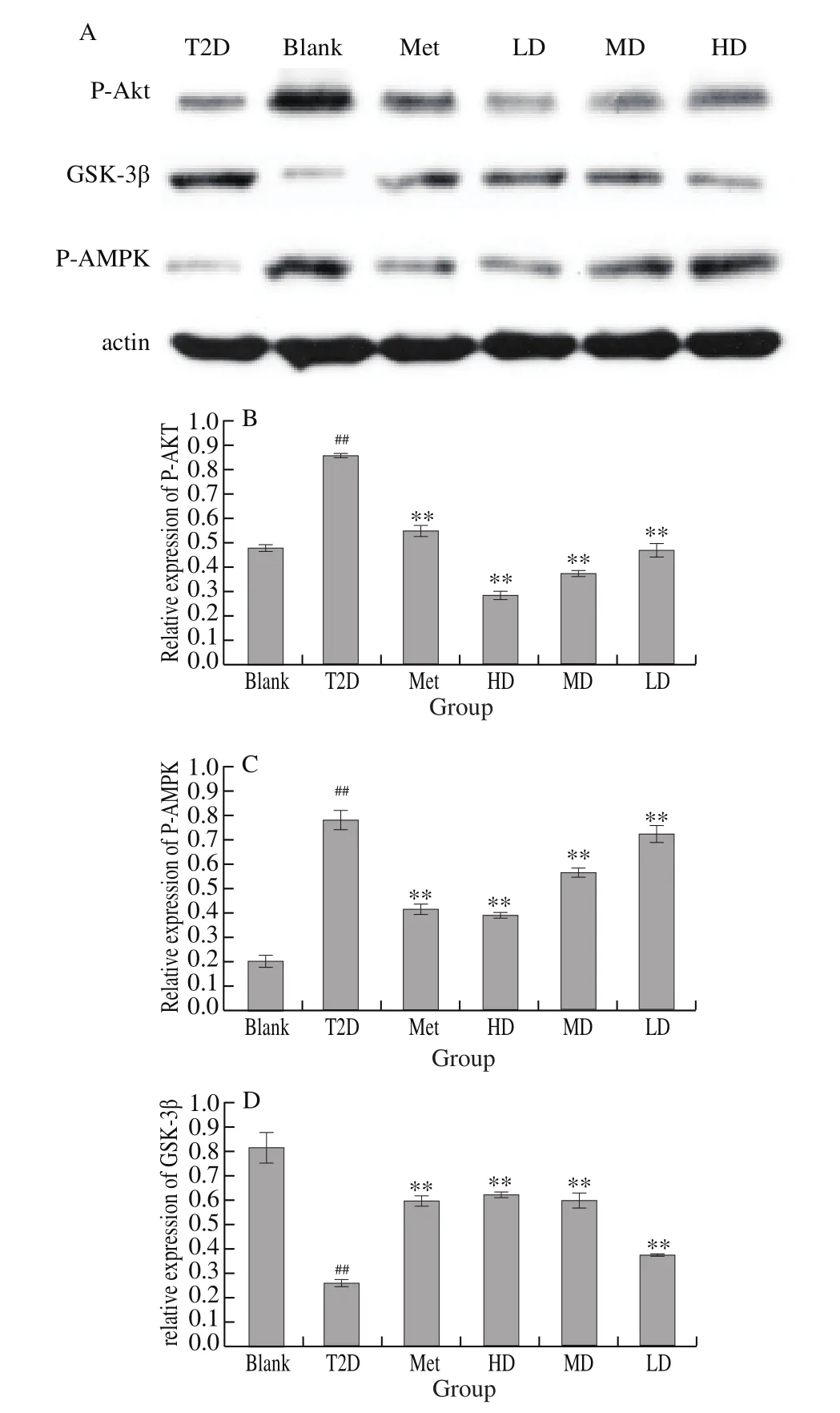
Fig. 5 Effects of DHM on relative protein expression of AKT/GSK-3β/AMPK in liver. The vehicle control is set as 1.0.Values is averages from three independent experiments. Data were shown as mean ± SD; ###P < 0.001, ##P <0.01, #P < 0.05, compared to normal control ***P < 0.001, **P < 0.01, *P < 0.05,compared to the vehicle group (diabetic vs. normal group). n = 8 in each group.
4. Discussion
Diabetes mellitus is a metabolic disorder, frequently associated with the development of micro- and macrovascular complications that include but are not limited to: cerebrovascular, cardiovascular,nephropathy, and neuropathy disease [18]. Moreover, it also gives a high risk of mortality and severely affects the quality of life [19].Increased body mass index and the associated insulin resistance in subjects at risk for diabetes might speed up the process that leads to β-cell destruction due to obesity-linked cytokines with inflammatory and/or immunomodulatory properties. Furthermore, obese individuals have shown to have increased susceptibility to infection and infectionrelated mortality [11,20]. We have previously reported that dietinduced overweight rats exhibited an altered immune response [1,21].Although decreased T-cell function has been observed in obese human subjects and genetically obese animals, the precise role of immune functions in obesity is still unclear. Jang et al. [22] showed that high-fat diets suppress the proliferation of splenic lymphocytes in rats for 10 weeks. Accordingly, to investigate the effects of DHM on blood glucose in high-fat diet fed STZ-induced diabetic mice, we examined the glucose lowering efficacy.
The DHM has been widely utilized for the management of diabetes and the present work was planned to assess its efficacy using type 2 diabetic rat models, in which combination induction method with high fructose and high fat diet and streptozocin (STZ) injection was employed. The established model could best mimics human type 2 diabetes in which obesity is highly related to β-cell dysfunction and insulin resistance, ensuing the late progression of the disease [23].Since the high fructose and high fat diet has been successfully used for developing insulin resistance, hyperinsulinemia, and obesity [24,25]. On the other hand, a low dose intraperitoneal injection of STZ is able to achieve an initial β-cell dysfunction observed in type 2 diabetes mellitus, which has been demonstrated as a better chemical inducer for diabetes than alloxan [26].
DHM at the tested doses in our work displayed significant antihyperglycemic effects. In addition, oral glucose tolerance test also confirmed its significant effects on insulin sensitivity, which may attribute to an enhanced glucose uptake by adipose tissue and muscle,and also related with the up-regulation of GLUT-4 transporters expression as well as the suppression of hepatic glucose production and increased insulin secretion [7]. Similar results were revealed in other previously published work as well [10]. It revealed that DHM also showed predominant impacts on the levels of homeostasis model of assessment of insulin resistance (HOMA-IR) and serum insulin as well, verifying potent antihyperinsulinemic influences and/or reductions on the resistance of insulin. HOMA-IR is an evaluation index widely accepted for insulin resistance and β-cell function in epidemiological studies [27,28]. The foregoing discussion therefore indicates that the observed antihyperglycemic effects of DHM are secondary to its insulin sensitizing effects. These experimental results are similar to those in published literature.Indeed, DHM has been reported to have similar effects [29]. The combination of hepatic steatosis, increased liver weights and increased hepatic index are some of the earliest features of type 2 diabetes [30]. Hepatic steatosis is often secondary to hepatic insulin resistance [31]. It is believed that increases in liver diacylglycerol(DAG) levels lead to protein kinase Cε (PKCε) activation and its consequent translocation to the cell membrane, which results in inhibition of hepatic insulin signaling. This then results in the development of hepatic insulin resistance [32]. The aforementioned events ultimately result in hepatic accumulation of fat i.e. hepatic steatosis with the consequent increase in hepatic weight and index.One can therefore argue that compounds in the extract cause a reduction in hepatic triglyceride content and consequently prevent hepatic steatosis by inhibiting one or more of these biochemical pathways. DHM possessed significant antidyslipidemic effects i.e.decreased total plasma cholesterol, LDL-C, serum triglyceride and increased HDL-C. The results obtained in this study are similar to those in published literature. Indeed, DHM was reported to possess significant antidyslipidemic effects [33]. These effects were directly attributed to the improvement in insulin signaling, besides other possible lipid-lowering mechanism acting in the cholesterol and/or fatty acids biosynthetic pathways [34]. To better understanding the molecular mechanism underlying the effects of DHM supplement diets on diabetic rats, we investigated the AMPK/Akt/GSK-3β signaling pathway. A lot of research literature has shown that AMPK is involved in the regulation of glucose metabolism [35]and it appears that AMPK activation required for the anti-diabetic effect of metformin [36]. Interestingly, DHM promoted AMPK phosphorylation and phosphorylated form of Akt. Supplementation of DHM to diabetic rats has significantly up-regulated the expression of p-AMPK and p-Akt. The rats treated with metformin showed similar results with no statistical difference with those treated with SOL. Kim and co-authors [37] have found that the expression levels of AMPK were significantly reduced in db/db mouse livers and curcumin increased the expression of these proteins, thereby playing a beneficial role in treating the complications of type 2 diabetes. The data obtained from Varshney, Gupta, and Roy [38] showed that kaempferol up/down-regulates AMPK and increased cell viability and anti-apoptotic activity in PA-stressed RIN-5F cells [38]. In our study, we also found that the expression level of p-Akt was significantly increased by phenolic compounds in insulin-resistance cell model [39], which is consistent with the results of present study. In our opinion, STZ-induced diabetes may be related to the activation of the AMPK/Akt signaling pathway. GSK3β is a critical substrate implicated in the regulation of glycogen synthesis. Subsequently, GSK3β phosphorylation plays a key role in insulin resistance. As shown in Fig. 5, a sharp decline of hepatic p-GSK3β expression was found in diabetic rats, as previously shown by Goedeke et al. [40]. This decline was significantly abolished when animals were fed metformin and DHM. Different phenolics have been shown to reactivate this effect in different insulin resistance models. For instance, catechin derived from green tea and flavonoids from cocoa increased the GSK3-β expression in the liver of insulin-resistant rats [41].In this line, this indicates that DHM might improve hepatic insulin resistance by inhibiting activation of GSK3β.
In conclusion, the results of this study validate the traditional use of this plant species in the management of diabetes mellitus and indicate that the main mechanism of action of these antidiabetic effects is via the modulation of adenosine AMPK/Akt/GSK-3β signaling pathway.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (NSFC, Grant No. 31801459; 31701520),Science and Technology General Projects of Fujian Province(2019J01393), Educational research project for young and middleaged teachers in Fujian Province (JT180116).
Conflict of interest
The authors declare no conflict of interest.
- 食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- Approaches to evaluate nutrition of minerals in food
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
- Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments
- Development of water-soluble zein colloid particles and in situ antibacterial evaluation by multiple headspace extraction gas chromatography

