Corrosion behavior of high-level waste container materials Ti and Ti–Pd alloy under long-term gamma irradiation in Beishan groundwater*
Qianglin Wei(魏强林), Yuhong Li(李玉红), Yanliang Huang(黄彦良),Dongyan Yang(杨冬燕), Bo Yang(杨波), and Yibao Liu(刘义保),‡
1School of Nuclear Science and Technology,Lanzhou University,Lanzhou 730000,China
2State Key Laboratory of Nuclear Resources and Environment,East China University of Technology,Nanchang 330013,China
3Institute of Oceanology,Chinese Academy of Sciences,Qingdao 266071,China
Keywords: γ-irradiation,corrosion,high-level waste,container material
1. Introduction
The geological disposal of spent nuclear fuel is considered as a promising method for the long-term storage of highlevel radioactive waste (HLW) by many countries.[1]China initiated the research on HLW disposal in 1985, with a mission to build a national repository in the middle of the 21st century. Beishan area, located in Gansu Province in northwestern of China, was selected as the most suitable location for HLW repository.[2]The disposal of container materials requires proper mechanical strength, great corrosion resistance and good irradiation shielding properties. Japan, Canada,France,Belgium,and Switzerland use carbon steel as the container material, while Sweden and Finland use copper as the inner material and cast iron as the outer material for the KBS-3 concept.[3]However,China has not yet determined materials for HLW disposal containers.[4]
Underground HLW disposal containers will be exposed to harsh environmental conditions for a long time and will be affected by groundwater erosion and intense irradiation field. In recent years, the corrosion behavior of different metal materials such as copper,stainless steel,and carbon steel has attracted a lot of attention.[5–11]Titanium and its alloys are considered as potential candidate materials for HLW disposal containers because of their excellent properties,such as good fracture toughness, high strength, and good corrosion resistance.[12]There are a lot of researches on the corrosion of titanium and its alloys, including pure titanium,[13–15]Ti–Pd alloy,[16,17]Ti-5%Ta-2%Nb alloy,[18]titanium grade 7,[19]and Ti–Cr–Nb ternary alloy.[20]The results show that at high temperature(T >500°C),the protective intense self-adherent oxide film mainly composed of TiO2and its sub-oxides Ti2O3,may also result in the formation of different titanium oxides (TiO2, Ti3O5, Ti2O3, and TiO). The most common type of titanium oxide is rutile TiO2.[21]The corrosion resistance of Ti and its alloys is the result of passivation oxide film(TiO2) formed spontaneously when the metal is in contact with oxygen.[22]
The container material is exposed to intense gamma irradiation field,and its surface may also bear the risk of corrosion of the surrounding groundwater. The study shows that gamma dose rate is also an important factor affecting corrosion.[23]The initial intensity of the gamma irradiation field on the buffer/over–pack surface of the super-container is expected to be 25 Gy·h−1.[3]The experimental data on low X65 carbon steel corrosion at different gamma irradiation field intensities show that the dose rate has little effect on the groundwater radiolysis when it is lower than a certain level (3 Gy·h−1).[24]Liu et al.[25]pointed out that medium γ-irradiation dose rate(2.98 kGy·h−1)can accelerate the corrosion rate of X65 grade low carbon steel by approximately 33%. Numerous experiments have shown that gamma irradiation accelerates the corrosion rate of carbon steel or iron-based alloy at a dose rate of 3 kGy·h−1or lower, but decelerates the corrosion rate at higher dose rate such as 13 kGy·h−1.[26]At a lower dose rate(450 R·h−1, 4.50 Gy·h−1), irradiation does not affect the excellent pitting resistance of Ti alloys and even promotes the crevice re-passivation. Different from 304L stainless steel,Ti-2, Ti-12, and Ti-7 do not have the ability to stimulate local corrosion. It remains passive even at a high dose rate, confirming that for dose rates as high as 104R·h−1(102Gy·h−1),the only effect of irradiation will be to accelerate the growth of films to a small extent.[27]Padovani et al.[28]analyzed the expected corrosion and environment-assisted cracking behavior of copper, carbon steel, and titanium in contact with relevant buffer materials, and under appropriate conditions, their confidence in the possible effectiveness of titanium as a long-term corrosion barrier will be greatly enhanced. According to the available data,there are few literatures about the effect of cumulative γ-irradiation on the corrosion behavior for titanium and its alloys.
As for the influence of the soaking medium, the effects of pure water,[29]granitic groundwater,[30]and single anion such as Cl−,[31]NO−3,[32]SO2−4[33]under irradiation and unirradiation have been studied. But there are few studies on the mechanism of corrosion behavior in Beishan groundwater under γ-irradiation. Although titanium and its alloys have high resistance to general liquids, local corrosion may be critical,and the actual groundwater composition around HLW disposal container is complex. There is still some uncertainty about the mechanism of how or whether irradiation affects the corrosion activity of metal materials. In order to better understand and predict these complex processes, scientific research programs are being carried out all over the world.[10]We have studied the corrosion behavior of Q235 carbon steel,titanium,and titanium alloys,through electrochemical methods without irradiation.[4,34]In this work, we further study the corrosion behavior of TA8-1 and TA2 under γ-irradiation by using Beishan groundwater as immersion medium. Our research results may provide reference for the selection of suitable materials for high-level waste disposal containers.

Fig.1. The images of(a)TA8-1 and(b)TA2 samples immersed in Beishan groundwater from left to right: (a1)fresh sample; [(a2), (a3), (a4), (b2), (b3),(b4)]unirradiated sample after 40,80,and 160 days,respectively;[(a5),(a6),(a7),(b5),(b6),(b7)]irradiated sample after 40,80,and 160 days,respectively.The colorless tube darkens gradually with the extension of irradiation time,which is the reason for irradiation discoloration.
2. Experimental procedure
2.1. Sample preparation
The titanium–palladium alloy(TA8-1)and pure titanium(TA2) used in this study are provided by the Institute of Oceanology,Chinese Academy of Sciences(CAS).The main chemical compositions of TA8-1 and TA2 provided are given in Table 1.
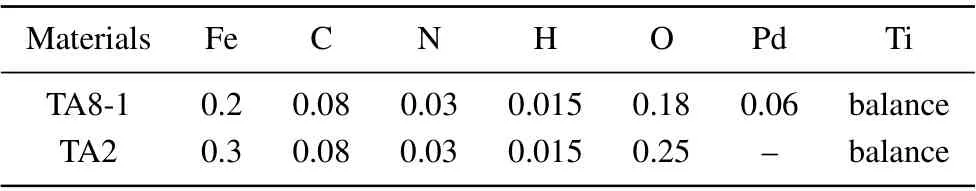
Table 1. Chemical composition of TA8-1 and TA2(in units of wt%).
Each TA8-1 and TA2 sample was cut into a short cylinder with Φ10 mm×10 mm. Then,the samples were cleaned with an acetone ultrasonic bath and then placed in a colorimetric tube, as shown in Fig. 1. Beishan groundwater was used as the experimental corrosion medium in this study and its chemical composition[35]was listed in Table 2, and the pH was adjusted to 8.22 before experiments. It can be seen that the color of the groundwater and the colorimetric tube changed obviously under the condition of γ-irradiation. With the extension of irradiation time,the original colorless tube gradually darkens,which is the reason for irradiation discoloration.[36]
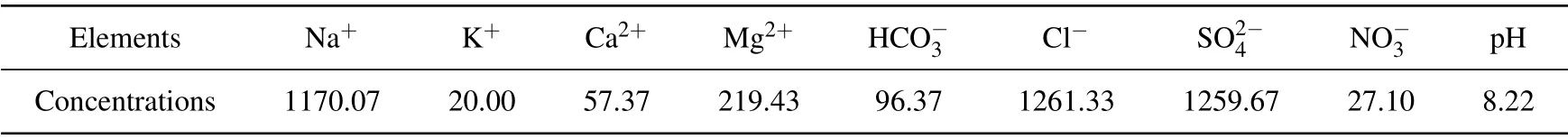
Table 2. Chemical composition of Beishan groundwater(in units of mg/L)and pH.
2.2. Corrosion experiments under irradiation
Corrosion experiments under γ-irradiation were carried out in60Co irradiator of Jiangxi Tianzhao Technology Development Co., Ltd, which contains 30 cylindrical60Co source bars(1.11×1016Bq)built in a 2.5-m×3.0-m rectangular hollow stainless steel cover (Fig. 2). In the course of the experiment, the60Co sources were raised from the water well storage position to the irradiation position, and then returned to the storage position by a lifting device after irradiation.The light energy of the sample irradiated by60Co irradiator is 1.173 MeV and 1.332 MeV,respectively. Before the irradiation experiments,the gamma dose rate at the sample position was calibrated using a potassium(silver)dichromate dosimeter. the result was 5.0 kGy·h−1when the60Co sources were raised.

Fig. 2. Schematic diagram of the 60Co irradiator, (a) profile diagram, (b)cross section diagram.
Each sample was cleaned three times and dried for 24 hours in a desiccator. The colorimetric tube was washed with distilled water and then filled with 50 mL of groundwater.Subsequently,each sample was placed in the colorimetric tube bottom and sealed with a plug, and then placed the irradiation position of the stainless-steel cover. Then, the60Co source was raised to the irradiation position for the irradiation test. In order to ensure the measurement parameters under the same conditions, three batches of colorimetric tubes(containing samples and groundwater) were placed in the irradiated position,respectively. The cumulative irradiation absorbed by the sample are 4.8, 9.6 or 19.2 MGy after 40, 80 or 160 days, respectively. The pH, conductivity and concentration of titanium ions of the solution were measured immediately after 160 days. And then the TA8-1 and TA2 samples were taken out and the cross section morphology and composition of the samples were analyzed by scanning electron microscope (SEM), energy dispersive spectroscopy (EDS), and x-ray diffractometer (XRD) methods. Accordingly, the unirradiated samples were prepared by the same method and preserved in our laboratory. All the tests were carried out at room temperature.
2.3. Analysis methods
Before and after the corrosion experiment, the hydrochemical parameters such as pH value and conductivity of groundwater were measured by water quality measurement instrument(Thermo Scientific Orion VERSA STAR).The concentration of Ti ion in groundwater samples was analyzed by inductively-coupled plasma mass spectrometry(ICP–MS).Scanning electron microscope (SEM, Nova Nano SEM 450 emission) and energy dispersive spectroscopy (EDS, X-Max 20)were used to analyze the surface morphology. In order to further study the composition of the corrosion layer, the surface of the sample was characterized by x-ray diffractometer(XRD, D/max-2400, Rigaku Corporation), which was identified on XRD(Ni filter,Cu-Kα)with the tube voltage of 40 kV,a tube current of 100 mA and a 2θ range from 10°to 80°(0.02°/step).
3. Results and discussion
3.1. Hydrochemical parameters
The pH value, conductivity, and Ti ion concentration of the groundwater under γ-irradiation and un-irradiation of TA8-1 and TA2 samples are shown in Fig.3.
Figure 3(a) shows the pH value of the groundwater as a function of irradiation–corrosion time. The pH value of the groundwater changed little under un-irradiation conditions,only decreased from 8.22 to 8.17(TA8-1)and 8.20(TA2)after 160 days. After that, the pH value almost remained constant. However,under long-term intense γ-irradiation,the pH value of the groundwater immersed in the two samples was more complex: The change trend of pH value of groundwater immersed with TA8-1 and TA2 samples was basically the same,and the change was not obvious in the first 20 days,and then decreased rapidly in about 100 days. The pH change rate of groundwater of TA8-1 is slightly lower than that of TA2 within 20 days–100 days,indicating that the corrosion rate of TA8-1 is relatively small. After 160 days,the groundwater became quite acidic and the pH values of groundwater reached 2.46 for TA8-1 and 2.44 for TA2 respectively, indicating that H+concentration in the groundwater reached to a constant value. One of the main reasons for the change of pH value is the decomposition of H2O by γ-irradiation. The γ-rays emitted by60Co can ionize H2O molecules,[32]the main radiolysis products are hydrated electrons, hydrogen atoms, oxygen atoms,H radicals,OH radicals,HO2radicals,activated water molecules,hydronium ions,H2molecules,O2molecules,and H2O2molecules.[37]The total effect of irradiation decomposition of H2O is to enhance the oxidizability of groundwater,in which OH radicals, HO2, O2, and H2O2are intense oxidants. These intense oxidants can oxidize low-valence oxides to high-valence oxides,[38]which is called cation hydrolysis.Metal cations react with water to produce metal oxides, hydroxides, or oxy/hydroxy ions. The main by-product of hydrolysis reaction is proton, which decreases the pH value of the solution, which is consistent with the results of No¨el et al.[39]
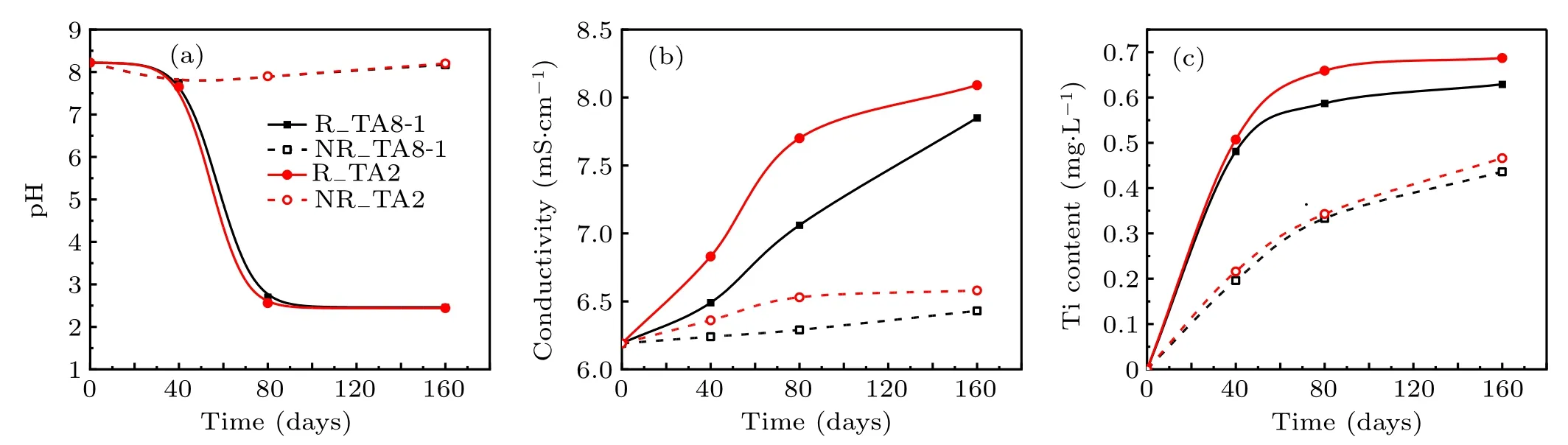
Fig.3. The(a)pH value,(b)conductivity,(c)Ti ions concentration of groundwater as a function of time under γ-irradiation and un-irradiation(note: R X:irradiation samples,NR X: un-irradiation samples,X stands for TA8-1,TA2,respectively).
In addition, the oxygen and nitrogen at the top of the colorimetric tube may also participate in the radiolysis reactions. Therefore, the formation of nitric acid in air can also be considered as a driving force to reduce the pH of the groundwater.[40]Moreover, the groundwater contains various inorganic anions (, Cl−, H, and) and cations(Na+,Mg2+,and Ca2+),the products of γ-irradiation may also have chemical and physicochemical processes with these inorganic ions,which may further affect the properties of groundwater.In this research,we have mainly focused on the changes of pH value,titanium ion concentration in solution and the formation of the corrosion layer. As for other properties involved need to be further studied.
In generally,due to the formation of Ti passive oxides,Ti interacts with the solution to produce TiO2(or mixtures of TiO and TiO2)in a closed disposal container environment after entering the groundwater. Further, the oxidized metal ions can be hydrolyzed to form hydrogen ions at the same time.[41,42]The reaction process can be expressed as follows.
Oxidation occurs when a sample is just put into solution:
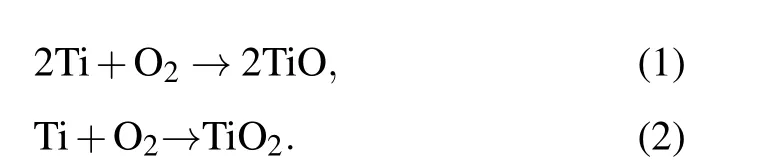
Next, the irradiation causes the acidification of the aqueous solution,resulting in the following corrosion behaviors:


Thus, these reactions are responsible for the decrease of pH.[43]It is well known that H+may accumulate in the groundwater and lead to a significant decrease of pH value.With the evolution of corrosion in the environment,corrosion products may form a protective layer on the sample surface,which slows down the rate of corrosion.[38]However, due to the presence of free H+in groundwater,the pH decreases significantly when H+interacts with electrons freely, and active metal dissolution can occur.[22]Therefore,the pH value is directly related to sample corrosion.
Figure 3(b) shows the conductivity of groundwater as a function of time under γ-irradiation and un-irradiation conditions. The conductivity of groundwater without γ-irradiation only increases slightly. On the contrary, the conductivity of groundwater changed significantly after γ-irradiation. At first,due to the protective oxidation film on the surface of the sample, the conductivity changes slowly. However, with the increase of cumulative irradiation dose,the protective layer was destroyed and the rate of corrosion increased gradually. Moreover,the change rate of groundwater conductivity for TA2 was larger than that of TA8-1 at the beginning,and reached almost equal after 160 days. This phenomenon can also be confirmed by the relationship of Ti ion concentration in groundwater as shown in Fig. 3(c). When Ti ions enter the groundwater, the conductivity of the groundwater will increase. It can be speculated that the corrosion rate of TA8-1 is slower than that of TA2,which may be due to the increase of Pd concentration in the passive film on Ti surface with the increase of groundwater exposure,resulting in the decrease of Ti dissolution rate.[16,44]
In Fig. 3(c), the initial increase rate of Ti ion concentration is faster,and then it gradually slows down after about 70 days in both irradiated and un-irradiated samples. After 70 days,the concentration of Ti ions in the irradiated groundwater solution was approximately twice as high as that of unirradiated solution. The increase of Ti ions in groundwater solution indicates the corrosion of samples,which may be caused by two factors. First of all,irradiation enhances the acidity of groundwater solution. The second possibility is that low pH causes an increase in the solubility of compounds that form a protective film on the sample surface,which may lead to further acceleration of material degradation in the storage container. From the above discussion, we can draw a conclusion that γ-irradiation can accelerate the corrosion of metal samples.
3.2. X-ray diffraction
The x-ray diffraction patterns are shown in Fig. 4 and were identified by comparison with the PDF standard of hexagonal Ti.
All the diffraction peaks of fresh TA8-1 and TA2 match well with the crystal structure of the hexagonal Ti, and a few TiO2peaks can still be observed during oxidation in air. No matter whether it is irradiated or not, the intensity of the Ti diffraction peak decreased obviously in both samples after soaking in groundwater for a period of time, and the other diffraction peaks appeared at 37°and 69°angles. By comparing with the standard value of PDF,the existence of the other peaks indicates that some other substances are generated, it was obvious that the corrosion layer formed on the surface of the un-irradiated sample is mainly composed of TiO2and TiO.[45,46]The corrosion layer formed on the surface of the irradiated samples is mainly composed of TiO2and TiO.However,outside the crevices of the irradiated samples,a layer of TiO is formed inside the TiO2layer,[21]and Ti itself is still the main component.
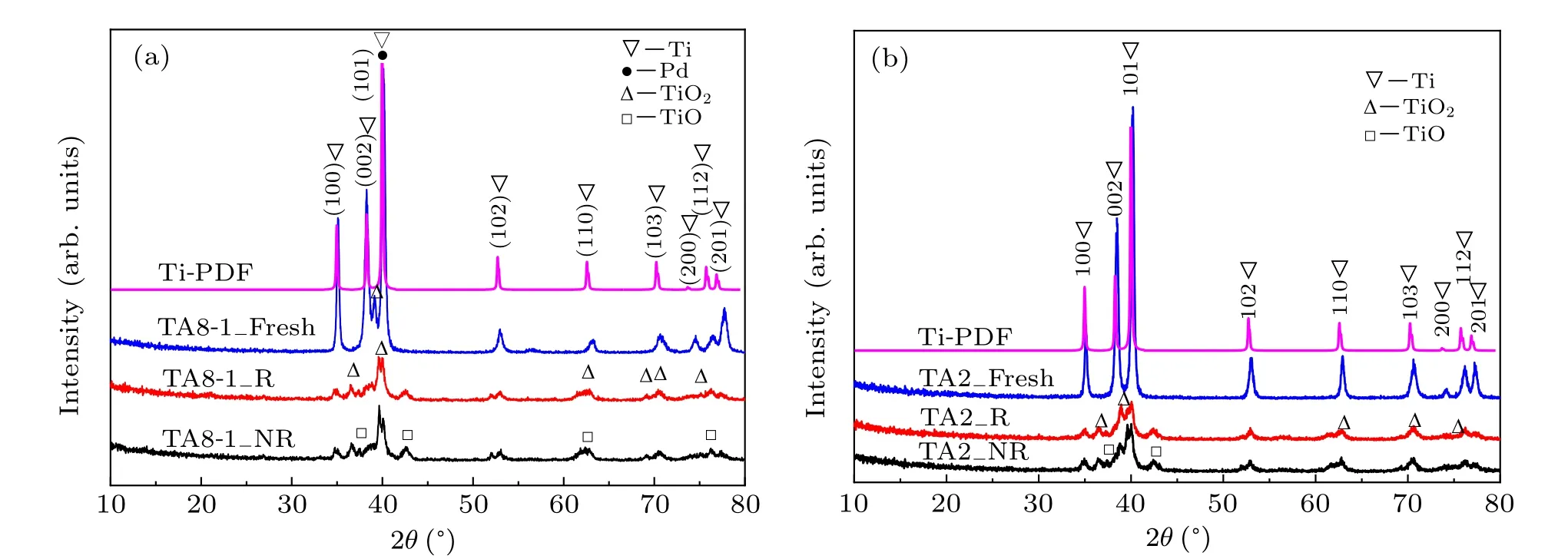
Fig. 4. XRD patterns of the (a) TA8-1 and (b) TA2 samples (note: Ti-PDF represents the standard spectrum of Ti, Fresh represents new sample, NR represents un-irradiation after 160 days,and R represents irradiation after 160 days).
3.3. SEM–EDS analysis
Figures 5 and 6 show the SEM–EDS images of the surfaces of un-irradiated and irradiated samples after 40,80,and 160 days,respectively. Fresh samples are also shown for comparison. The surface of the fresh sample is smooth, and its main components are Ti and O for both TA8-1(Fig.5(i))and TA2(Fig.6(i)). With the extension of irradiation time,a dense corrosion layer is gradually formed on the surface of sample and becomes uneven.The x-ray diffraction analysis shows that an oxide corrosion layer is formed on the surface of the sample. However,due to the enhanced oxidization of the groundwater under γ-irradiation, the corrosion layer was gradually destroyed,which leads to further corrosion. These results are consistent with the change trend of pH value,conductivity and titanium ion concentration of groundwater shown in Fig. 3.Compared with the fresh sample, the oxygen content of the corroded surface increases,indicating that the sample surface is gradually oxidized.
Moreover, it can be clearly seen that, whether irradiated or not,the bubble-like corrosion products are produced on the surface of the two kinds of samples in groundwater,however,the irradiated samples produced more bubble-like corrosion products than the un-irradiated ones. The results show that the corroded TA8-1 and TA2 samples are not uniform. However,after the formation of stable corrosion layers(amorphous and crystalline oxide)as protective films,there was no further corrosion, which confirms the conclusion of Nelson et al.[47]Therefore,TA8-1 and TA2 are promising container materials.
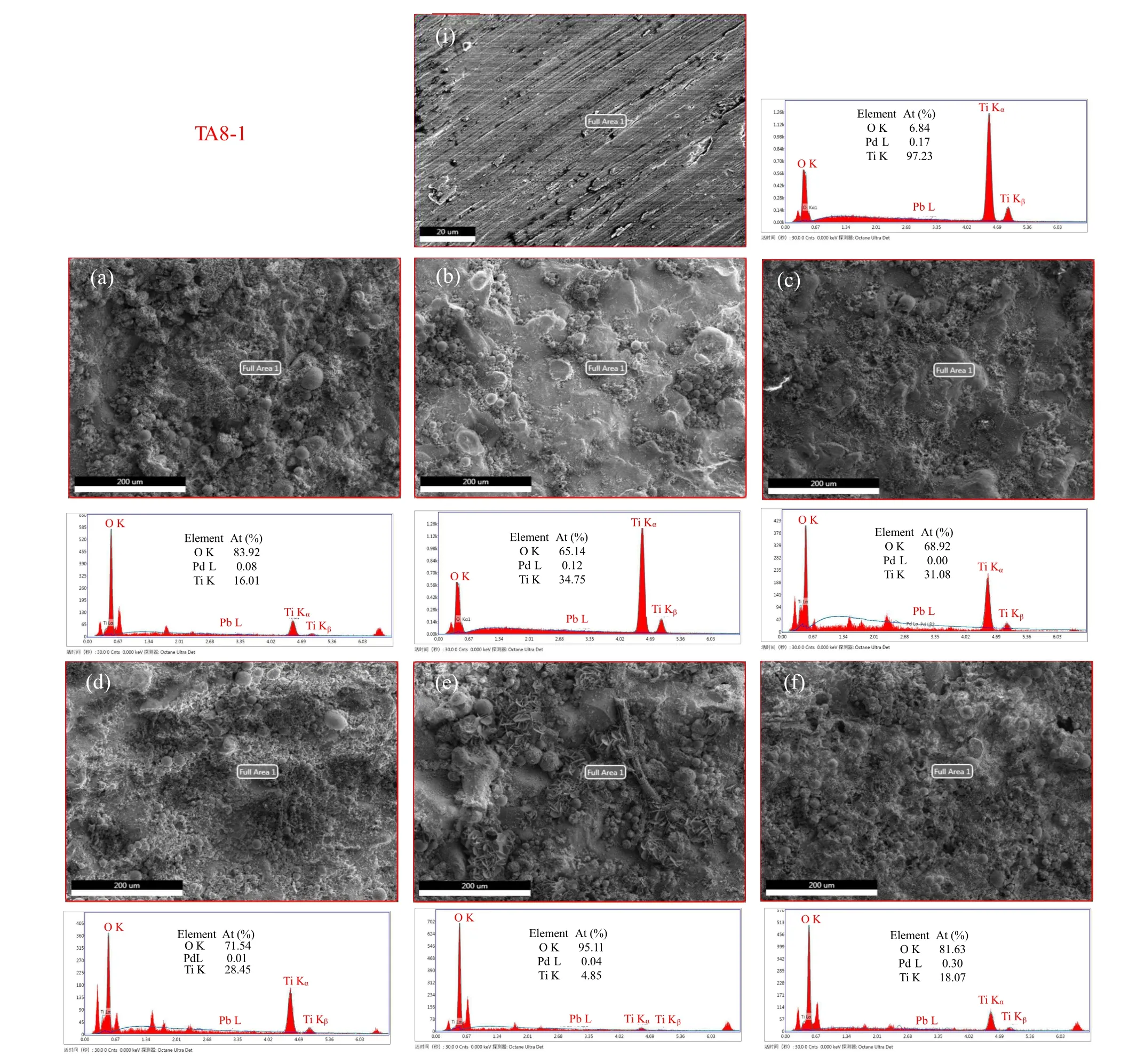
Fig.5. SEM–EDS micrographs of the TA8-1 sample surface. (i)Fresh,(a)irradiated 40d,(b)irradiated 80d,(c)irradiated 160d,(d)non-irradiated40d,(e)non-irradiated80d,and(f)non-irradiated160d.

Fig.6. SEM–EDS micrographs of the TA2 sample surface. (i)Fresh,(a)irradiated 40d,(b)irradiated 80d,(c)irradiated 160d,(d)non-irradiated40d,(e)non-irradiated80d,and(f)non-irradiated160d.
4. Conclusion
The corrosion behavior of TA8-1 and TA2 samples in Beishan groundwater under long-term intense γ-irradiation was studied. The results show that γ-irradiation can significantly change the corrosion environment of groundwater.The pH value,conductivity,and titanium ion concentration of groundwater change significantly under irradiation,but not obvious without irradiation. It indicates that irradiation can accelerate the corrosion of samples in groundwater. In addition,TA8-1 and TA2 are mainly local corrosion in groundwater,and an oxide layer of TiO2or/and TiO2is formed on the surface of the sample, which is finally oxidized into TiO2, forming a stable corrosion layer to prevent further corrosion of metals.Therefore,TA8-1 and TA2 are suitable to be used as container materials for high-level waste disposal containers.
Acknowledgment
We would like to thank Wei Guo of Jiangxi Tianzhao Technology Development Co., Ltd. for providing cobalt source equipment for the irradiation experiment. We also thank Dr. Yunlong Lian and Dr. Saeed for their guidance on pictures and language smoothing in this paper.
- Chinese Physics B的其它文章
- Process modeling gas atomization of close-coupled ring-hole nozzle for 316L stainless steel powder production*
- A 532 nm molecular iodine optical frequency standard based on modulation transfer spectroscopy*
- High-throughput identification of one-dimensional atomic wires and first principles calculations of their electronic states*
- Effect of tellurium(Te4+)irradiation on microstructure and associated irradiation-induced hardening*
- Effect of helium concentration on irradiation damage of Fe-ion irradiated SIMP steel at 300 °C and 450 °C*
- Optical spectroscopy study of damage evolution in 6H-SiC by H+2 implantation*

