Evolving role of endoscopy in inflammatory bowel disease: Going beyond diagnosis
Paulina Nunez F, Noa Krugliak Cleveland, Rodrigo Quera, David T Rubin
Abstract Inflammatory bowel disease, encompassing Crohn’s disease (CD) and ulcerative colitis, are chronic immune-mediated inflammatory bowel diseases (IBD) that primarily affect the gastrointestinal tract with periods of activity and remission.Large body of evidence exist to strengthen the prognostic role of endoscopic evaluation for both disease activity and severity and it remains the gold standard for the assessment of mucosal healing. Mucosal healing has been associated with improved clinical outcomes with prolonged remission, decreased hospitalization,IBD-related surgeries and colorectal cancer risk. Therefore, endoscopic objectives in IBD have been incorporated as part of standard care. With the known increased risk of colorectal cancer in IBD, although prevention strategies continue to develop, regular surveillance for early detection of neoplasia continue to be paramount in IBD patients’ care. It is thanks to evolving technology and visualization techniques that surveillance strategies are continuously advancing.Therapeutic endoscopic options in IBD have also been expanding, from surgery sparing therapies such as balloon dilation of fibrostenotic strictures in CD to endoscopic mucosal resection of neoplastic lesions. In this review article, we discuss the current evidence on the use of endoscopy as part of standard of care of IBD, its role in surveillance of neoplasia, and the role of interventional endoscopic therapies.
Key Words: Inflammatory bowel disease; Endoscopy; Crohn’s disease; Ulcerative colitis;Therapeutic endoscopy; Surveillance
INTRODUCTION
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn's disease (CD) are immune-mediated inflammatory diseases that primarily affect the gastrointestinal tract with periods of activity and remission[1 ,2 ]. The diagnosis of IBD requires endoscopic and histological evaluation of the intestinal mucosa, combined with the proper clinical presentation and setting[3 ]. Endoscopy allows us to differentiate CD from UC and define its severity by visualization of the colonic mucosa, its vascular pattern, mucosal lesions, as well as patterns locations of inflammation including assessment of involvement of the terminal ileum and perianal region[4 ].
In the absence of effective treatment, intestinal inflammation is often progressive and cumulative, leading to complications such as strictures, fistulas that require surgery, and in the long term, the risk of dysplasia or colorectal cancer (CRC)[5 ]. There is also evidence that such cumulative damage may result in mucosal dysfunction, with dysmotility and hypersensitivity[6 ]. Therefore, endoscopic control has become part of the objective treatment pillar of IBD and mucosa healing is a preferred goal of treatment[7 ].
In this review we will focus on the usefulness of endoscopy as a therapeutic objective, in follow-up and surveillance to detect and to prevent the development of dysplasia and CRC, and its evolving utility in interventional therapies.
ENDOSCOPY TO ASSESS DISEASE ACTIVITY
Mucosal healing is recommended as a therapeutic objective in patients with IBD. This objective is associated with a better prognosis, lower rates of hospitalizations, lower risk of relapse and the need for surgery[8 ,9 ]. After the STRIDE consensus (selecting therapeutic targets in inflammatory bowel disease), it is recommended to evaluate the colonic mucosa looking for the resolution of ulcers and the friability of the mucosa 6 -9 mo after starting therapy in CD and 3 -6 mo in UC[10 ].
Other reviews have supported this approach as well[11 ,12 ]. It has been suggested that endoscopic remission in UC should be defined as a Ulcerative Colitis Endoscopic Index of Severity (UCEIS) of 0 or a Mayo Endoscopic Score (MES) of 0 or 1 . However,in follow-up studies, a higher percentage/risk of relapse has been observed in patients who had reached a MES 1 index compared to the group with a MES 0 index (36 .6 %vs9 .4 % with P < 0 .001 )[13 ]. On the other hand, an endoscopic response has been defined as a decrease in ≥ 1 degrees of the MES or a decrease in ≥ 2 points of the UCEIS[14 ,15 ].
The Crohn's Disease Endoscopic Index of Severity (CDEIS) and Simple Endoscopic Score for Crohn's Disease (SES-CD) are validated and reproducible indices in CD[16 ].However, remission thresholds have been arbitrarily determined considering endoscopic remission with a SES-CD 0 -2 index and after surgery a Rutgeerts i0 -i1 [17 ,18 ]. Endoscopic response is defined as a decrease greater than 50 % in SES-CD or CDEIS[19 ] (Figure 1 ). STRIDE-II maintained endoscopic criteria for UC and CD[20 ].

Figure 1 Endoscopic index in inflammatory bowel diseases[13 -22 ]. The Simple endoscopic score Crohn´s disease: Sum of values of the four variables for the five bowel segments (rectum, left colon, transverse colon, right colon and ileum). UCEIS: Ulcerative colitis endoscopic index of severity; SES CD: Simple endoscopic score Crohn´s disease; IBD: Inflammatory bowel diseases.
One of the most important postoperative endoscopy studies was the multicenter POCER (postoperative Crohn's endoscopic recurrence), which included 17 centers in Australia and New Zealand[21 ]. Authors recommend performing a control endoscopy six months after surgery, regardless of the risk factors for recurrence, since approximately 60 % may recur (80 % if they have high risk factors vs 30 % in those with low risk). In this control endoscopy the anastomosis and the terminal ileum are to be evaluated and assessed for recurrence. High risk factors for recurrence include the following: disease in those under 30 years of age, a penetrating phenotype, presence of perianal disease or having two or more surgeries[22 ]. The therapeutic objectives in endoscopy are fundamental to avoid future complications of the disease, and are summarized in Figure 2 .
ENDOSCOPY FOR SCREENING AND SURVEILLANCE FOR COLORECTAL NEOPLASIA
CRC is the third most frequent neoplasm in the general population, with an incidence of 1 .8 million new cases per year, being the second cause of cancer mortality in the world[23 ]. IBD presents a higher risk of CRC, and traditionally it was considered that patients with IBD had a 2 % increase in CRC risk at 10 years of disease, 8 % at 20 years and 18 % after 30 years of evolution[24 ]. However, the estimated risk of neoplasia in recent years has been lower, possibly due to more effective therapies[25 ] as well as better prevention strategies. A subsequent meta-analysis identified a 2 .4 -fold risk of CRC in UC patients, with a higher risk in male patients, younger age at diagnosis and extensive disease. Cumulative absolute risk of developing CRC was 0 .4 % at 10 years and 1 .1 %- 5 .3 % at 20 years[26 ].
Other risk factors to consider are a family history of CRC or its association with primary sclerosing cholangitis[27 ]. Studies in CD have identified risk factors similar to those in UC, including diagnosis at an early age, prolonged disease, distal location,and penetrating phenotype[28 ]. Perianal fistulas have also been identified to have a risk of neoplastic transformation[29 ] (Table 1 ).

Figure 2 Endoscopic objectives in inflammatory bowel diseases treatment[8 -22 ]. CCR: Colorectal cancer; UC: Ulcerative colitis; CD: Crohn’s disease; IPAA: Ileal-pouch-anal anastomoses; SES-CD: Simple endoscopic score Crohn´s disease; UCEIS: Ulcerative colitis endoscopic index of severity.
A screening endoscopic evaluation for dysplasia or colon cancer has been recommended 8 years after diagnosis in UC patients with an extension beyond the rectum or in CD patients with an extension over 30 % of the colon or that compromises more than one segment[30 ]. In 2017 , a Cochrane systematic review reported that those patients who were in endoscopic surveillance had a lower mortality associated with CRC compared to those who were not followed up [8 .5 % vs 22 .3 %, respectively; odds ratio (OR) 0 .36 , 95 % confidence interval (CI): 0 .19 -0 .69 ][31 ].
Endoscopic surveillance requires optimal control of the disease, including mucosal healing. This will allow for early recognition of neoplasia[3 ]. It has even been recommended that surveillance should be performed with fecal calprotectin levels under 100 μg/g[32 ] to improve visualization. It should not be forgotten that optimal bowel preparation is essential for the detection of lesions[33 ].
Dysplasia is defined as a neoplastic alteration of the intestinal epithelium that remains restricted within the basement membrane, without invasion of the lamina propria. These lesions can be low (LGD) or high grade (HGD). The distinction between these two depends on the distribution of nuclei within the mucosa. LGD maintains hyperchromatic nuclei located in the basal half of cells, whereas HGD presents nuclear stratification and loss of cell polarity[34 ].
Lesions found can be polypoid or flat, the latter being the most frequent. Flat lesions are associated with higher rate of neoplasia, common in regions that are or have been involved by IBD[35 ]. These lesions tend to be subtle and can be multifocal, requiring meticulous and trained endoscopists[36 ]. Furthermore, since dysplasia can be difficult to distinguish from epithelial regeneration secondary to inflammation[37 ], it is recommended that biopsies are evaluated by two expert pathologists[38 ].
DYSPLASIA DETECTION METHODS
Dye-based chromoendoscopy (DCE) is an image-enhanced endoscopic technique in which topical dyes such as methylene blue or indigo carmine are applied allowing a detailed view of the mucosa and a targeted evaluation of suspicious lesions[39 ]. It has reported a higher performance in detecting dysplasia in the analysis per patient OR 2 .05 (95 %CI: 1 .26 -3 .35 ) and analysis by type of lesion OR 2 .79 (95 %CI: 2 .08 -3 .73 )[40 ].On the other hand, Feuersteinet al[41 ] observed that DCE was more effective in identifying dysplasia compared to standard white light endoscopy (WLE), but without reaching significant differences compared to high-definition WLE (HD-WLE).Recently, a retrospective analysis, has shown no difference in the detection of dysplasia using DCE compared to HD-WLE, although withdrawal times were longerwith DCE (24 .6 min vs 15 .4 min, P < 0 .001 )[42 ].
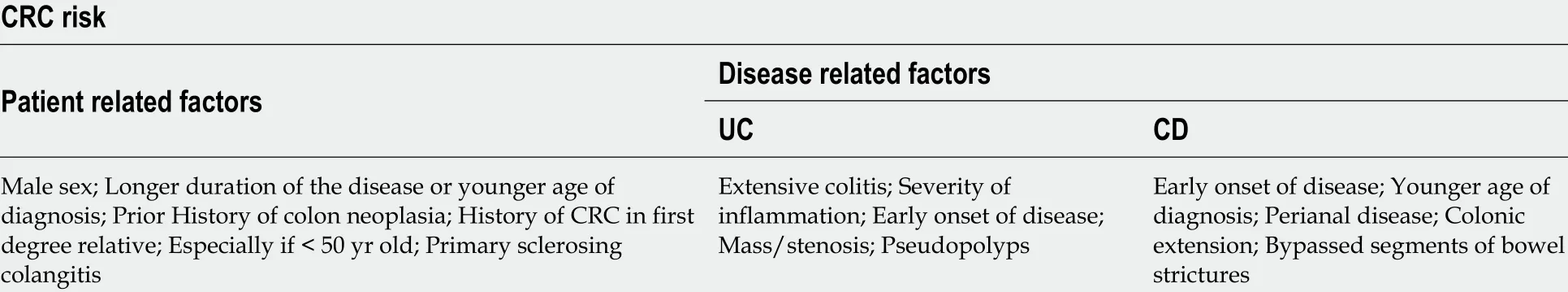
Table 1 Risk factors for colitis associated neoplasia[26 -29 ]
SCENIC (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus)recommends a surveillance study with high-definition colonoscopy or chromoendoscopy when standard white-light exam are performed[30 ]. The American College of Gastroenterology 2019 guidelines suggest targeted biopsies as an option with highdefinition scopes. This approach is evolving as the technology is advancing. In the absence of augmenting imaging by high-definition or chromo, systematic sampling of the mucosa with random biopsies are recommended for every 10 cm of colon[3 ,37 ].
Virtual chromoendoscopy (VCE) [which includes narrow banding imaging(Oympus NBI), i-SCAN (Pentax) and Fujinon Fuji Intelligent Chromo-Endoscopy] is an optical imaging technique that uses filters to enhance the contrast of both the mucosa and the superficial vasculature, allowing a better evaluation of the mucosa.Bisschopset al[43 ], conducted a multicenter study with UC patients comparing DCEvsNBI. No significant difference was reported between these techniques in detecting neoplastic lesions (OR 1 .02 , 95 %CI: 0 .44 -2 .35 , P = 0 .964 ). The SCENIC consensus guidelines state that VCE should not replace DCE. Undoubtedly, larger studies are lacking to evaluate the usefulness of these techniques. The 2019 ACG guidelines recommend the use of DCE or NBI for the surveillance of dysplasia (conditional recommendation, low quality of evidence)[44 ].
MANAGEMENT OF DYSPLASIA
Once dysplasia has been identified, its respectability is essential to interrupt the carcinogenic sequence and thus reduce the incidence of CRC. The different management guidelines indicate that active surveillance and endoscopic follow-up should be performed according to the type of dysplasia found and the patient's risk factors. In Figures 3 and 4 the subsequent management is summarized. In selected patients, segmental resection without proctocolectomy is possible[45 ].
ENDOSCOPY FOR THERAPEUTIC INTERVENTIONS
Currently, therapeutic endoscopic interventions are considered in four areas: stenosis,fistulas, complications associated with surgery and neoplasms associated with colitis[46 ]. We will review the latter in a separate chapter.
Stenosis
For its diagnosis, an endoscopic evaluation and radiologic exams are required, which may be a magnetic resonance enterography or an abdomen-pelvis computed tomographic enterography[47 ]. It is important to record the number of stenoses, their location, their composition (inflammatory, fibrotic), morphology, size, as well as the detection of complications such as abscesses or fistulas[48 ].
There are three endoscopic therapeutic options in those stenoses smaller than 5 cm;endoscopic balloon dilation, endoscopic stricturotomy or stent placement, the latter with a very low level of evidence[49 ]. Endoscopic balloon dilation would have a lower risk of bleeding, but a higher risk of perforation. However, retrospective studies have shown it is a safe technique in patients with CD, with more than 40 % of patients asymptomatic and without requiring surgery in a subsequent follow-up[50 ].Endoscopic stricturotomy is in evolution and may be an effective procedure in treating fibrotic, distal or anastomotic stenosis. The technique is based on an electro-incision,allowing control of the depth and location of the cut, and with a lower risk of perforation. In a small reported experience surgery free survival in IBD to non-IBD patients undergoing endoscopic stricturotomy, there was no statistically significant difference between the groups[51 ].

Figure 3 Inflammatory bowel diseases surveillance[3 ,24 -28 ]. CCR: Colorectal cancer; IBD: Inflammatory bowel diseases; UC: Ulcerative colitis.

Figure 4 Algorithm for the management of dysplasia[3 ,37 ,42 -46 ]. LGD: Low grade dysplasia; HGD: High grade dysplasia; CE: Chromoendoscopy.
Endoscopic injections of steroids or anti-tumor necrosis factor agents in conjunction with endoscopic balloon dilation have been reported in series of cases with the intention of reducing the need for future dilation with inconsistent results[52 ,53 ].
In recurrent or refractory stenoses, self-expanding metal stents, covered metal stents or biodegradable metal stents have been used with good results[54 ,55 ]. These can be placed endoscopically with or without fluoroscopic guidance but must be maintained for at least 4 wk[56 ].
Fistulas
The penetrating phenotype may be primary or a result of a long-standing CD. The goals of endoscopic treatment are drainage, closure of the fistulas and preventing them from becoming complex[48 ]. In a cohort of 29 patients, about 90 % achieved resolution of their fistulas by endoscopic fistulotomy[57 ]. This type of therapy might be performed in superficial, short and enteroenteric fistulas[56 ]. In addition, it is possible to close the fistula endoscopically by means of a clip, avoiding the formation of abscesses. This has been reported to be achieved with through-the-scope clips or overthe-scope clips, even with reports in perianal fistulas[48 ]. Further work is needed in this area, with clarification of risks, benefits, and approach to combination with medical interventions.
Post-operative complications
Post-surgery complications may present as dehiscence of the suture/staple line or present later with stenosis of the anastomosis, causing obstructive problems[58 ].Endoscopic management of suture dehiscence has been described in clinical cases or case series, reporting lumen integrity in over 80 %[59 ]. Approximately 11 % of patients with UC require an ileal pouch anal anastomoses for their management[60 ], where strictures may develop in anastomosis, in the pouch or in the afferent loop.Postoperative strictures can be managed endoscopically with balloon dilations or stricturotomy. Endoscopic interventions should be avoided in periods of increased inflammation given the increased risk of perforation[21 ].
CONCLUSION
Endoscopic evaluation is essential to achieve current therapeutic goals in IBD, which focus on mucosal healing[61 ,62 ]. In cases in which the objectives are not achieved,endoscopic assessments direct therapeutic optimization in order to achieve them.Importantly, endoscopic assessment has a key role in prognostication and disease monitoring to control modifiable risk factors for worse clinical outcomes, with a focus on control of inflammation and prevention of complications. Among these, the incidence of CRC has a significant impact on patient morbidity and mortality, and its early diagnosis is a crucial element in IBD management. The evolution of endoscopic visualization techniques, such as the use of VCE and DCE, allow a better detection and characterization of premalignant endoscopic lesions, avoiding the risks of advanced stages[63 ]. Additionally, evolving endoscopic therapies allow greater minimally invasive therapeutic options for the treatment of stenosis and fistulas as an alternative to surgical management with lower morbidity.
 World Journal of Gastroenterology2021年20期
World Journal of Gastroenterology2021年20期
- World Journal of Gastroenterology的其它文章
- Pancreatitis after endoscopic retrograde cholangiopancreatography: A narrative review
- Cyclophosphamide-associated enteritis presenting with severe protein-losing enteropathy in granulomatosis with polyangiitis: A case report
- Breakthroughs and challenges in the management of pediatric viral hepatitis
- Association between oral contraceptive use and pancreatic cancer risk: A systematic review and metaanalysis
- Understanding celiac disease monitoring patterns and outcomes after diagnosis: A multinational,retrospective chart review study
- Role of modern radiotherapy in managing patients with hepatocellular carcinoma
