Maillard reaction in Chinese household-prepared stewed pork balls with brown sauce:potentially risky and volatile products
He Li, Xiangyi Tang, Chunjian Wu, Shujuan Yu,c,d,*
a School of Chemical Engineering and Technology, North University of China, Taiyuan 030052, China
b College of Food Science and Engineering, South China University of Technology, Guangzhou 510640, China
c Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, Guangzhou 510640, China
d Overseas Expertise Introduction Center for Discipline Innovation of Food Nutrition and Human Health (111 Center), Guangzhou, China
ABSTRACT
The stewed pork balls with brown sauce (SPB-BS) in China is well known for its delicacy and preferred by consumers. Maillard reaction (MR) is widespread in SPB-BS due to the use of sugar, meat and sauce in the thermal process. However, there is a lack of research on its risk and flavor by MR. By solid phase extraction combined with HPLC-MS, 4 kinds of harmful compounds including acrylamide (AA), heterocyclic aromatic amines (HAAs), 4-methylimidazole (4-MEI) and furan were analyzed in SPB-BS and their amounts ranged 0.05-0.50 mg/kg. The quantitative formula was proposed to evaluate the risk value of the SPB-BS, after taking into account the content, acceptable daily intake (ADI) and carcinogenicity of each compound. The risk values were in range of 0.57-37.93, suggesting that the risk caused by MR in SPB-BS was low. By head space-solid phase microextraction combined with GC-MS, 57 volatile compounds in SPB-BS were identified with the dominant contribution of alcohols, aldehydes, acids and esters. Based on the structures of these compounds and the composition of SPB-BS, lipid oxidation and MR are inferred to be responsible for the formation of the harmful and volatile compounds. In addition, the added sauces and oil provides the main precursors to form the harmful and volatile compounds in SPB-BS, so it is necessary to point out a balance between them in the further study.
Keywords:
Stewed pork balls with brown sauce
Maillard reaction
Harmful compounds
Volatiles
HS-SPME-GC-MS
1. Introduction
Food-related mutagens play a major role in human carcinogenesis and about one third of cancer is caused by diet [1]. Recently, foodrelated risks especially those related to Maillard reaction (MR) have attracted increasing attention because of the widespread distribution of MR in the food thermal processing industry [2]. Several hazardous compounds consisting of acrylamide (AA), heterocyclic aromatic amines (HAAs), 4-methylimidazole (4-MEI) and furan are generated from MR during food heating processing [3,4].
AA, a neurotoxin and potential carcinogen, has been found in various thermally processed foods such as potato chips, biscuits,and coffee [5]. Because of its neurotoxicity, carcinogenicity and genotoxicity, it was classified as “Group 2A” (probably carcinogenic to humans) by International Agency for Research on Cancer (IARC)in 1994 [6] and as a Category 2 carcinogen and Category 2 mutagen by European Commission [7] as well as a substance of “very high concern” by European Chemical Agency in 2010 [8]. HAAs were primarily reported in 1977 by professor Sugimura and his colleagues in meat as a result of normal domestic cooking processes [9] and identified as a group of potent mutagenic/carcinogenic compounds associated with heat-processed meat foods [10,11]. As three common HAAs, 2-amino-3-methylimidazo[4,5-f] quinolone (IQ), 2-amino-3,4-dimethyl-3H-imidazo[4,5-f] quinolone (MeIQ) and 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) were classified as“Group 2A” and “Group 2B” by IARC, respectively [12]. 4-MEI,which is generated during the caramelization process [13] has attracted a great deal of attention among federal and state regulatory agencies because of its carcinogenicity and presence in foods and beverages [14,15]. A toxicological study performed by the National Institute of Environmental Health Sciences of USA [16] showed that 4-MEI can induce alveolar/bronchiolar adenoma and carcinoma in male and female mice. Furan is a potential human carcinogen that can be formed in abroad range of foods processed at high temperatures,such as coffee, baby foods, bread, and snacks [17]. Although it is still unclear what the risks are associated with the current intake levels of dietary furan, furan mitigation in foods may be considered as a challenge in the prevention of human diseases, such as cancer[18]. Furan and 4-MEI [19] were classified as “Group 2B” (possibly carcinogenic to humans) by IARC.
Stewed pork balls with brown sauces (SPB-BS) are the traditional meat foods and popular dish in China. The MR can easily occur due to the presence of sugars and amino acids or proteins, which were derived from streaky pork, sucrose and brown sauce during the cooking process of SPB-BS [20]. MR can produce some compounds that have a negative impact, thus researchers should pay attention to the risks from MR due to the complex reaction occurred in SPBBS. Liu et al. [21] reported that the maximum amount of AA was 42.4 μg/kg in Chinese SPB-BS after addition of sugar, soy sauce and other seasonings. However, the MR is also one of the main pathways during the thermal processing of meat, leading to the formation of flavor, which is an important sensory attribute for consumers to judge the quality and acceptability of foods. For years, volatiles were found in various MR-related food products, such as beef [22], pork [23], soy sauce [24,25] and MR models [26,27]. More than 1 000 volatiles have been identified from meat products [28], including alkanes, alcohols,aldehydes, ketones, acids, esters, characteristic nitrogen and sulfurcontaining compounds. The order quality of aroma-active compounds was also characterized using in gas chromatography-olfactory-mass spectrometry (GC-O-MS) and omission test [29,30].
To the best of our knowledge, only a few studies were reported to analyze the detriment and flavor compounds changing during the preparation of Chinese SPB-BS. The main objective of this study were: (i) to estimate the amounts of the harmful compounds caused by MR during the preparation of SPB-BS; (ii) to conduct a trial to quantify risk values by a formula; and (iii) to identify the main volatile compounds in the preparation of SPB-BS, which might lay the groundwork for the discriminatory control of MR in food thermal processing to avoid harm and promote flavors.
2. Materials and Methods
2.1 Chemicals and reagents
Methanol and acetonitrile were all HPLC grade and obtained from Merck (Damstadt, Germany) and OCEANPAK (VstraGtaland,Sweden), respectively. Hexane (≥ 97.0%) and ethyl acetate (≥99.5%) were all supplied by Sinopharm Chemical Reagent Co., Ltd.(Shanghai, China). AA, formic acid (88%), acetic acid (99.8%),anhydrous sodium acetate (99.0%) and tri-ethylamine (99.0%) were all purchased from Tianjin Kermel Chemical Reagent Co., Ltd.(Tianjin, China). Ammonium hydroxide was obtained from Thermo Fisher Scientific Co., Ltd. (USA). HAAs including IQ, MeIQ and PhIP were purchased from Toronto Research Chemicals Inc (North York, Ontario, Canada). C7-C30n-alkane and 4-MEI standards were all purchased from Sigma-Aldrich (St Louis, MO, USA). Furan was obtained from Aladdin Shanghai Macklin Biochemical Co., Ltd.(Shanghai, China). The sucrose, edible blend oil and brown sauce soy were purchased from Chinese Light and Sugar group (Guangzhou,China), LEE KUM KEE group (Hong Kong, China) and Yihaikerry group (Guangzhou, China), respectively. Phenethyl acetate was bought from JiuDing Chemical Co., Ltd (Shanghai, China).
2.2 Preparation of stewed pork balls with brown sauces
The tested streaky pork was bought from local market randomly.The streaky pork was processed by the flowing procedure (Fig. 1)and 3 replicates of each treatment were run. For each treatment,portion (about 3 cm × 3 cm × 1.5 cm) of the meat was cut and about(30.0 ± 0.5) g was weighed (sampling S1). A sample was tempered to an initial temperature of (25 ± 2) °C. Then all pieces of meat were soaked in 900 mL water in a pan or wok and heated for 10 min at 120 °C(sampling S2). Bail out the meat from the pan, remove the water in the pan and evaporate to dryness. 15 g edible blend oil was poured into the pan and heated for 5 min at 180 °C. The streaky pork was fried for 5 min/side and was turned once during frying (sampling S3).Afterwards 10 g brown sauce soy and 10 g sucrose were added in the pan and were stir-fried for 5 min (sampling S4). About 800 mL water was poured into the pan and heated to boiling. Then the mixture was stewed for 30 min at 120 °C. Add 2.5 g edible salt and turn to high heat (200 °C), stir evenly until all sauce is absorbed, dish up (sampling S5). Each sample after cooking was cooled and was grinded by a homogenizer (AUX HX389, AUX group Co., Ltd., Ningbo, China).

Fig. 1 Cooking procedure of stewed pork balls with brown sauce (SPB-BS). S1, 25 °C; S2, 120 °C; S3, 180 °C; S4, 120 °C; S5, 200 °C.
2.3 Potential health risks of SPB-BS
2.3.1 Determination of AA in SPB-BS
AA was determined according to the method described by Omar et al. [31] with some modifications. In briefly, each thoroughly homogenized sub-sample (1.0 g of each sample) was weighed into 50 mL centrifuge tube. 5 mL of formic acid aqueous solution (0.1%,V/V) was added to extract AA and 5 mL of chloroform-methanol (2:1,V/V) solution was added for de-fatting process. The centrifuge tubes were shaken vigorously for 5.0 min using a vortex-2 Genie mixer.An aliquot of 10 mL acetonitrile was added followed by a mixture of 5 g anhydrous magnesium sulfate and 1.0 g sodium chloride. The tubes were vortexed immediately for 1.0 min and then centrifuged at 5 000 r/min for 10 min (CR22G, Hitachi Limited Co., Ltd, Tokyo,Japan). The chloroform was discarded. The middle layer containing the AA was transferred into a 10 mL centrifuge tube containing 150 mg basic Al2O3. The mixture was then vortexed for 30 s and centrifuged at 4 000 r/min for 3.0 min. The upper supernatant was transferred into a glass vial and evaporated to dryness under a gentle stream of nitrogen gas. The residue was reconstituted with 0.5 mL of methanol and then filtered through a 0.22 μm syringe filter (Ameritech Technology Co., Ltd., Tianjin, China).
The AA was analyzed by an Atlantis dC18column for HPLC (a Waters 600 pump, a Waters 2707 auto-sampler, and a Waters 2998 diode array detector (Waters, USA)) connected to an LCQ-Fleet ion-trap mass spectrometer (MS, Thermo Fisher Scientific, USA)analysis. The inject volume is 20 μL. Mobile phase A was composed of formic acid aqueous solution (0.1%, V/V), and mobile phase B was composed of methanol. The gradient program was as follows: 0.5% A and 99.5% B. The flow rate was 0.3 mL/min in isocratic mode. The electrospray ionization (ESI) MS operating conditions included an electro-spray voltage of 3.0 kV and drying gas flow and sheath gas flow were 9 and 11 mL/min, respectively. The sheath gas (N2)flow rate at 40 arbitrary units, auxiliary gas (N2)flow rate at 15 arbitrary units. The tube lens was set at 85 V, capillary temperature at 275 °C and mass range m/z was 50-100. Acrylamide was quantified using m/z 71. The calibration curve (y = 15 543x + 176.7, R2= 0.998 1) for quantification prepared in meat matrix was used to compensate for the occurrence of matrix effect. The limit of detection (LOD) of AA was 4.5 μg/kg.
2.3.2 Determination of HAAs in SPB-BSS
About 2.0 g sample was weighed and soaked in 5 mL acetonitrile and n-hexane in a centrifuge tube (50 mL). Then they were shaken for 3 min in a vortex mixer and ultrasonically extracted for 20 min in the ultrasonic system (SB-5200DT, Ningbo ScienTz Biotechnology Co.,Ltd., Ningbo, China). The mixtures were centrifuged at 8 000 r/min for 20 min and the acetonitrile layer was collected in a glass tube. The residue was extracted repeatedly using 5 mL acetonitrile and the two extracts were combined.
Samples were cleaned up by a solid phase extraction (SPE)procedure according to the Wang et al. [32]. The PCX column(60 mg/3 mL, Agela Technologies, Tianjin, China) was used and pre-equilibrated with 3 mL methanol and 3 mL ultrapure water. All extracts were passed through the PCX column at a rate of about 1 drops/s until air came through the column. 3 mL of hydrochloric acid solution (0.1 mol/L) was passed through the column, and it was then washed with 2 mL of methanol at a rate of about 1-2 drops/s until air came through the column. The hydrochloric acid and methanol solution were all discarded. The sample was eluted by passing 6 mL of methanol/ammonia water solution (9/1, V/V) at a rate of 1 drops/s through the column. The collected eluate was then evaporated to dryness by a gentle stream of nitrogen gas at 45 °C, and the residue was reconstituted with 1.0 mL of methanol. After mixing thoroughly,the reconstituted residue was filtered through a 0.22 μm nylon filter membrane for analysis by HPLC-MS.
A 20 μL portion of the final extract was injected into an Atlantis dC18column for HPLC-MS analysis. Mobile phase A was composed of water/acetic acid (0.1%, V/V) at pH 3.6 (adjusted with 35% of ammonium hydroxide), and mobile phase B was composed of methanol. The gradient program was as follows: 5% B, 0 min; 5%-25% B, 0-20 min; 25% B, 20-25 min; 25%-100% B, 25-35 min;100%-5% B, 35-45 min; and 5% B, 45-50 min. The mobile phase was delivered at 0.3 mL/min. The ESI-MS operating conditions included an electro-spray voltage of 5.0 kV and nitrogen sheath and auxiliary gases set to 35 and 15 (arbitrary units), respectively. The temperature of the heated capillary was set to 275 °C. For quantitative analysis, the selected ion for IQ, MEIQ and PhIP detection was 199.2→184.0 (30V), 213.3→198.0 (25V) and 225.3→210.0(30V), respectively. The calibration curves for IQ, MeIQ and PhIP quantification were y = 98 389x + 5 658 (R2= 0.998 8), y = 47 595x -1 844 (R2= 0.996 3) and y = 50 014x + 7 390 (R2= 0.992 3),respectively. The LODs of them were 7.0, 9.5 and 3.5 μg/kg,respectively.
2.3.3 Determination of 4-MEI
4-MEI was extracted from the samples using a procedure based on a previously described methodology [33] with some modifications.For sauces, a sample of sauces (0.5 g) was weighed into a 10 mL volumetric flask, and water was added up to the mark, while for SPBBS, a sample of meat (3 g) was mixed with 3 mL water and 7 mL acetonitrile on a Vortex mixer for 3 min and ultrasonically extracted for 20 min. The mixtures were centrifuged at 8 000 r/min for 20 min and the upper layer was collected by a glass tube. The typical volumes of solutions used for SPE were as follows: 0.8 mL for sauces and 1.0 mL for SPB-BS.
The solutions were passed through a PCX cartridge (preconditioned by 3 mL methanol and 3 mL water) after the addition of 1 mL of 0.1 mol/L HCl solution, and then the column was washed with 1 mL methanol. The retained compounds were eluted out with 6 mL of methanol/ammonia (95/5, V/V). The collected extracts were dried using a rotary evaporator at 45 °C, then the residues were dissolved with 1 mL methanol for 4-MEI analysis. The HPLC-MS/MS system was used to detect 4-MEI. Chromatographic separation was accomplished with a Waters XBridge BEH Shield RP18 Column(3.5 μm, 3.0 mm × 150 mm) using mobile phase A (10 mmol/L of ammonia in high-purity water) and mobile phase B (methanol) in a gradient programme with a flow of 0.3 mL/min: 0-10 min, 5% B; 11-15 min, 5% B to 100% B; 15-20 min, 100% B. In the mass spectrometer, ESI in positive ion mode was used at a capillary temperature of 300 °C, a spray voltage of 3.0 kV and a vaporizer temperature of 275 °C. The sheath gas was nitrogen at 35 psi. For quantitative analysis, the selected ion for 4-MEI detection is 83→41,83→42, and the collision energy was set at 33 kV. The calibration curve and LOD of 4-MEI was y = 403 058x - 5 202 (R2= 0.997 8)and 2.0 μg/kg, respectively.
2.3.4 Determination of furan in SPB-BS
The method described by Sarafraz-Yazdi et al. [34] was used to determine furan level in SPB-BS. All samples were stored at 4 °C to prevent possible losses of furan until analysis. Refrigerated meat samples (0.5 g) were mixed with 9.5 mL cold water in a 20 mL vial,which contains an appropriate amount of sodium chloride and then the vial was immediately closed with a septum before analysis. The analytical conditions were as follow: the gas chromatograph (GC)was equipped with a DB-Wax capillary column (30 m × 0.25 mm ×0.25 μm) (Agilent Technologies, Inc., CA, USA); the flow rate of nitrogen gas (> 99.999%) was 1.0 mL/min. The front inlet and detector temperature were controlled at 200 and 250 °C, respectively.The oven temperature was programmed as follows: started at 50 °C,holding 2 min, a 10 °C/min ramp to 200 °C and then isotherm for 5 min with the total running time of 22 min. The air and hydrogen flow rate for FID were 250 and 25 mL/min respectively. The calibration curve and LOD of furan was y = 403 058x - 5 202 (R2=0.997 8) and 2.0 μg/kg, respectively.
2.4 Volatile compounds in SPB-BS
The sample preparation and solid-phase micro-extraction (SPME)technique were used according to the previous methods [24,35]with some modifications, respectively. Aliquots (5 g) of SPB-BS and 10 mL ultrapure water were transferred into 20 mL gas-tight glass vessels. Prior to analysis, 40 μL phenethyl acetate solution(internal standard, 1 000 mg/L in dichloromethane) were added and mixed. After being equilibrated at 60 °C for 30 min, the sample was extracted with a 75 μm carboxen/polydimethylsiloxane fibre (CAR/PDMS, Supelco, Inc., Bellefonte, PA) for 30 min with continuous heating and agitation. After extraction, the SPME device was removed from the sample bottle and inserted into the injector port of the gas chromatography-mass spectrometry (GC-MS) for 3 min to desorb determinants. Each sample was extracted in triplicate. In all cases, CAR/PDMS fibre should be conditioned by heating in a gas chromatography injector port for 60 min and then desorbed for 10 min at 250 °C before extraction to prevent any contamination.
Analyses were performed using GC-MS system equipped with a GC, a Trisplus automated sampler and a quadrupole DSQ II MS(Thermo Finnigan, San Jose, CA). Separation was performed with a DB-5MS column (30 m × 0.25 mm i.d. × 0.25 μm thicknesses,Agilent Technologies, Wilmington, DE, USA). The carrier gas was ultrahigh-purity helium (99.999%) at a constant flow of 1.0 mL/min.During desorption, the oven was at 40 °C. After desorption, the oven was maintained at 40 °C for a further 2 min and then the temperature was raised at 4 °C/min to 280 °C for 5 min. The injector port was in splitless mode. The injector, ion source and transfer line temperature were maintained at 250, 230 and 230 °C, respectively. The mass detector was operated in the electron impact mode with ionization energy of 70 eV and a scanning range of 35-500 m/z. A series of n-alkanes (C7-C30) were run under the same conditions to obtain the linear retention index (LRI) values for the aroma compounds.The aroma compounds were identified with NIST08 and Wiley275 libraries and by their LRI from our experiment.
2.5 Statistical analysis
Statistical analyses were carried out using Minitab 15 (Minitab Inc., State College, PA, USA) and statistical product and service solutions (SPSS) 13.0 (IBM spss Inc., New York, USA) analytical software. P < 0.05 was identified as being statistically significant at a 95% confidence level.
3. Results and discussion
3.1 Amounts of harmful compounds and their possible formation path in SPB-BS
It is well known that meat samples had more protein and the brown sauces had more reducing sugar and free amino acids (FAA)[20]. Hence, the MR will easily occur and thus some harmful compounds were generated in the SPB-BS processing [4,36]. Our present study found 4 kinds of harmful compounds in the SPB-BS strongly implied that MR has occurred. The amounts of AA, three HAAs, 4-MEI and furan in SPB-BS shown in Fig. 2 were in the range of 0.051-0.50 mg/kg. AA is mainly existing in several heat-treated carbohydrate-rich foods, such as French fries, coffee and other starchy products [5]. The amount of AA is lower than other Maillard reaction products (MRPs) in the SPB-BS due to its low content of starchy materials; and this is consistent with reports from Liu et al. [21]. AA is only detectable in S5 (Fig. 2), in other words, it is generated after adding the sugar and sauces which consist of the precursor of the AA formation.

Fig. 2 Content variations of (a) AA, (b) 4-MEI and (c) furan during cooking of stewed pork balls with brown sauce (SPB-BS) (n = 3).
Only the PhIP was detectable in S5, but the content was lower than the methodological limit of quantification. This result revealed that HAAs were more easily generated when the food contact directly with the open flame (baking) or surface of high temperature metal(frying). During preparing S3, HAAs have not been detected due to the lack of sugars or another precursor. It is generally accepted that IQ-types HAAs and their derivatives (methylated forms) are suggested to be formed from creatinine, sugars, free amino acids, and some di-peptides through Maillard reaction and Strecker degradation upon heating [10]. For S4, 800 mL water was added onto the meat,and the mixture was stewed at a relatively low temperature (lower than 100 °C due to the water presence). Hence, the low temperature cooking inhibits the formation of HAAs in S4. The AA and HAAs were not detectable in the heated sauces, so the possibility they come from sauces can be ruled out.
As presented in Fig. 2, the 4-MEI was detectable in S4 and S5.It has two main sources: on the one hand, the 4-MEI came from the brown sauces itself, because many studies have reported that 4-MEI is ubiquitous in brown sauces [33]. The 4-MEI content of sauce used herein was (0.76 ± 0.10) mg/kg. On the other hand, sufficient MR precursors are brought in sauce and generated from the protein hydrolysis of meat, and thus the 4-MEI can be generated from the MR during cooking [13].
With the continuous processing of pork balls, the amount of furan was increased gradually from 0.049 mg/kg (S3) to 0.092 mg/kg(S5). These results might be caused by the multiple formation paths of furan. Based on the composition of the SPB-BS system,3 mechanisms [37] for furan generation in SPB-BS were proposed and illustrated in Fig. 3. For S3, the furan was mainly generated from the oxidative degradation of polyunsaturated fatty acids (PUFAs) in the edible oil (Fig. 3A) and the thermal degradation reaction of some amino acids in the meat (Fig. 3B). In the S3, only several amino acids(for example serine) can simultaneously transform to the acetaldehyde and glycol-aldehyde by thermal degradation reaction. The acetaldehyde and glycol-aldehyde can generate furan by aldol condensation and cyclization. While in the S4 and S5, the furan was formed not only by path A and path B, but also by Maillard reaction with the addition of sugar and brown sauces. Some reducing sugars (for example glucose)from brown sauces can generate furan through the thermal degradation reaction (Fig. 3C). In addition, some amino acids (for example aspartic acid, alanine and threonine) can only produce acetaldehyde, so in these two systems, the furan was formed with the increasing of glycol-aldehyde from the degradation of reducing sugars.
3.2 Quantitative assessment the potential risks
In order to evaluate the exposure of risks, the following formula was proposed and used firstly to describe each and total risk values of MRPs in the SPB-BS.

Where T is the comprehensive risk value; Tkis the single risk value of each compound; A is the weighting value according to the classification by IARC (kg/d) (Table 1); c is the concentration of each compound in SPB-BS (μg/kg); mlis the acceptable daily intake(ADI, μg/d) (Table 1); n is the number of MRPs. Fig. 4 shows the total risk value of SPB-BS during cooking. The main compounds contributing to the risk value of SPB-BS are furan, 4-MEI, and AA in S3, S4 and S5, respectively. Taking AA as an example, eating 100 g of braised pork is equivalent to 5.0 μg of AA consumption. Although it exceeds the ADI (2.7 μg/d, Table 1), considering that it is not consumed every day and has a metabolic effect, AA in SPB-BS is still a lower risk compared with potato chips and grilled asparagus [38].Moreover, these values vary widely according to individual consumer habits, and in some cases (consume SPB frequently) they probably reach levels of concern for human health.

Table 1Content of studied 8 compounds in SPB-BS during cooking (n = 3).

Fig. 3 Potential paths of furan from polyunsaturated fatty acids (path A), amino acids (path B) and reducing sugar (path C) in SPB-BS.
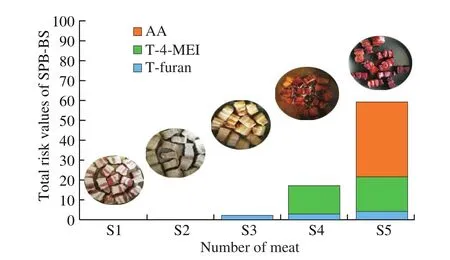
Fig. 4 Contribution of risk value in stewed pork balls with brown sauce(SPB-BS) during cooking.
It is scientifically incorrect to evaluate the food safety by taking into account a single factor of content. The present study serves as a proof-of-concept for safety assessment on food, thinks that comprehensive consideration of the content, toxicity and ADI of these compounds is essential for food safety. For example, the 4-MEI content is higher than AA, but the risk value of AA is higher than 4-MEI due to its lower ADI value. In spite of this, procedures and methods for risks assessment on food safety are very sophisticated and the quantification of the valuation results is difficult; this study might suggest a possibility of safety assessment. In addition, some potentially harmful compounds, also generate from MR, such as 5-hydroxymethylfurfural (HMF) [39] and advanced glycation endproducts (AGEs) were not detected in this paper. That is because there is controversy about the harmfulness of these compounds and their limitation is not ruled.
3.3 Volatile compounds in SPB-BS
To determine the impact of cooking process on the volatile compounds in the SPB-BS, a total of 57 volatile compounds were identified and shown in Table 2. These compounds were grouped according to their chemical structure as 9 alkanes, 4 alcohols, 8 aldehydes, 4 ketones, 7 acids, 11 esters, 7 furans and pyrazines and 7 others. All volatiles detected are ranked in order of decreasing relative contents of S5: alcohols > esters > acids > aldehydes >furans and pyrazines > alkanes > others > ketones. Compared the changing trends of these volatiles (form S1 to S5), three resultoriented conclusions can be obtained. One is that alkanes, ketones and partial aldehydes were mainly come from raw pork balls, which are depended on the breeds, sources and rearing conditions of pig[23]. They were decreased significantly by the boiling (S2) and frying (S3) process. The increasing of 2 and 3-methylbutanal and phenylacetaldehyde in S4 may be explained by the addition of sauce.This result is in agreement with the previous report [24]. Another is that the amounts of alcohols, acids and fractional aldehydes and esters in the SPB-BS were mainly come from brown sauce. This could be confirmed by the composition of previously examined Korean and Chinese soy sauces [25,40]. The edible blend oil may be responsible for another part of esters, which is added into the SPBBS during S3. Results also show that the contents of total alcohols and aldehydes in S5 were lower than S4. The reason may be that some alcohols and aldehydes were evaporated during the process of reducing liquids from S4 to S5. At last, some furan-like and nitrogen-containing compounds such as pyrazines identified in our study indicated that MR is actually occurred and contributed to form flavor compounds. Further, the free amino acids play an important role in generating harmful and flavor compounds by MR during SPB-BS processing. Not only that, some other compounds including pentadecane, nonanoic acid, 3-hydroxydodecanoic acid,arachidonic acid and glycocyamidine were only found in S5,which inferred that some complex reactions may be occurred.
Table 2 also shows a total of 31 aroma-active compounds found from the SPB-BS during preparation [30,41-43]. These compoundsincluded 2 alkanes, 3 alcohols, 6 aldehydes, 1 ketone, 3 acids, 4 esters, 7 furans and pyrazines and 5 others compounds [29]. Among these compounds, most of alcohols and aldehydes could contribute to the aroma of “malty” or “almond”, a large proportion of acids and esters could contribute to the aroma of “fruity” or “floral” and the aroma of “caramel” or “roasted nuts” was mainly responsible by the furans and pyrazines compounds. In addition, the aroma of “sulfur”or “cooked vegetables” was contributed to others compounds, which including 2-methylthiophene, dimethyl trisulfide and 3-acetyl-1H-pyrroline. As shown in Fig. 5, the sensory analysis of the 5 samples during preparing SPB-BS revealed significant differences for selected descriptors evaluated. The adding sauce and oil contributed equally to the aroma of “fruity”. The aromas of “almond” and “caramel” were mainly formed by the addition of soy sauce (the cyan and green line in Fig. 5). Most of these aroma-active compounds observed in present work have been previously reported in soy sauce [39,40]. Moreover,some compounds including furans and pyrazines contributing to the aroma of “caramel” or “roasted” may be generated from MR during the meat heating. Some new aroma compounds such as pentadecane,acetyl-propionic acid, nonanoic acid, 3-hydroxydodecanoic acid,arachidonic acid and glycocyamidine were firstly reported in SPB-BS.However, several bitter compounds including furfural and 5-methyl-2-furancarboxaldehyde formed from MR were also identified in present work [44]. Because it is complicated and difficult to elucidate the formation path of volatiles from MR in SPB-BS; the presence of multiple reactants as well as the dynamic conditions all contributes to a complex chemical landscape. In summary, some aldchydes,esters, furans and pyrazines are the characteristic aroma components in SPB-BS. Aldehydes, with highly volatile and low threshold, play an important role in the odor characteristics [45]; esters usually contributed a fruity and oily ordor; furans and pyrazines are mainly derived from Maillard reactions with a low threshold, and even small amounts have a greater impact on flavor [46].

Fig. 5 Sensory analysis of the 5 samples during preparing SPB-BS.

Table 2Mean concentration of volatile compounds of SPB-BS during cooking process by HS-SPME-MS.
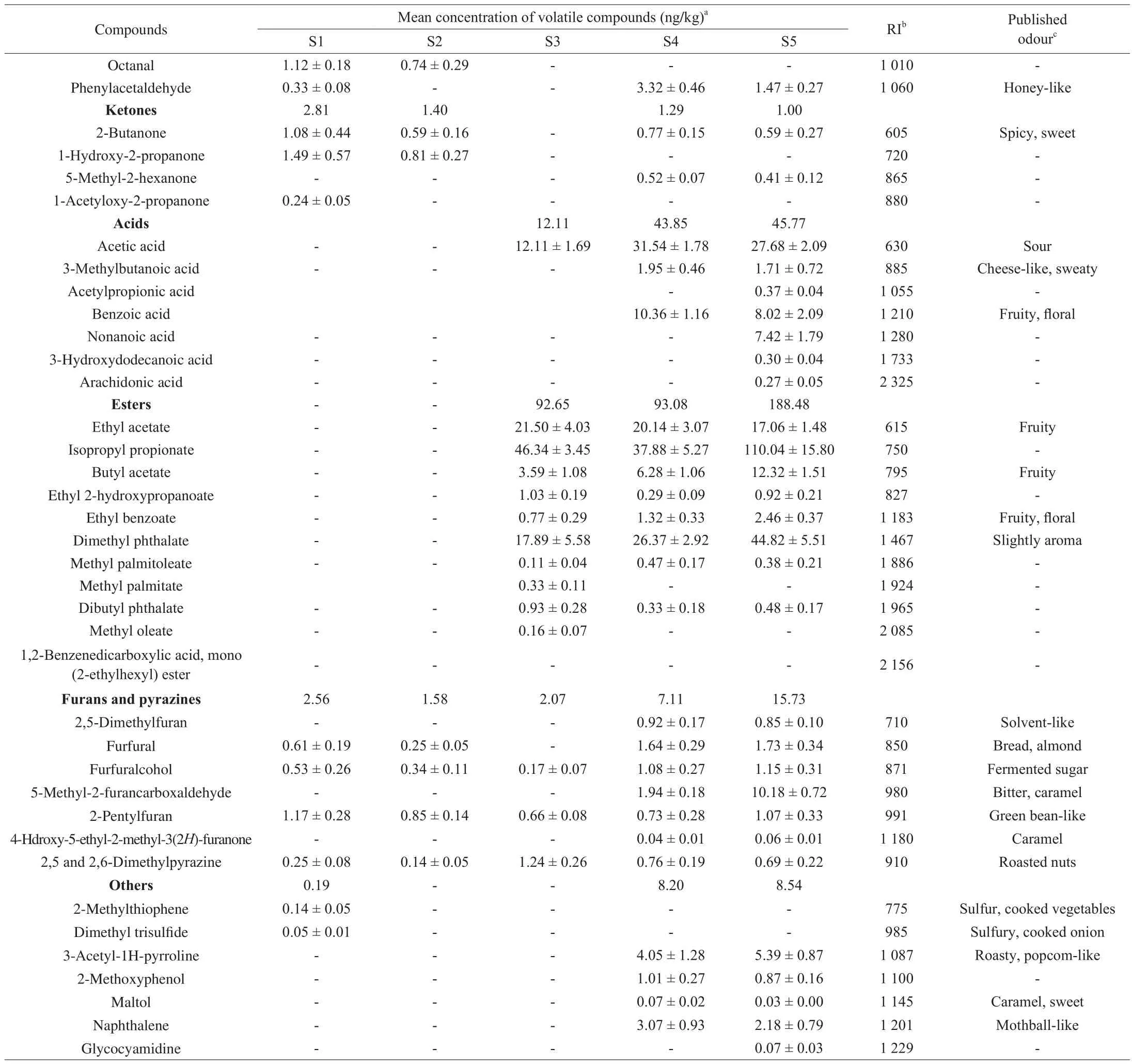
Table 2 (Continued)
Combined with the above harmful compounds, it is really important to control the MR in home cooking. Researches have reported that the suitable home cooking conditions can lead to low MRPs generation and pleasant colors [47], and affect the fatty acid composition [48]. There are some effective inhibitors to limit the accumulation of risks compounds in model systems, but it may simultaneously affect other organoleptic properties. Therefore, a further study needs to conduct to control the MR in SPB-BS by adding some materials containing biologically active substances to find a balance between risk and aroma, which is more important and significative to guide the SPB-BS cooking and rational diet in China.
4. Conclusion
In SPB-BS, selected MRPs content was in the range of 0.05-0.50 mg/kg. Results indicated that the addition of sauces provides the main precursors and enhances the formation of selected harmful compounds. Several sources of these compounds in SPBBS were inferred based on their structures and the composition of this system, lipid oxidation and Maillard pathways. The proposed quantitative formula might suggest a possibility to evaluate the risk value of food products and the values vary widely according to individual consumer habits, and in some cases (consume SPB frequently) they probably reach levels of concern for human health.A total of 57 volatiles were identified in SPB-BS using HS-SPMEGC-MS. Results showed that alcohols, aldehydes, acids and esters could contribute to the aroma of SPB-BS and most of them are mainly generated from sauces, oil and MR.
Conflict of Interest
The authors declare to have no conflict of interest.
Acknowledgements
All authors acknowledge the financial support from the National Science Foundation of China (Grant No. 31771931), the start-up funds for scientific research at North University of China (304-1101285714),the Science and Technology Planning Project of Guangdong Province of China (No.2014B020205001 and No. 2013B051000015) and the 111 Project (B17018).
- 食品科学与人类健康(英文)的其它文章
- Bioactive compounds and probiotics-a ray of hope in COVID-19 management
- Approaches to evaluate nutrition of minerals in food
- The role of glutamine in supporting gut health and neuropsychiatric factors
- Anti-hyperglycemic effects of dihydromyricetin in streptozotocin-induced diabetic rats
- Aroma profile of two commercial truffle species from Yunnan and Sichuan, China:inter- and intraspecific variability and shared key compounds
- Stability of phenolic compounds and drying characteristics of apple peel as affected by three drying treatments

