Role of modern radiotherapy in managing patients with hepatocellular carcinoma
Liang-Cheng Chen, Hon-Yi Lin, Shih-Kai Hung, Wen-Yen Chiou, Moon-Sing Lee
Abstract Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. Several treatment options are available for managing HCC patients,classified roughly as local, local-regional, and systemic therapies. The high postmonotherapy recurrence rate of HCC urges the need for the use of combined modalities to increase tumor control and patient survival. Different international guidelines offer treatment recommendations based on different points of view and classification systems. Radiotherapy (RT) is a well-known local-regional treatment modality for managing many types of cancers, including HCC.However, only some of these treatment guidelines include RT, and the role of combined modalities is rarely mentioned. Hence, the present study reviewed clinical evidence for the use of different combined modalities in managing HCC,focusing on modern RT's role. Modern RT has an increased utility in managing HCC patients, mainly due to two driving forces. First, technological advancement(e.g., stereotactic body radiotherapy and advanced proton-beam therapy) enables precise delivery of radiation to increase tumor control and reduce side effects in the surrounding normal tissue. Second, the boom in developing target therapies and checkpoint-blockade immunotherapy prolongs overall survival in HCC patients, re-emphasizing the importance of local tumor control. Remarkably, RT combines with systemic therapies to generate the systemic therapy augmented by radiotherapy effect, a benefit now being actively investigated.
Key Words: Hepatocellular carcinoma; Stereotactic body radiotherapies; Radiotherapy;Guideline; Combined treatment; Immunotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver,ranking as the sixth leading cause of cancer incidence and the fourth most common etiology of cancer death worldwide; overall, around 953000 cases were diagnosed in 2017 globally[1]. The majority of HCC patients are found in the Asia-Pacific region,and around 75%-90% of cases are associated with chronic viral hepatitis (mainly hepatitis B and C) infection[2-4]. Therefore, the management of HCC requires considering detailed information from three main domains together: Cancer characteristics, liver function reserve, and overall patient condition.
Several treatment modalities are available for managing HCC patients; these modalities are generally classified as local, local-regional, and systemic therapies.Local therapy includes surgical resection, ablative therapies, and radiotherapy (RT).Ablative therapies include radiofrequency ablation (RFA)[5-7], percutaneous ethanol injection therapy[8], cryoablation[9], and microwave[10]; these modalities can be performed through percutaneous, laparoscopic, or open approaches. Local-regional or so-called arterially directed therapies include trans-arterial embolization (TAE), transarterial chemoembolization (TACE)[5], drug-eluting beads (DEB)-TACE[11-13], transarterial radioembolization (TARE) with yttrium 90[14,15], and hepatic arterial infusion chemotherapy (HAIC)[16-18]. Systemic therapy includes target therapy (e.g.,sorafenib[19], lenvatinib[20], and regorafenib[21]) and immunotherapy (e.g., nivolumab[22]); more notably, the use of several such types of agents has been investigated aggressively.
The preferred curative surgical modalities include liver resection and liver transplantation, generating a 5-year overall survival rate of up to 70%-75%[23,24]. RFA is another frequently used curative modality, with a 5-year overall survival rate of around 40%-70%[24]. However, local control of RFA is decreased in patients with large tumors, especially those larger than 3 cm[25,26]. It is recognized that only 10%-30% of HCC patients are candidates for curative surgical options because most HCCs are diagnosed at an intermediate or advanced stage[27,28]. Even though many treatment options can be chosen for HCC patients and each treatment modality has seen advancement in past decades, 5-year overall survival rates are still unsatisfactory,being less than 20%[29].
A high treatment failure (i.e., recurrence) rate is reported in the heterogeneous group of patients with intermediate- to advanced-stage HCC. Five-year tumor recurrence rates are more than 50% after liver resection and may be up to 80% after RFA[30]. This high recurrence rate includes patients who underwent TACE or some other singular local treatment modality. Of note, intrahepatic recurrence is the most common recurrence pattern. This unsatisfactory tumor control rate suggests that combining different locoregional therapies may improve treatment outcomes[31-33].This review focuses mainly on RT's current role in managing HCC, not only as a monotherapy but also as an essential part of combined modalities, especially stereotactic body radiotherapy (SBRT)/stereotactic ablative radiotherapy (SABR).
CURRENT TREATMENT GUIDELINES FOR HCC
Currently, useful treatment guidelines for managing HCC include recommendations from the National Comprehensive Cancer Network (NCCN)[34], the American Association for the Study of Liver Diseases (AASLD)[35], the European Association for the Study of the Liver (EASL)[36], the Japan Society of Hepatology (JSH)-HCC[37], the Korean Liver Cancer Association (KLCA) and National Cancer Center (NCC)[38], the National Health and Family Planning Commission (NHFPC) of the People’s Republic of China HCC guideline[39], and the Taiwan Liver Cancer Association (TLCA)[40].
In practice, these guidelines implemented different classification systems to stratify HCC patients for appropriate management. For example, the NCCN grouped patients as potentially resectable/transplantablevsunresectable/inoperable to guide treatment options. The AASLD and EASL guidelines used the Barcelona clinic liver cancer(BCLC) stage to lead management recommendations. The KLCA and NCC guidelines used a modified Union for International Cancer staging system adapted from the Liver Cancer Study Group of Japan[41,42]. Both the JSH-HCC and TLCA guidelines adopted hepatic functional reserve, extra-hepatic metastasis, vascular invasion, tumor number,and tumor size to guide treatment choice using a step-by-step manner and the Chinese guideline added general health status to the previously-mentioned risk factors[37,39,40]. Due to the variety of classification systems used in these different guidelines, we summarized treatment recommendations according to the BCLC stage in Figure 1.
COMBINED MODALITY FOR HCC
None of the guidelines mentioned above well declare the role of the combined treatment strategies used frequently in daily practice. Clinically, the choice of different combined modalities is based not only on guidelines or evidence, but also on the individual patient’s condition, the liver function preservation, the tumor characteristics, and the treatment perspective including the availability of resources within the facility and the therapeutic ratio. Overview of therapeutic options and the consideration behind the combination of different modalities for liver cancer are illustrated in Figure 2.
For very early and early stage HCC
Some anatomic locations of HCC, such as tumors adjacent to the gallbladder, liver hilum, bowel, stomach, and other critical organs, may limit the use of RFA as an intervention[43]. On the other hand, RT can be delivered to tumors that arise from any location, so that it can compensate for or combine with RFA.
For intermediate stage HCC
Many patients receive TACE as their first local-regional therapy. However, TACE alone seldom achieves satisfactory tumor control[44]. Therefore, several combined modalities have been reported to increase treatment outcomes, and they are subdivided into three main categories, as follows.
Local-regional plus local therapy:TACE combined with RFA achieves a complete response (CR) rate of 55-65% at the time of the first or second post-treatment check[31,32]. TACE combined with conventionally fractionated radiotherapy (CFRT)demonstrates a better 1-year survival rate than TACE alone[45]. Remarkably, TACE combined with SBRT shows promising results[46]. In contrast, the role of HAIC in conjunction with RT is still under investigation[47].
Local-regional plus another local-regional therapy:TACE combined with TARE has been reported as a safe and effective treatment modality for bi-lobar HCC[48].
Local-regional plus systemic therapy:This type of combination includes such options as TACE combined with target therapy[44,49,50], TARE combined with target therapy[51-53], TACE combined with immunotherapy[54-56], and TARE combined with immunotherapy[57].
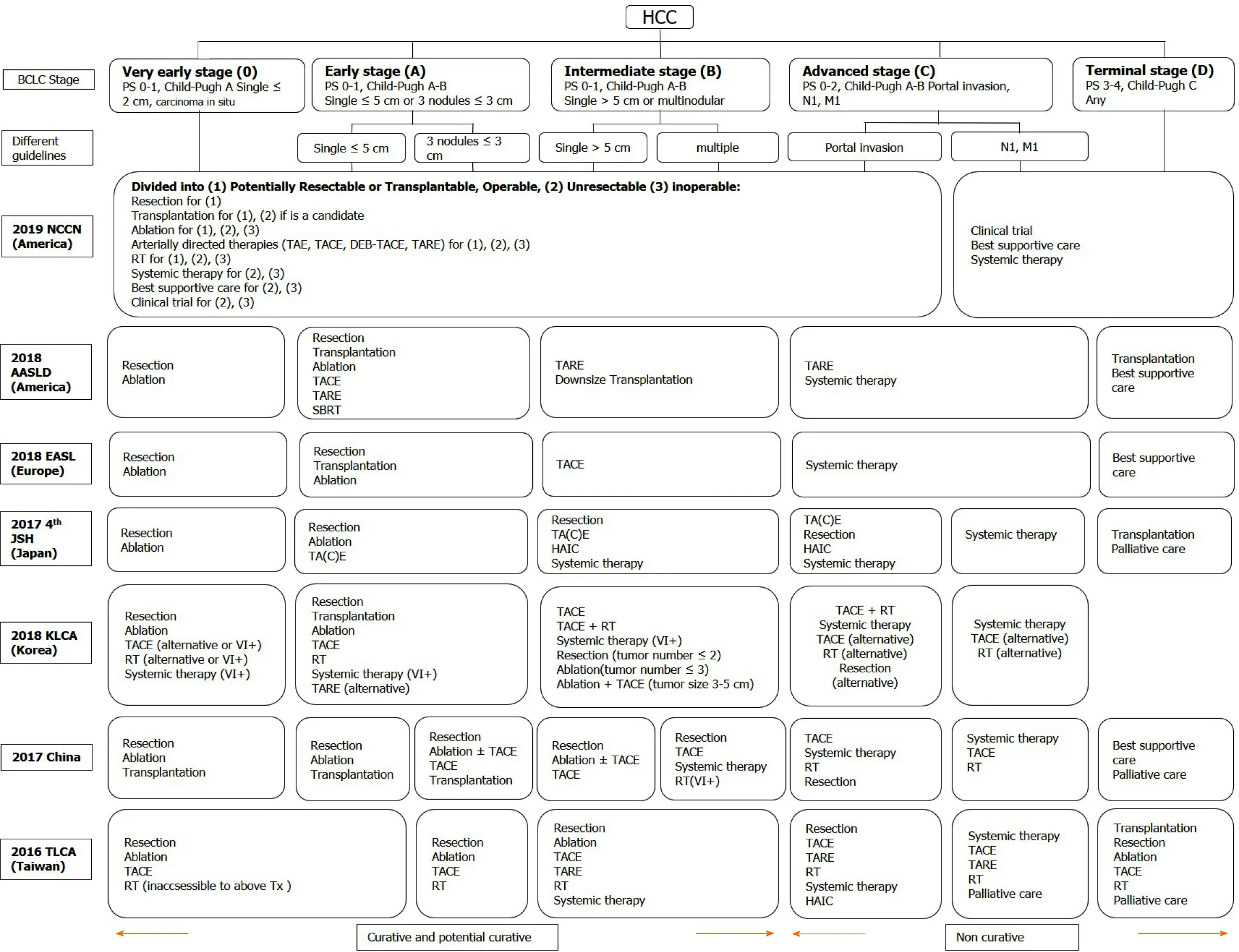
Figure 1 Treatment recommendations modified in different guidelines according to the Barcelona clinic liver cancer stage. HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer; PS: Performance status; TA(C)E:Trans-arterial (chemo)embolization; TARE: Trans-arterial radioembolization; DEB-TACE: Trans-arterial chemoembolisation with drug-eluting beads; RT: Radiotherapy; SBRT: Stereotactic body radiation therapy; HAIC: Hepatic arterial infusion chemotherapy; NCCN: National Comprehensive Cancer Network; AASLD: American Association for the Study of Liver Diseases; EASL: European Association for the Study of the Liver; JSH: Japan Society of Hepatology; KLCA: Korean Liver Cancer Association; TLCA: Taiwan Liver Cancer Association; Tx: Treatments; VI+: Positive vascular or bile duct invasion.
For advanced HCC
Only limited HCC patients are responsive to immune checkpoint inhibitors, and a combination of these with RT may enhance the immune response; this phenomenon is named the systemic therapy augmented by radiotherapy (STAR) effect[58]. Overall,RT or SBRT combined with other treatment modalities has been applied increasingly in HCC patients.
THE ROLE OF RT IN HCC
RT was used as a salvage or palliative treatment in the past, and only a few guidelines mention the role of RT. However, in the modern era, RT is indicated across all stages (i.e., from very early to end-stage HCC)[34,35,37,40]. Notably, RT can be used as a single therapy or as an essential component of a combined modality. Current treatment recommendations based on the BCLC stage and RT's potential roles are summarized in Figure 3.
Different RT techniques
Photon therapy: The most commonly available treatment beam of RT is photons. In managing patients with HCC, several photon-beam delivery systems of external-beam radiation therapy (EBRT) are clinical available, such as conventional fractionated RT(CFRT), hypo-fractionated RT (HFRT), and SBRT. CFRT is usually delivered with daily fractions of 1.8-2 Gy, and HFRT is characterized by a large daily dose (i.e., > 2 Gy) in the context of precise RT. Clinically, HFRT is a useful strategy for improving dose intensity and then tumor control. Both CFRT and HFRT can be delivered by using three-dimensional conformal radiotherapy (3DCRT), intensity-modulated radiotherapy (IMRT), and Volumetric-modulated Arc Therapy (VMAT).
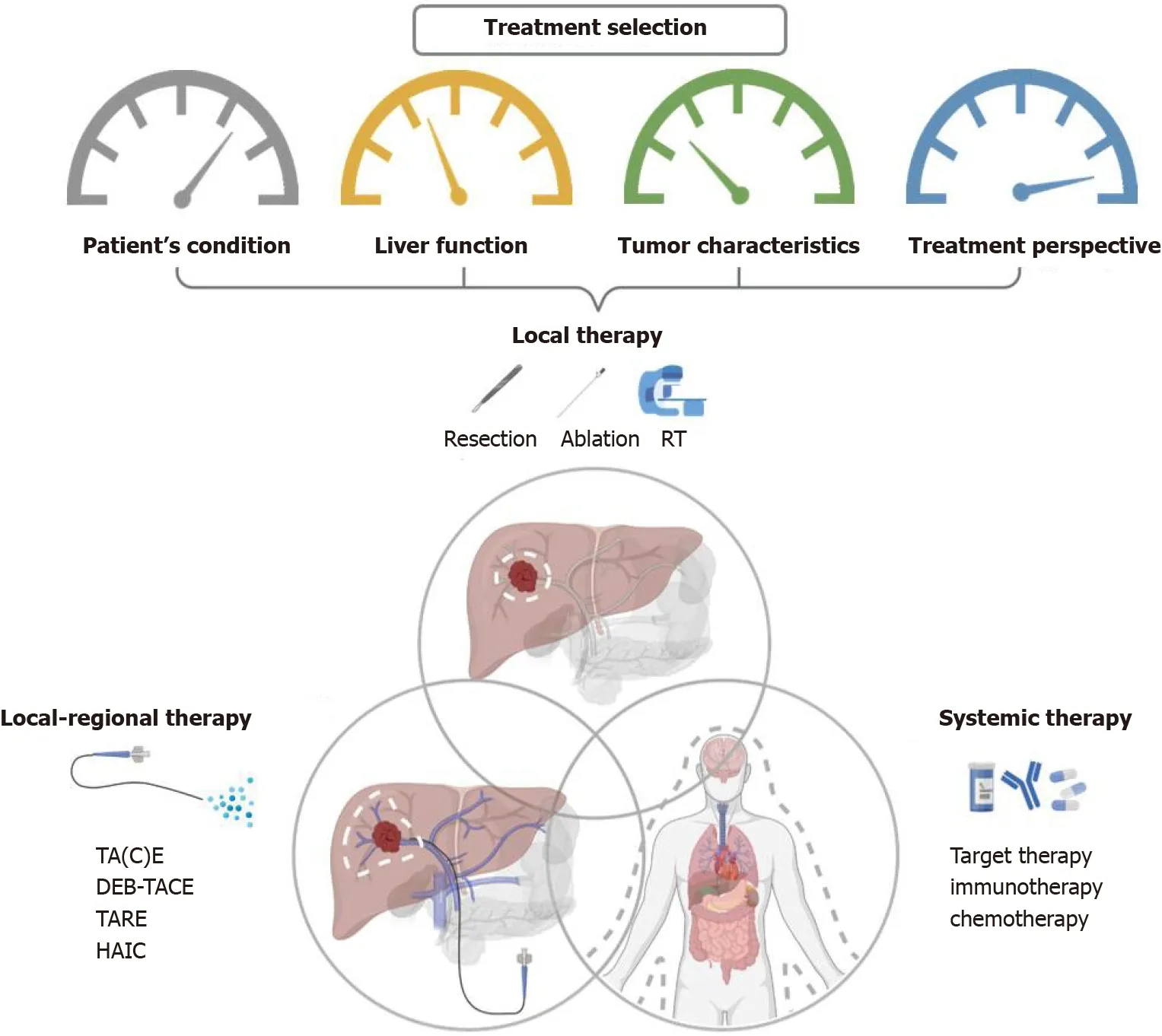
Figure 2 Overview of therapeutic options and the consideration behind the combination of different combined modalities for liver cancer.RT: Radiotherapy; TA(C)E: Trans-arterial (chemo)embolization; TARE: Trans-arterial radioembolization; DEB-TACE: Trans-arterial chemoembolisation with drugeluting beads; HAIC: Hepatic arterial infusion chemotherapy.
Remarkably, SBRT, or so-called SABR, is an advanced technique of EBRT that delivers a very high dose of irradiation in a very precise way in a limited number of treatment fractions (i.e., usually 3-6 fractions and > 5 Gy per fraction) over a treatment course of 1-2 wk. For more focused and accurate delivery of SBRT, advancements across the whole RT department should be provided, including imaging, immobilization, target delineation, treatment planning, on-board image guidance, and respiratory motion management (RMM). Only advanced IMRT or VMAT with or without non-coplanar beam designs can be used for delivering SBRT. These advancements result in better dose distribution, deliver a higher dose within the tumor, and generate a rapid dose fall-off outside the target. Thus, SBRT can improve tumor control and reduce the irradiation dose to the surrounding normal tissue, to decrease RT toxicity. Owing to this double benefit of enhancing therapeutic gain, SBRT is highly recommended in managing HCC patients treated with curative intent.
For patients who cannot be treated successfully with SBRT, CFRT combined with two or more advanced irradiation techniques, such as combined VMAT and Simultaneously Integrated inner-Escalated Boost (SIEB), may be helpful to achieve a better therapeutic gain (i.e., better tumor control with minimal RT toxicity) compared to conventional CFRT, including for elderly HCC patients who have inoperable disease[59].
Proton therapy:Charged particle irradiations, including proton-beam therapy (PBT)and carbon-ion RT, have unique dosimetric characteristics. That is, they eliminate the low-dose bath volume distal to the target area that is associated with photons. This elimination is because the characteristic Bragg peak of charged particles deposits irradiation energy mainly within the targeted tumor area and results in a near-zero dose beyond the end of its path[60]. Therefore, charge particle irradiation represents an excellent option for improving normal liver sparing and minimizing side effects such as radiation-induced liver disease (RILD). It also makes possible dose escalation for curing unresectable huge HCC.
Parket al[61] reported that dose escalation could enhance HCC tumor control. Kimet al[62] further confirmed that proton dose escalation is safe and effective; they suggested an EQD2 ≥ 78 Gy-equivalents (GyE) could be delivered to achieve reasonable tumor control. According to the tumor location, the University of Tsukuba proton team developed different PBT dose protocols[63-65]. Extrapolating on the concept of a lung cancer SBRT “No Fly Zone”[66], peripheral liver tumors located at >2 cm from the hepatic portal region or gastrointestinal (GI) tract can be treated with hypofractionated proton 66 GyE in 10 fractions. On the other hand, for tumors located within 2 cm adjacent to the hepatic portal region, small doses per fraction with 72.6 GyE in 22 fractions should be considered. For tumors located within 2 cm of the GI tract, 77.0 GyE in 35 fractions may be given[63-65].
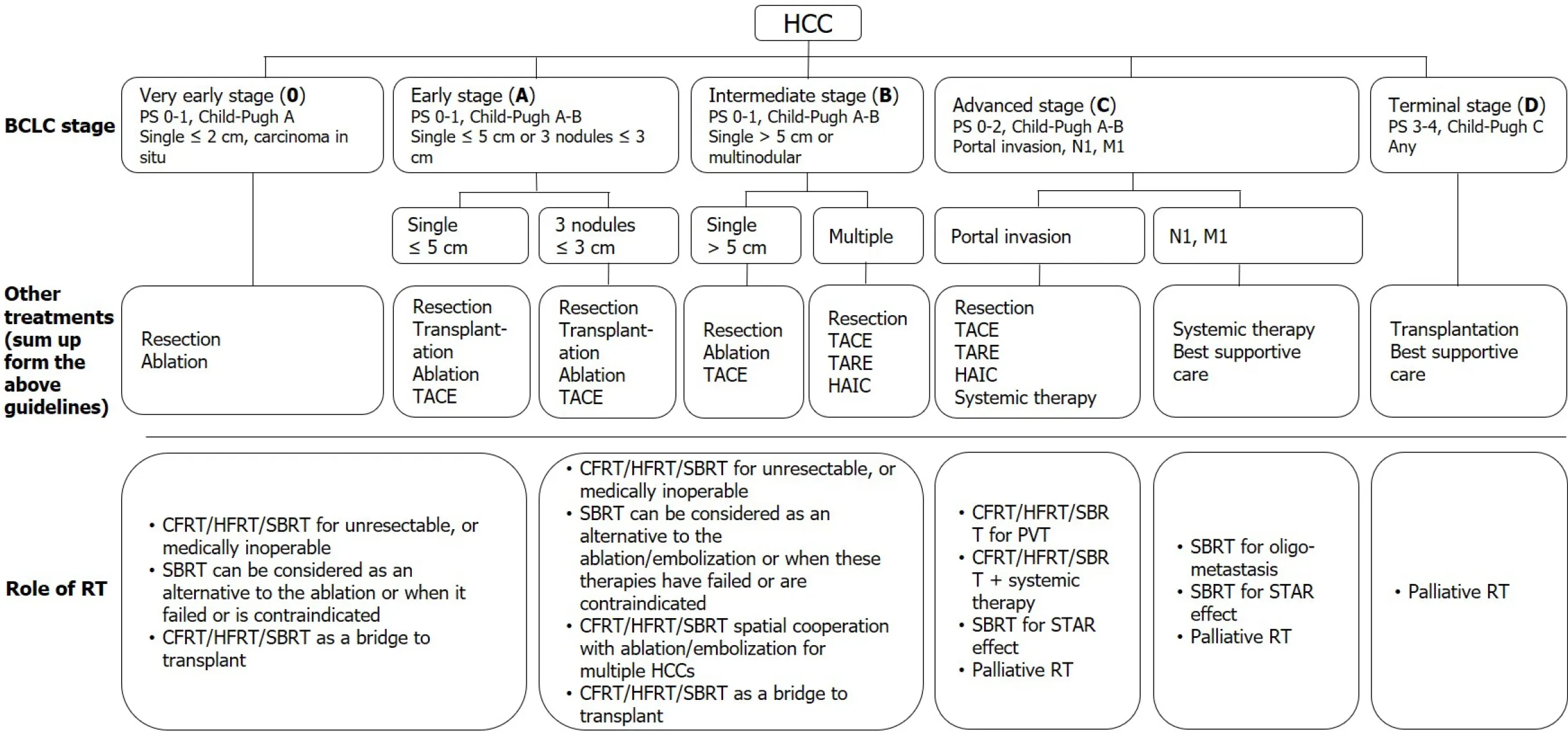
Figure 3 Current treatment recommendations based on the Barcelona clinic liver cancer stage and the potential roles of radiotherapy. HCC: Hepatocellular carcinoma; BCLC: Barcelona clinic liver cancer; PS: Performance status; TACE: trans-arterial chemoembolization; TARE: Trans-arterial radioembolization; HAIC: Hepatic arterial infusion chemotherapy; RT: Radiotherapy; CFRT: Conventionally fractionated radiotherapy; HFRT: Hypo-fractionated radiotherapy; SBRT:Stereotactic body radiation therapy; PVT: Portal vein thrombosis; STAR effect: Systemic therapy augmented by radiotherapy effect.
Several studies report using PBT for localized HCC with excellent local control ranging from 80% to 100%, even for huge unresectable HCCs, due to dose escalation and sparing of more liver function[67-69]. Furthermore, Sanfordet al[70] reported that the overall survival (OS) benefit of proton-RT over photon-RT might be due to decreasing the incidence of RILD. Hsiehet al[71] further identified the predictors of RILD in HCC patients treated with PBT beyond the conventional concept of minimizing the mean liver dose. A "volume-response" relationship between unirradiated liver volume (ULV)/standard liver volume (SLV) and RILD was found: For Child-Pugh A patients, it was < 50%; for Child-Pugh B patients, it was < 30%[71].
Both photon and PBT can achieve high rates of local control with acceptable toxicity.However, PBT has better potential to deliver a higher dose while maximizing the volume of unirradiated liver. Clinically, the reduced normal liver dose achieved by PBT is not critically required for all patients, since some may benefit from a smaller irradiated target volume when normal liver constraints can be met. The 2018 Miami Liver Proton Therapy Conference reached the consensus that the patients who should be strongly prioritized for PBT include those with: At least Child-Pugh B cirrhosis, a high tumor-to-liver ratio (i.e., larger tumor size or smaller uninvolved liver volume), a greater number of tumors, or prior RT to the liver[72]. The dose comparison of proton therapyvsSBRTvsconventional RT for liver tumors are illustrated in Figure 4.
The role of RT in very early and early stage HCC
Very early and early stage HCCs include those with BCLC classification 0-A, as follows: Carcinoma in situ; a single tumor of ≤ 2 cm, a single tumor of ≤ 5 cm, or three tumors of < 3 cm; and tumor burden laying within the Milan criteria, with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 as well as Child-Pugh classification A-B[73]. For these HCC patients, although the standard of care is still surgery and RFA, definitive SBRT is a potential third curative treatment modality for medically inoperable, technically unresectable, and difficult to RFA conditions; more notably, it can serve as a bridge to liver transplantation[26,74].
The role of RT in intermediate stage HCCs
Intermediate stage HCCs include those with BCLC classification B, as follows: Multitumors, a single tumor of > 5 cm, good patient condition (i.e., PS 0-1), as well as good liver reserve (i.e., Child-Pugh A-B). Tumor burden can be further subdivided, as follows: (1) Beyond Milan criteria but within the Up-to-7 criteria; and (2) Tumors beyond the Up-to-7 criteria[75]. For these patients, only limited cases can be treated by surgery or RFA. Several combined-modality approaches, including local therapy,local-regional therapy, and systemic therapy, have been reported in conjunction with CFRT and SBRT[76,77].
The role of RT in advanced stage HCCs
Advanced stage HCCs include those with BCLC classification C, with the criteria of portal vein invasion, inferior vena cava/heart invasion or thrombosis, lymph node metastasis, distant metastasis, and Child-Pugh A-B. SBRT or conventional RT may be applied in conjunction with other local, local-regional, and systemic therapies to serve as a potentially curative or palliative treatment[76-78].
The role of RT in terminal stage HCCs
Terminal stage HCCs include those with BCLC classification D, with the criteria of Child-Pugh C or ECOG PS 3-4. For these patients, SBRT with careful planning is safe as a bridge to liver transplantation in selected patients with a Child-Pugh score of ≥ 8.Additionally, SBRT or conventional RT can be used to treat symptoms[79-81].
THE ROLE OF SBRT IN MANAGING PATIENTS WITH HCC
SABR/SBRT
Recently, clinical evidence has rapidly grown for the use of SBRT in managing all stage HCC patients, with curative, potentially curative, or palliative intent[33,77,78,82].Prospective clinical trials have demonstrated that SBRT effectively treats HCC,resulting in satisfactory local control, ranging from 75% to 100% at 1 year and 65% to 100% at 2 years[33,77,78]. Local control of HCC using SBRT is typically defined as no progression or no recurrent disease within the irradiated field according to the Response Evaluation Criteria in Solid Tumors (RECIST) or its modification(mRECIST)[83-85]. Moreover, SBRT showed a benefit of limited toxicity, with a severe late toxicity rate of < 15%; thus, SBRT is considered a safe modality for treating elderly patients[86]. In the literature, most patients treated with SBRT have Child-Pugh A disease and limited lesions (often < 3 tumors), and delivering SBRT in Child-Pugh B patients increases toxicity rates[80]. However, if dose modification is done to meet the more conservative (i.e., strict) normal tissue constraints, SBRT may be allowed for patients with small HCCs with Child-Pugh B or those with relatively larger tumors with a Child-Pugh score of 7[80].
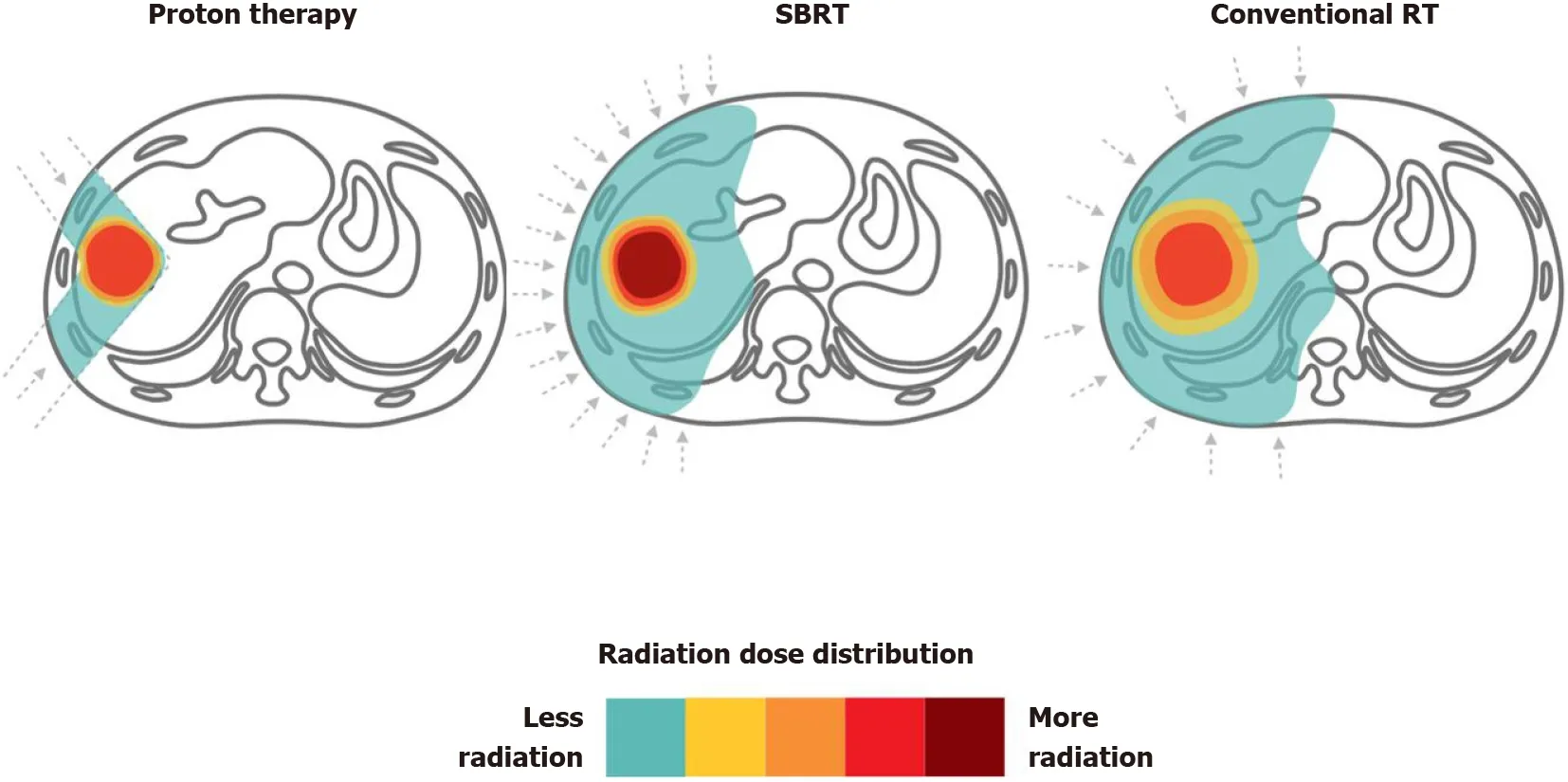
Figure 4 Proton therapy vs stereotactic body radiation therapy vs conventional radiotherapy for liver tumors. Dose distributions for a proton(left), stereotactic body radiation therapy (middle) and conventional radiotherapy (right) hepatocellular carcinoma radiotherapy plan are illustrated for comparison. RT:Radiotherapy; SBRT: Stereotactic body radiation therapy.
SBRT as an alternative treatment modality to ablation therapy (i.e., RFA)
Ablative therapy is curative in treating small tumors (i.e., ≤ 3 cm) that locate in a feasible location, achieving excellent local control rates of around 70-90%; these results are similar to that of surgical resection[87]. Under such conditions, ablative therapy is considered an alternative treatment to surgical resection or liver transplantation.
Recently, SBRT showed comparable outcomes with RFA. Wahlet al[26] compared treatment outcomes between SBRT and RFA; they declared that both are effective for patients with inoperable HCC, with comparable freedom from local progression(FFLP) and comparable OS rates. The 1- and 2-year FFLP rates of SBRT were 97.4%and 83.8%vs83.6% and 80.2% for RFA, respectively. The 1- and 2-year OS rates were also similar between the two treatment modalities. Remarkably, for larger tumors of ≥2 cm, SBRT demonstrated a better FFLP than that of RFA [hazard ratio (HR), 3.35; 95%confidence Interval (CI): 1.17-9.62;P= 0.025][26].
Haraet al[88] used propensity score matching (PSM) to assess the pre-treatment characteristics of BCLC stage, computed tomography (CT) status, and tumor size; they reported comparable 3-year OS rates between RFA and SBRT. Kimet al[89] also applied PSM to compare treatment results of SBRT and RFA. Two-year FFLP rates were 74.9% for the SBRT group and 64.9% for the RFA group. Taking these data together, SBRT demonstrates an emerging role as a curative treatment modality that is an alternative to ablative therapy for managing HCC patients.
SBRT as an alternative or adjuvant therapy to arterially directed therapies
Among arterially directed therapies, TACE is the most widely used treatment modality for managing HCC patients with an intermediate stage, applied in 50%-60%of patients[90]. However, TACE alone demonstrates unsatisfactory local control. This unacceptably low response rate suggests the use of local therapy, such as SBRT, as an alternative or adjuvant therapy to improve local control.
SBRT as an alternative therapy to TACE:Sapiret al[91] used PSM with inverse treatment weighting probability to compare SBRT and TACE in HCC patients with 1-2 tumors. They found that SBRT demonstrated better 1- and 2-year local control rates when compared with TACE: 97% and 91%vs47% and 23% (HR, 66.5; 95%CI: 18.99-233.0;P< 0.001), respectively. However, the difference in OS did not significantly differ between the two treatment modalities.
SBRT combined with TACE:Several retrospective studies and reviews have demonstrated increased local control rates by adding conventional RT to TACE[45,92,93]. However, studies using SBRT to replace conventional RT are scarce because SBRT is a new technique.
A pilot study and preliminary results of prospective studies also confirmed the safety and efficacy of SBRT in patients with HCC that failed to respond to TACE[94-96]. Kanget al[46] published a phase-II trial that enrolled 47 patients who received TACE 1 to 5 times before SBRT. The result showed a good response, with a 2-year local control rate of 94.6%. Moreover, 38.3% of patients achieved a complete response within 6 mo. However, there is still no published data from head-to-head trials that compare TACE plus SBRT with TACE alone. Several clinical trials are ongoing (ClinicalTrials.gov Identifier: NCT02762266, NCT02323360, NCT02323360,NCT02794337, NCT02921139).
SBRT combined with TARE:Previously, there has been much concern over increasing radiation-related toxicity when using SBRT after TARE. Hardy-Abelooset al[97] recently reported that TARE followed by SBRT has comparable safety and efficacy to TACE followed by SBRT.
SBRT as a bridge to liver transplantation
One of the preferred gold-standard treatments for managing HCC patients is liver transplantation, but only a few patients have a chance to receive a transplant due to an insufficient supply of donor livers[23]. Therefore, several local and local-regional therapies for HCC have been used to bridge care in patients seeking a transplant, to delay tumor progression[24]. Several studies have shown that SBRT may be an excellent alternative to conventional therapies as a bridge to transplantation[81,98].Sapisochinet al[98] reported 1-, 3-, and 5-year actuarial patient survival rates from the time of listing of 83%, 61%, and 61% in the SBRT group, respectively, rates which were comparable with those of the TACE or RFA groups.
SBRT for macroscopic vascular invasion or portal vein thrombosis
In the past decades, two landmark randomized trials revealed that sorafenib yielded modest survival prolongation in patients with portal vein thrombosis (PVT)[99,100].However, the response rate was unsatisfactory (only 2%). CFRT has been widely used in advanced HCC with macroscopic vascular invasion or PVT because it can be delivered regardless of tumor location, and major vessels demonstrate high radiation tolerance[24,78,79].
Rimet al[101] published a meta-analysis and systematic review which compared 3DCRT, TARE, and SBRT in patients with PVT. No significant differences in OS were observed among the three treatment modalities, but SBRT demonstrated the highest response rate of around 70%. More notably, toxicities of more than grade 3 were rare in the SBRT group[101]. These data revealed that SBRT could be safely applied in patients with PVT, with a better response rate than CFRT.
SBRT and sorafenib
SBRT compared with sorafenib:Bettingeret al[102] used PSM to compare SBRT and sorafenib. SBRT showed a superior survival benefit to that of sorafenib: median overall survival was 17.0 mo (range, 10.8-23.2)vs9.6 mo (range, 8.6-10.7), respectively, with or without adjusting for different baseline characteristics. A cost-effectiveness study also reported that SBRT had better cost-effectiveness than sorafenib for patients with advanced HCC[103].
SBRT combined with sorafenib:Bradeet al[104] initiated a phase-I trial to evaluate the combination of sorafenib and SBRT. Sorafenib was delivered before, during, and after SBRT. The researchers found that concurrent use of SBRT with sorafenib significantly increased side effects,e.g., grade 3+ bowel toxicity and tumor rupture.Thus, they did not recommend using this combination outside of a clinical trial. A clinical trial (RTOG 1112) is ongoing to compare SBRT followed by sorafenib with sorafenib alone in patients with advanced HCC (ClinicalTrials.gov Identifier:NCT01730937).
SBRT combined with immunotherapy
SBRT as an immunostimulator:Preclinical data demonstrated that RT could augment the intra-tumor cell surface expression of immunogenicity markers (e.g., dendritic cells) and enhance therapeutic efficacy[105-107]. Recently, immunotherapy combined with RT is an active field in managing HCC and has shown promising results[106,108,109]. SBRT may stimulate the release of tumor antigens and increase antigen-presenting cells to enhance the immune response to cancer cells provided by the immunotherapy[110]. This effect is also termed STAR[58] or “immunotherapy and stereotactic ablative radiotherapy (ISABR)”[111]. Investigations exploring in detail the underlying mechanisms of these effects are ongoing aggressively. Potential mechanism of SBRT combined with systemic therapy to induce the STAR effect(ISABR) for liver tumors is illustrated in Figure 5.
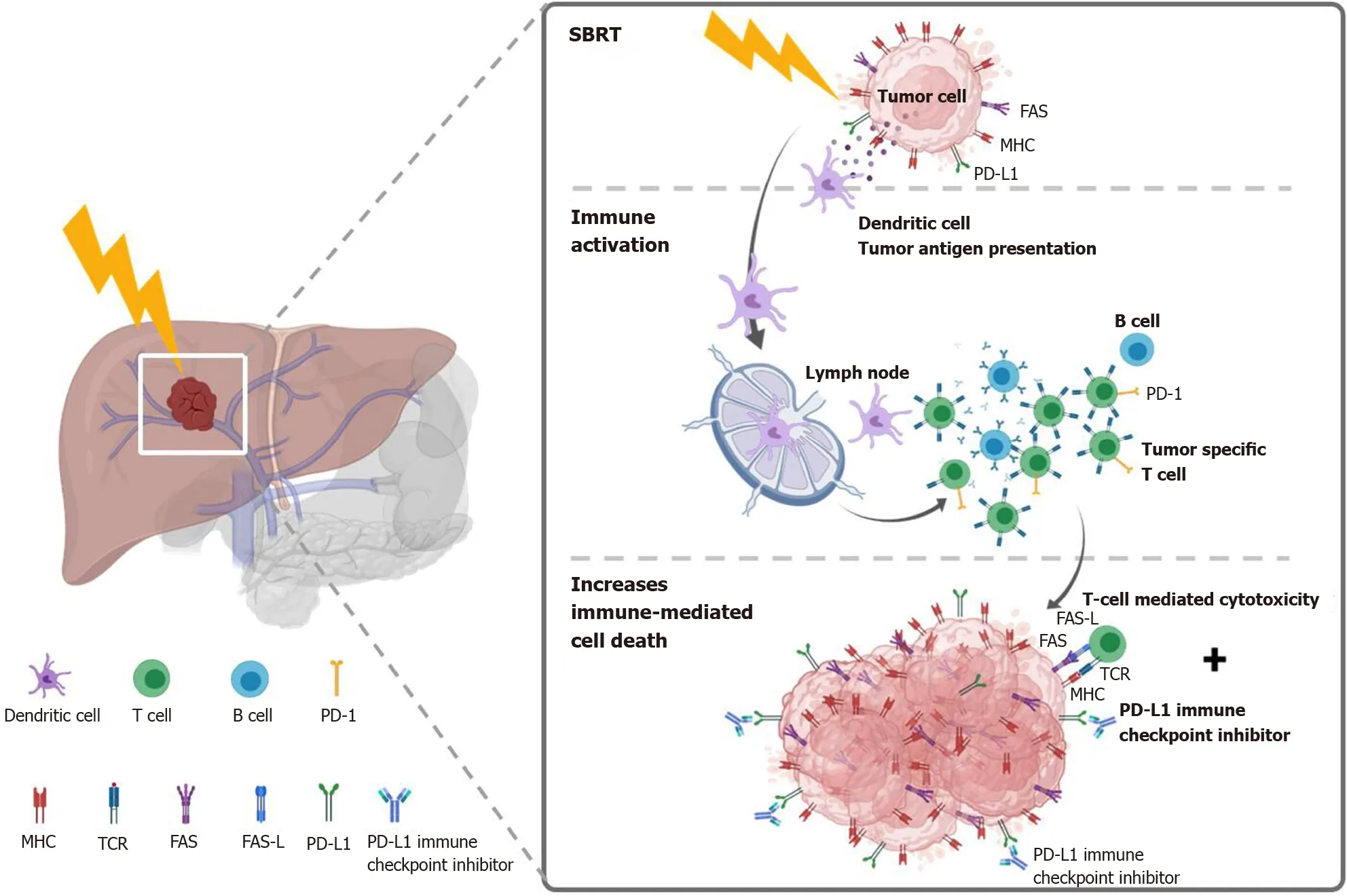
Figure 5 Potential mechanism of stereotactic body radiation therapy combined with systemic therapy to induce the systemic therapy augmented by radiotherapy effect (also known as immunotherapy and stereotactic ablative radiotherapy) for liver tumors. Stereotactic body radiation therapy (SBRT) induces antigen release and immunogenic cell death, activation of several transcription factors and signal pathways, as well as dendritic cell antigen presentation and maturation, resulting in proliferation of tumor-specific T cells and immune-mediated cytotoxicity. SBRT combined with Immune-checkpoint inhibitors augmented the tumoricidal effect by upregulates major histocompatibility complex and FAS on tumor cells, increasing susceptibility to T-cell-mediated cell death. MHC: Major histocompatibility complex; TCR: T cell receptor; FAS-L: FAS ligand.
SBRT is more immunogenic than conventional RT:SBRT is more immunogenic and has a beneficial outcome than the conventionally fractionated RT[112,113]. By using ablative SBRT in a mouse model, Leeet al[113] observed that a 20-Gy single fraction dose leads to a reduction in the tumor burden in both primary and distant metastases in a CD8+ T-cell-dependent fashion. However, conventionally fractionated irradiation showed a CD8+-depleted condition. In another preclinical study that compared soluble PD-L1 (sPD-L1) between the SBRT and conventional RT groups[114], Kim and colleagues found that the sPD-L1 Level increases persistently for 1 mo in the SBRT group. In comparison, it increased initially after irradiation but decreased after 1 mo in the conventional RT group.
Potential optimal SBRT dose and treatment sequences for induced immunogenicity:The optimal window of radiation immunogenicity is determined by the levels of double-strand DNA (dsDNA)vsTrex1[115,116]. SBRT doses up to 10-12 Gy (e.g., 8 Gy x 3 fractions) can up-regulate dsDNA accumulation in cancer cellsviathe cGAS/STING pathway, turning on RT-driven immune responses. However, higher doses above 12-18 Gy, such as 20-30 Gy in a single fraction, may induce the exonuclease TREX1, which down-regulates the immunogenicity by degrading cytosolic DNA, turning off RT-driven immune responses[115]. Otherwise, increasing the dose to higher than 10 Gy per fraction can rapidly reduce the tumor blood perfusion, leading to a tumoricidal effectviasevere vascular damage[117].
The optimal sequence of SBRT and immunotherapy is still unclear. Both concurrent and sequential combination have been applied[118-120]. In other cancers, Wegneret al[121] found that OS was improved when immunotherapy was given more than 3 wk after initiating SBRT/SRS in patients with stage IV non-small-cell lung cancer. In a study designed by Tanget al[120], SBRT was given either concurrently or sequentially with ipilimumab in patients with advanced solid tumors. However, there are limited studies specifically focused on HCC. Chianget al[122] added nivolumab at 2 wk after completed SBRT and continuously applied every 2 wk until disease progression.Currently, the optimal timing of SBRT and immunotherapy remains under active investigation.
Clinical data and ongoing trials for SBRT combined with immunotherapy:Recently,two phase-II studies, CheckMate 040[123] and KEYNOTE-224[124], have shown that the anti-PD-1 immune checkpoint inhibitors of nivolumab and pembrolizumab demonstrate favorable tumor responses (15%-20%) in managing HCC patients. The two agents have been approved by the Food and Drug Administration (FDA) for patients previously treated with sorafenib. A phase-III randomized trial, KEYNOTE-240[125], then compared pembrolizumab and placebo in 413 patients previously treated with sorafenib. A median OS was 13.9 mo for pembrolizumabvs10.6 mo for placebo (HR, 0.781; 95%CI: 0.611-0.998;P= 0.0238) and the median PFS was 3.0vs2.8 mo (HR, 0.718; 95%CI: 0.570-0.904;P= 0.0022). However, neither study endpoint reached statistical significance according to the trial-specified criteria (one-sided significance threshold,P= 0.0174, for the final analysis). Another phase-III trial,CheckMate 459, comparing nivolumabvssorafenib as first-line treatment in advanced HCC, is ongoing (ClinicalTrials.gov Identifier: NCT02576509). Abstracts of CheckMate 459 in the 44thEuropean Society for Medical Oncology Congress revealed that the primary endpoint of OS was statistically insignificant, but the objective response rate was double in the nivolumab group (15%vs7%, respectively)[126].
Although the immune checkpoint inhibitor's objective response rate was higher than that of sorafenib, 15%-20% is still relatively insufficient[123-125]. Hence, the combination of immunotherapy with SBRT has been proposed to improve the efficacy of immunotherapy, to combine the direct tumoricidal effects with the immunogenic STAR effect[110,111,127,128]. As a novel combined treatment modality, only one published HCC study is currently available, revealing encouraging results. Chianget al[122] reported 5 retrospective cases with unresectable HCC treated with SBRT 27.5-35 Gy in 5 fractions, followed by nivolumab. The median follow-up time was 14.9 mo.An objective response rate of 100% was reported, with 2 complete response and 3 partial responses. The 1-year OS and LC rates were both 100%. In addition, several phase-I or -II trials that combine SBRT and immunotherapy are ongoing (Clinical-Trials.gov Identifier: NCT03817736, NCT03316872, NCT03482102, and NCT03203304).
SBRT for metastatic disease
SBRT becomes much more important in managing cancer patients with oligometastatic disease[129-134], including those with HCC[76]. Recently, a landmark trial applied SBRT to manage oligometastatic cancers, defined as primary controlled tumors with only 1-5 metastatic lesions, PS ≤ 1, and life expectancy > 6 mo[135]. Median OS of the SBRT group was better than that of the control group (41vs28 mo; HR, 0.57; 95%CI:0.30-1.10;P= 0.090)[135]. Therefore, it is reasonable to treat more aggressively those HCC patients with oligometastasis, oligo-progression, and oligo-recurrence,i.e., treat with potentially curative intent by using SBRT if individual conditions allow.
How to combine SBRT?
Clinically, managing HCC patients with a combination of different treatment modalities, including SBRT, remains a challenge. Information from several domains should be judged together carefully, such as the individual patient's condition, the tumor location/characteristics, liver function reservation, facility resources, and the irradiation techniques available. A multidisciplinary evaluation before initiating treatment is crucial[34]. Moreover, to individualize the treatment combination into the best available option, clinicians must discuss options with patients and their families,using shared decision making (SDM)[136].
COMBINED MODERN RT TECHNIQUES FOR PATIENTS IN WHOM SBRT CANNOT BE DONE SAFELY
As mentioned above, for vulnerable patients with unresectable bulky HCCs (i.e., > 5 cm), clinicians may hesitate to use SBRT because of the potentially greater risk of severe non-classic RILD or GI tract toxicity[61,137]. Under such conditions, a combination of modern irradiation techniques, such as VMAT, RMM[138], and modified simultaneously integrated boost (SIB)[139-142], has been reported to be useful.
In the literature, SIB dose prescription has been recommended for dose escalation[143]. The original SIB prescription delivers different homogenous doses to separate target regions in the same fraction numbers[144]. That is, the high-,intermediate-, and low-risk target volumes are simultaneously irradiated with high,intermediate, and low doses, respectively, per fraction (Figure 6A).
Recently, a modified SIB technique simultaneously applied a heterogeneous dose per fraction on peripheral (lower dose per fraction,e.g., traditional 200 centigray [cGy])and intra-tumor zones (escalated higher dose per fraction,e.g., 240 cGy) (Figure 6B)[139-143]. Modified SIB simultaneously delivers an intra-tumor boost dose to the irradiated tumor's inner region to maximally enhance the possibility of tumor control in treating bulky tumors. This type of planned intra-tumor heterogenous dose distribution of modified SIB differs from the original SIB. This modified SIB has shown a potential role in managing bulky tumors, such as bulky pelvic tumor[139], retroperitoneal mass[140], breast cancer[141], and liver tumor[142,143,145]. Recently, higher doses per fraction,e.g., 2.4 Gy on peripheral and 3 Gy on intra-tumor boost zones, have been prescribed for managing very bulky tumors[145].
To gain better tumor control without the cost of increasing irradiation-associated critical organ toxicity, a type of combined SIB and simultaneously integrated protection (SIP) was developed (Figure 6C)[146,147]. In conjunction with an SIB to the intra-tumor volume, SIP includes an attenuated dose per fraction on the overlapping sites of PTV and the extended critical organ volume, to gain a double benefit from clinical irradiation. Several unresectable bulky cancers have benefitted from this modern RT dose prescription technique, including HCC[142,146,148].
Following this line of dose prescription, a secondary-modified SIB technique (i.e.,SIEB; Figure 6D) was developed[59]. SIEB secondly remodeled the modified SIB to further expand the double benefits of SIB in managing unresectable bulky tumors.That is, SIEB simultaneously maintained an intra-tumor escalated boost of the modified SIB to gain maximal tumor control (e.g., 220-240 cGy per fraction). Moreover,SIEB includes prescribing a planned attenuated peri-gross-tumor dose to all adjacent normal tissues (e.g., 120-150 cGy per fraction). Note that this intended protection is not limited only to critical organs/structures[59]. As a result, in theory, SIEB's therapeutic gain is further enhanced even over that of modified SIB (Figure 6B) or combined SIB and SIP (Figure 6C). However, prospective randomized clinical trials should be conducted to confirm the effectiveness of SIEB in managing patients with unresectable HCC.
One topic for further investigation in modified SIB and SIEB is whether the intratumor boost volume should be guided by using metabolic images, such as positron emission tomography (PET)[149,150]. For example, F-18-labeled fluoromisonidazole PET/CT images have been used to guide an SIB-escalated boost to the hypoxia tumor region[150]. However, using metabolic images to guide the intra-tumor boost volume has some limitations in daily clinical use, such as resource availability and the dynamic change in the PET-guided increased-uptake region during the treatment course of irradiation. As a result, multiple sessions of PET images and adaptive RT treatment planning may be required. Hence, in practice, it is feasible to deliver an intra-tumor boost to the geometric-central zone to maintain treatment efficacy,enhance tumor control, and minimize irradiation toxicity[59,143,145].
CONCLUSION
Currently, two driving forces have come together to improve the treatment efficacy of RT in HCC. One is technological advancement, which enables the precise delivery of SBRT, increasing tumor control and reducing the side effects to the surrounding normal tissue. The other is the boom in the development of target therapies and checkpoint-blockade immunotherapy, which prolongs HCC patients' survival and reemphasizes the importance of local tumor control. Currently, the role of RT in HCC treatment is actively being investigating in combination with systemic therapies to generate the STAR effect.
Remarkably, the development and use of combined modalities to increase liver reserve and patient tolerance may have the best chance to improve treatment outcomes. For HCC patients with any stage disease, RT plays a crucial role, whether delivered alone or in combination with other treatment modalities, because it is not limited by tumor location. Currently, a lack of level-III evidence is the main barrier to recommending SBRT as a standard of care in most international treatment guidelines.In this regard, several clinical trials of SBRT for HCC are ongoing. In the modern era,the role of photon- and proton-based precise RT in managing patients with HCC is shifting continuously from palliative to curative intent.
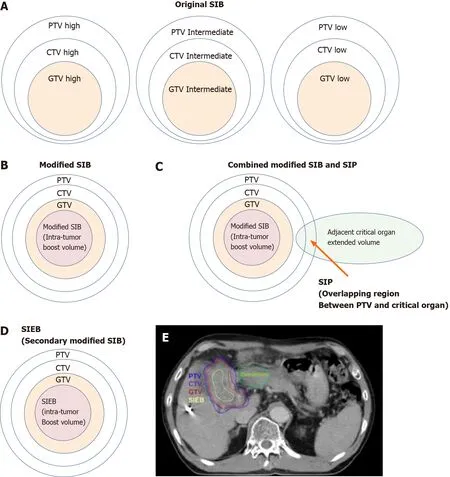
Figure 6 Cartoon and case illustrations of simultaneously integrated boost, modified simultaneously integrated boost, combined simultaneously integrated boost and simultaneously integrated protection, and simultaneously integrated inner-escalated boost doseprescription techniques. A: A simple cartoon figure representing original simultaneously integrated boost (SIB) dose prescription. Original SIB is prescribed in different doses per fraction to different target regions according to the risk levels of recurrence. For example, uniform doses per fraction may be given at planning target volume (PTV) high, intermediate, and low with 240 centigray (cGy) (simultaneously integrated boost), 180-200 cGy (traditional dose per fraction), and 160 cGy(inferior to the traditional dose) in the same fractions, respectively; B: A simple cartoon figure representing a modified SIB dose prescription. Traditionally,radiotherapy is prescribed as a uniform dose per fraction (e.g., 200 cGy) on PTV, which provides a homogeneous dose to cover clinical target volume (CTV) and gross target volume (GTV). Recently, to maximally enhance the possibility of tumor control, a modified SIB technique is applied using a planned non-homologous dose distribution, i.e., escalating a simultaneous intra-tumor boost (e.g., 220-240 cGy per fraction) in addition to a traditional covering dose to the PTV (e.g., 200 cGy per fraction) in the same treatment fractions; C: A simple cartoon figure representing combined modified SIB and simultaneously integrated protection (SIP) dose prescription. To reduce treatment toxicities to adjacent critical organs/tissue, SIP was developed in conjunction with modified SIB. SIP prescribes an inferior-totraditional dose per fraction, e.g., 150 cGy, to the overlapping region between the PTV and the extended critical organ volume (as shown by the long arrow); D: A simple cartoon figure representing simultaneously integrated inner-escalated boost (SIEB) dose prescription. For further enhanced therapeutic gain (i.e., increased tumor control and decreased treatment toxicity) in managing patients with unresectable liver tumors, we applied a secondary modified SIB (also termed SIEB). SIEB further escalates the intra-tumor boost (e.g., 240-260 cGy per fraction) based on a planned generally attenuated peri-tumor dose (e.g., 120-150 cGy per fraction delivered to the PTV), administered simultaneously. The intra-tumor SIEB boost volume is delineated as a uniform-inner-shrinkage area from the GTV with a margin of 1-10 mm (i.e., a geometrically central zone), depending on the tumor size, the intensity of the dose escalation, the level of liver preservation, the closeness of the gastrointestinal organs to the irradiation targets, and the patient's condition. Note that an additional most-inner SIEB boost volume with the highest dose per fraction (e.g., 260-300 cGy or higher) might be considered for highly selected patients with very bulky tumors. E: A clinical case with SIEB dose prescription. The blue, purple,and red lines show the PTV, CTV, and GTV of the irradiating target, respectively. In this case, based on the physician’s choice and the patient’s condition, a dose per fraction of 120 cGy was prescribed to the PTV, 150 cGy to the CTV, and 200 cGy to the GTV. Finally, in the yellow-outlined region, 280 cGy per fraction was simultaneously delivered to the SIEB boost volume. A total of 30 fractions were given, generating total doses levels of 3600 cGy, 4500 cGy, 6000 cGy, and 7400 cGy to the PTC, CTV, GTV, and SIEB boost volume, respectively. Note that the most peripheral dose per fraction of 120 cGy was chosen mainly due to a very close distance between the PTV and an adjacent critical organ, i.e., the duodenum. This short-distance closeness could easily lead irradiation to harm the duodenum under the context of daily organ motion. SIB: Simultaneously integrated boost; SIP: Simultaneously integrated protection; SIEB: Simultaneously integrated inner-escalated boost; PTV: Planning target volume; CTV: Clinical target volume; GTV: Gross target volume.
ACKNOWLEDGEMENTS
We gratefully acknowledge all RT staff members of the Buddhist Dalin Tzu Chi Hospital and Tzu Chi Medication Foundation. We also thank the contribution of all RT members, including Buddhist Hualien Tzu Chi Hospital, Far Eastern Memorial Hospital and China Medical University Hospital who involved in inter-hospital HCC research program TASABR trial registered prospectively on ClinicalTrials.gov (Trial Identifier: NCT02921139).
 World Journal of Gastroenterology2021年20期
World Journal of Gastroenterology2021年20期
- World Journal of Gastroenterology的其它文章
- Deep learning for diagnosis of precancerous lesions in upper gastrointestinal endoscopy: A review
- State of machine and deep learning in histopathological applications in digestive diseases
- COVID-19 in normal, diseased and transplanted liver
- Upregulation of long noncoding RNA W42 promotes tumor development by binding with DBN1 in hepatocellular carcinoma
- Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study
- Inflammatory bowel disease in Tuzla Canton, Bosnia-Herzegovina: A prospective 10 -year follow-up
