Analysis of the adverse reactions of atezolizumab:A real-world study based on FAERS database
Hao Liu,Yujing Zhang,Jingyi Li,Rong Yan (✉)
1 Department of Surgical Oncology,The First Affiliated Hospital of Xi'an Jiaotong University,Xi'an 710061,China
2 College of Stomatology,Xi'an Jiaotong University,Xi'an 710061,China
Abstract Objective In this study,we aimed to determine the incidence of adverse drug reactions (ADRs) of atezolizumab,identify ADR signals that are significantly related to atezolizumab,and provide a reference for the rational use of atezolizumab in the clinic through the statistical analysis of its adverse drug events(ADEs) reported in the American Food and Drug Administration (FDA) Adverse Event Reporting System(FAERS) database.Methods In total,4796 cases of atezolizumab ADEs reported in the American FAERS database from 2017 to 2019 were retrospectively analyzed.Results The top three ADEs were febrile neutropenia (3.7%),anemia (2.9%),and acute renal failure(2.3%).In addition,the incidence rates of some ADEs were significantly different according to sex and age.The systematic organ classification of atezolizumab ADEs involved 32 systems,among which the top three were blood and lymphatic system disorders (585 cases,12.2%),gastrointestinal disorders (433 cases,9.0%),and infections and infestations (401 cases,8.4%).The reporting odds ratio (ROR) method was used to detect the ADR signals of atezolizumab.The ROR (95% confidence interval) of the top ADE,febrile neutropenia,was 39.236 (33.757-45.604).In addition,we found 121 cases of complications associated with immune-related ADEs.Conclusion The ADRs of atezolizumab reported in the FAERS database were consistent with those mentioned in the instructions for atezolizumab use,suggesting that atezolizumab has an acceptable and controllable drug effect.
Key words:atezolizumab;adverse reactions;Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database;rational drug use
Currently,three main global databases are used worldwide to collect information about adverse drug events (ADEs):the World Health Organization (WHO)-Vigibase,the European Information System for Suspected Drug Adverse Reactions (Eudravigilance),and the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS).The FAERS database has been open to the public since 2004,and data are updated quarterly.The large amount of data included in the FAERS database can be effectively used for post-market safety monitoring and risk evaluation.In addition,the number of ADE cases as well as detailed information about the ADEs for each drug,including age,gender,combination therapy,and outcome,can be found in the FAERS database.The mining and analysis of large-scale ADE databases allow us to determine the incidence rate of several ADEs and provide a reference for the rational clinical drug use.Because of its rich content,extensive coverage,and large scale,the FAERS database has an important research value in drug safety evaluation[1].
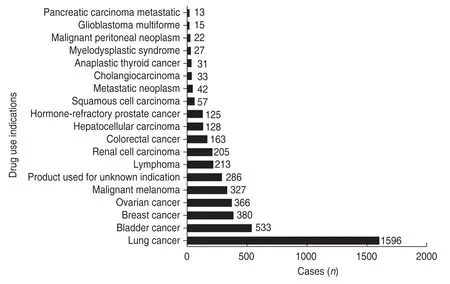
Fig.1 Distribution of the top 20 indications for atezolizumab use.For a more intuitive impression,we unified small cell lung cancer and non-small cell lung cancer as lung cancer
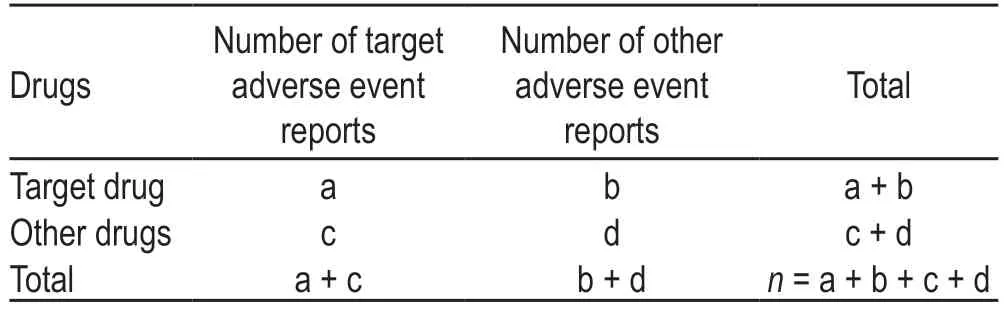
Table 1 Four-grid table of the ratio imbalance measurement method
Atezolizumab is the first clinically approved synthetic immunoglobulin (Ig) G1 monoclonal antibody developed against programmed death-ligand 1 (PD-L1).Because of its favorable safety and efficacy,it was approved by the US FDA in 2016 as a second-line treatment for advanced non-small-cell lung carcinoma (NSCLC) and urothelial bladder cancer[2-4].Fig.1 shows the top 20 indications for atezolizumab use among 4796 cases of atezolizumab ADEs reported in the FAERS database.These data may indirectly reflect the worldwide use of atezolizumab for the treatment of various tumors.However,with the increasingly widespread use of atezolizumab in clinical practice,concerns about the adverse drug reactions(ADRs) associated with its use have gradually emerged.In this study,we aimed to reveal the incidence of atezolizumab ADRs,identify the ADR signals that are significantly related to atezolizumab,and provide a reference for its rational use in the clinic.We analyzed a total of 4796 atezolizumab ADE cases reported in the US FAERS database from 2017 to 2019.
Methods
Data sources and processing
Data used in this study were obtained from the US FAERS database.The US FAERS database is a spontaneous reporting system database that does not require a reporter to prove the causal relationship between drugs and reported ADEs and does not require the report to include sufficient information to evaluate the causal relationship between drugs and ADEs.
The generic name of the target drug is“atezolizumab”(Tecentriq®).We exported the atezolizumab ADE reports for the period from the first quarter of 2017 to the fourth quarter of 2019 from the FAERS database,removed duplicate data,and used the Medical Dictionary for Regulatory Activities (MedDRA) terminology to standardize the ADR description in the report.After data cleaning,a total of 4796 reports of atezolizumab ADEs were obtained for statistical analysis.
Signal detection and analysis
In this study,descriptive statistical analysis was used to analyze the 4796 reports of atezolizumab ADEs extracted from the US FAERS database.The data included clinical manifestations,organs and systems involved,and major ADR signals.The reporting odds ratio (ROR) method,which is widely used for ADR signal mining,was employed to detect the main ADRs related to atezolizumab.This method has a high sensitivity and can eliminate a large number of deviations.The algorithm was based on a four-grid table as listed in Table 1.The formula used for calculating the ROR is as follows:ROR=(a/c)/(b/d);ROR 95% confidence interval (95% CI)=.The signal detection standard was set as follows:the ROR value should be ≥ 3,and the lower limit of ROR 95% CI should be > 1 to indicate a suspicious signal.The stronger the ADR signal,the stronger the correlation between the drug and the ADR.
Results
ADE overview
Of the 4796 ADE reports included,4787 included the source region;among these,1298 reports (27%) were from the United States and remaining were from Japan (539 cases),France (413 cases),Spain (387 cases),and China(309 cases).The vast majority of reports (approximately 70%) were from economically developed Western countries,whereas the remaining reports were mainly from Southeast Asia,followed by South America.No reports were obtained from Africa and the Middle East.This difference in the source of ADE reports might be attributed to differences in the frequency of atezolizumab use and the development of the ADE reporting systems in different regions.
In 2017,the number of reported ADEs of atezolizumab in the four quarters was 322,320,244,and 299,respectively;in 2018,there were 344,389,447,and 501 reports,respectively;and in 2019,there were 465,435,447,and 583 reports,respectively.Therefore,the annual atezolizumab ADEs reported in the FAERS database showed a year-on-year upward trend from 2017 to 2019,whereas the quarterly distribution was more uniform.However,potential differences in the quarterly distribution of ADE reports require further investigation.
Among the 4796 ADE reports,sex was effectively recorded in 4702 reports (98%),including 2597 males(55.23%) and 2123 females (45.15%).In addition,age was recorded in 4601 reports (95%) and could be divided into four groups:8 cases < 18-years old,532 cases between 18-and 50-years old (including 18-years old),1761 cases between 50-and 65-years old (including 50-years old),and 2300 cases ≥ 65-years old,indicating that most of the reported atezolizumab ADEs occurred in patients aged more than 50-years old.However,this did not necessarily imply that men or individuals aged more than 50-years old are more likely to have ADRs related to atezolizumab use because the incidence of atezolizumab use might differ according to sex and age,in addition to the presence of other potential confounding factors.
Reporting frequency of the major atezolizumab ADEs
The reporting frequency of ADEs can reveal the incidence rate of ADRs and reflect the impact of ADRs on the patients’ health,thus indicating the key direction for risk management[2].In this study,4796 cases of ADEs related to atezolizumab use were analyzed.Based on the number of ADEs,the top 20 reported ADEs included febrile neutropenia (179 cases),anemia (140 cases),acute renal failure (111 cases),diarrhea (83 cases),death (78 cases),colitis (73 cases),and elevated alanine aminotransferase (70 cases;Fig.2).Notably,51 cases of skin-related ADRs with similar nature but different names,including rash,dermatitis,eczema,and empyema,were reported.
In addition,we used the unique identification codes of ADEs,the number of ADEs,sex,age,country and region,indications,adverse reaction outcomes,and other indicators to exclude data that might be reported by the same person at different times;however,very few records of ADEs reported by the same person to the FAERS database could not be excluded because some individuals might have used atezolizumab at different ages.Accordingly,4023 cases of atezolizumab-related ADEs were included in this analysis and divided into three age groups:532 reports between 18-and 50-years old (including 18-years old),1761 cases between 50-and65-years old (including 50-years old),and 2300 cases ≥65-years old.The difference in the incidence of ADEs according to different ages and sex was analyzed using chi-squared test (Tables 2 and 3).
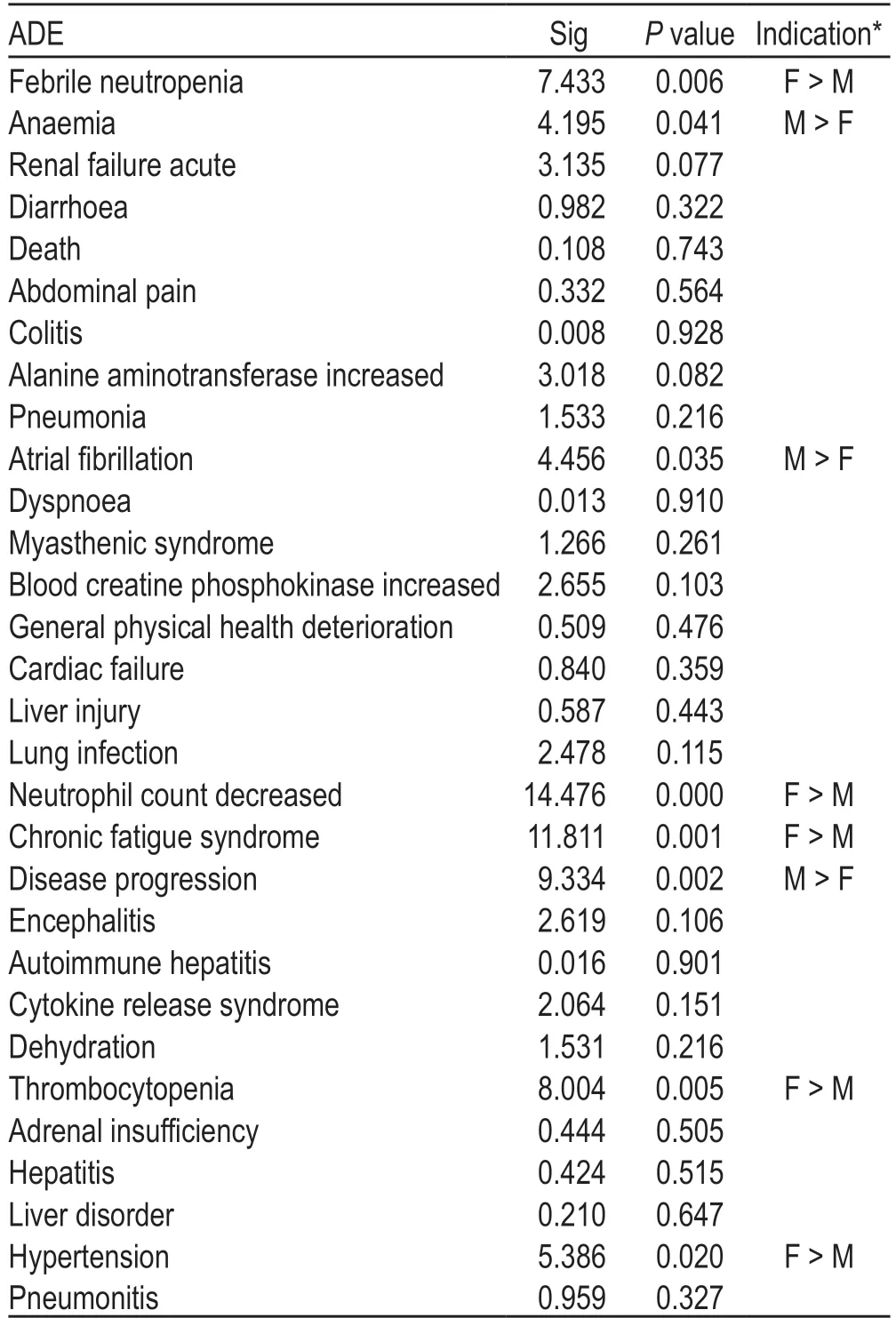
Table 2 Differences in the incidence rate of the top 20 ADEs by sex
In addition,chi-squared test was performed using the three age groups for each ADE and showed significant differences among different age groups (Table 4).
Involved organs and systems
The MedDRA is a multi-axial,five-tiered hierarchical terminology used by regulatory authorities and biopharmaceutical industry for the coding (classification)of clinical data in ADE/ADR reports.Therefore,it has become an international standard terminology for ADR reports[5].According to the MedDRA terms,a total of 4796atezolizumab ADEs were recorded and classified using the ADE Preferred Terminology (PT) and Systematic Organ Classification (SOC) codes.The details of the ADEs of each SOC that contained PT > 100 are shown in Fig.3 and Table 5 (the percentage base of“proportion”in Table 5 is 4796 ADEs).The number of“product usage problems”and“incorrect product management approaches”was 9.There was no relevant description in the MedDRA,which might be because of terminology changes due to the MedDRA biannual updates[2,6].The ROR method was used to detect the signal strength of ADEs,and five ADEs with strong ADR signals were selected from the top tenADEs:febrile neutropenia,anemia,renal failure acute,colitis,and elevated alanine aminotransferase (Fig.4).
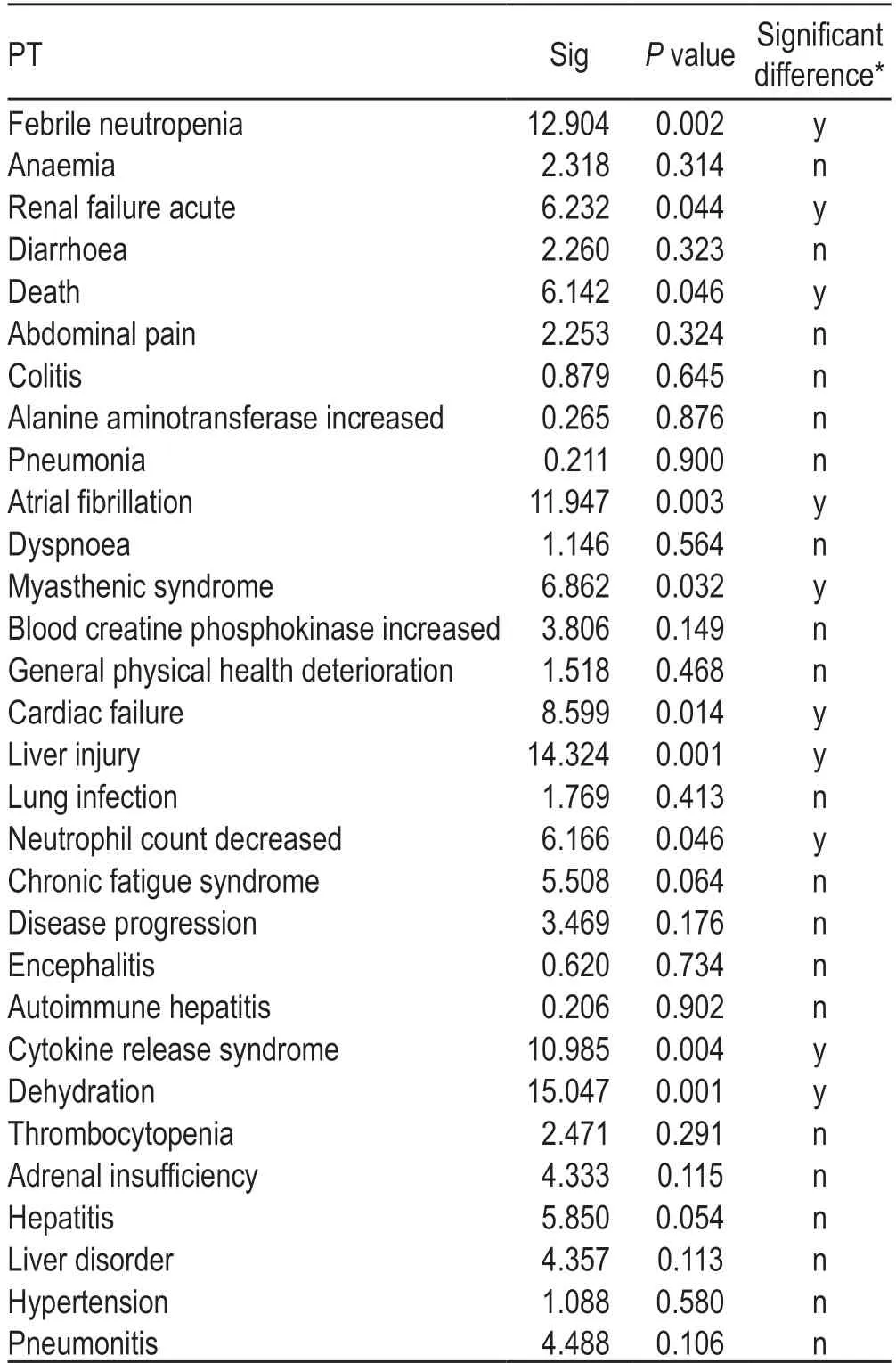
Table 3 Differences in the incidence rate of the top 20 ADEs by age
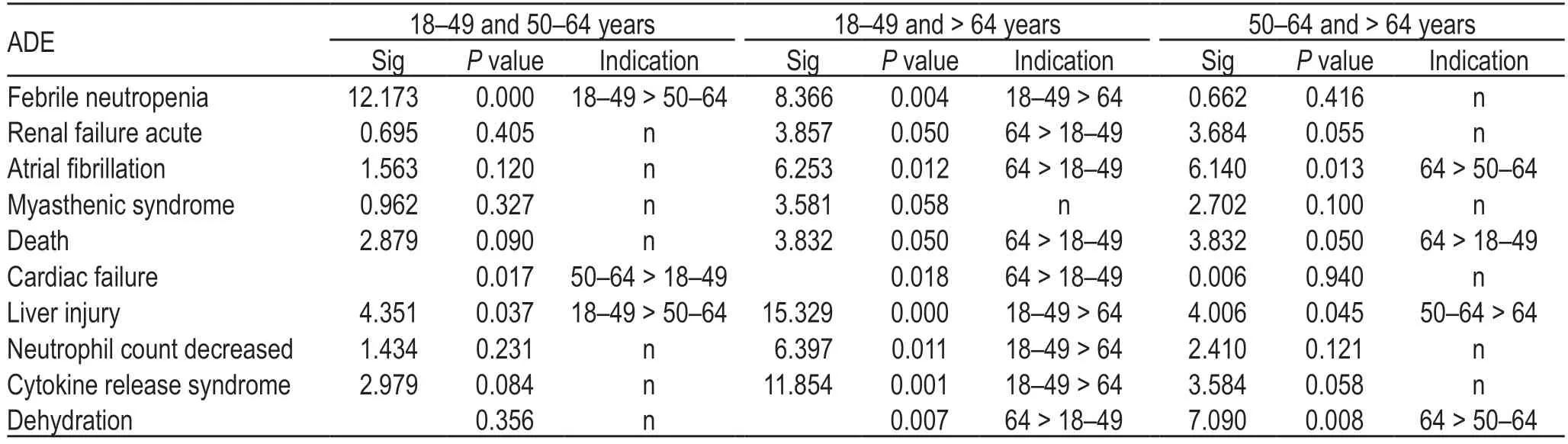
Table 4 Incidence rate of each ADE in the three age groups
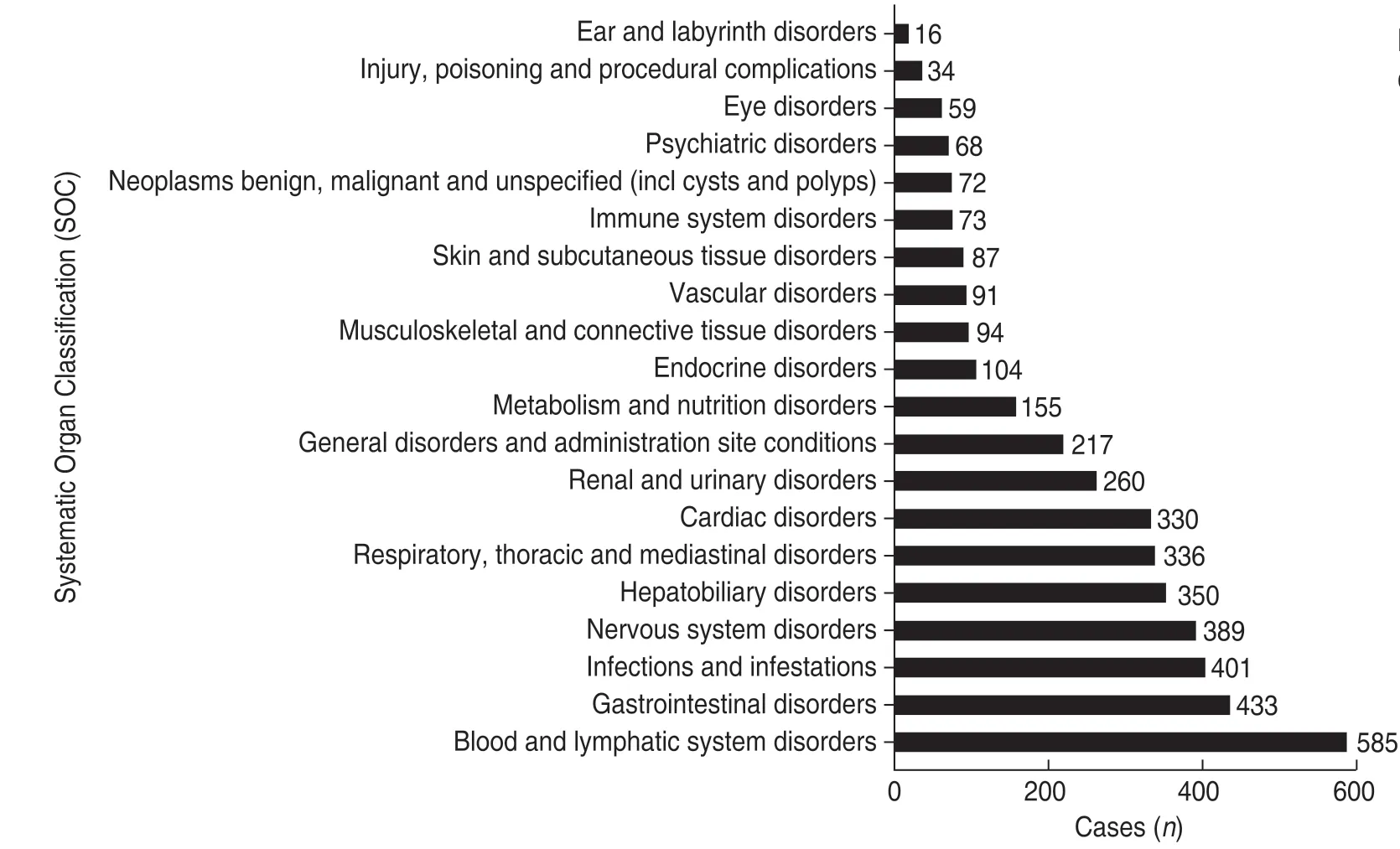
Fig.3 The systematic organ classification (SOC) of ADEs
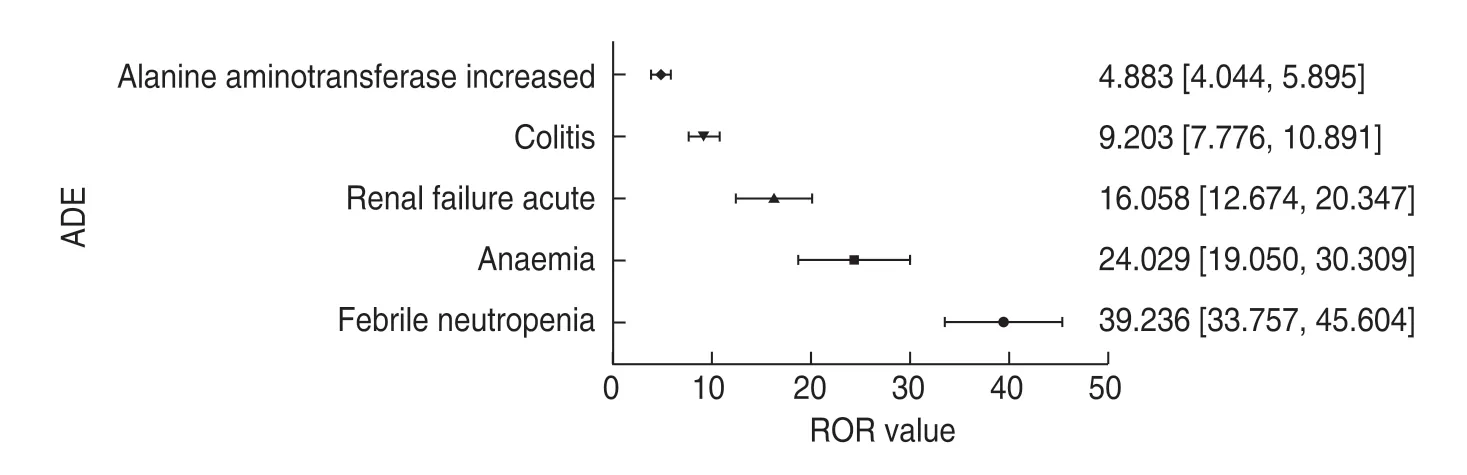
Fig.4 ADE signal detection value (ROR,95% CI).Notes:Data on the right side of the graph show the ROR values with 95% CI of each ADR
IrAEs-related complications
There have been some reports of atezolizumab-related autoimmune pancreatitis and hepatitis.Although these adverse reactions are uncommon,they pose a great threat to the patient life.Therefore,we extracted the immunerelated ADE (irAE)-related complications from the 4796 reports of ADRs.We found that the incidence of irAErelated complications was low.In particular,a total of 121 cases of 14 irAEs-related complications were reported(Fig.5).In addition,there were several ADEs that might indicate irAEs-related complications,including nine cases of amylase increase that might indicate pancreatitis and three cases of hepatitis.
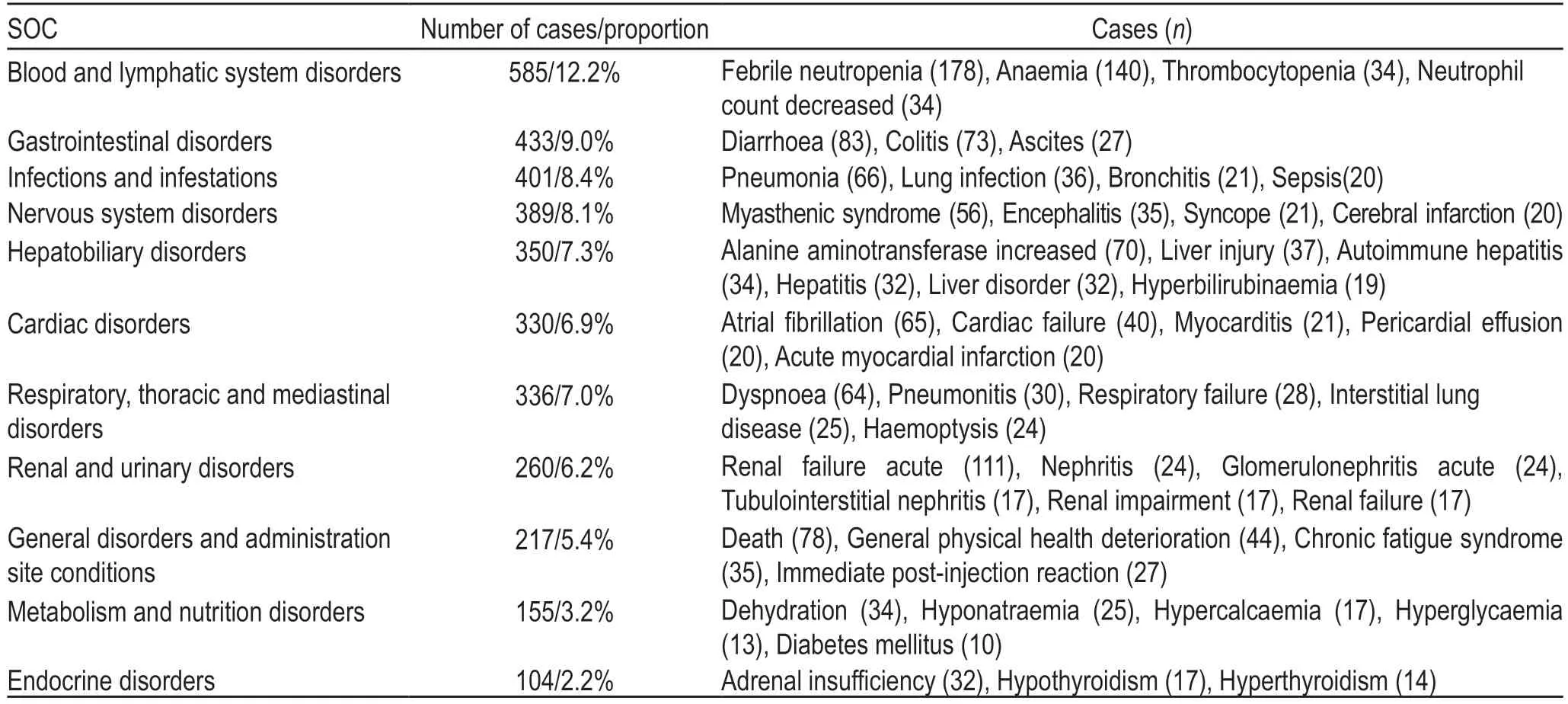
Table 5 The major ADEs of each SOC
Discussion
Atezolizumab has been previously shown to exhibit acceptable and controllable tolerance.Therefore,it is a valuable treatment option for patients with NSCLC,melanoma,urological tumors,and breast cancer who progress during or after chemotherapy[7-10].
As an immune checkpoint inhibitor (ICI),atezolizumab(anti-PD-L1) may subsequently lead to various autoimmune manifestations with a specific clinical spectrum called irAEs[11].These effects result from an overall immune enhancement,and thus,they may affect any body system;however,they mainly involve the skin,colon,lungs,endocrine glands,and liver[12]and are related to the tumor types[13].Some rare complications,such as atezolizumab-induced photo-distributed bullous pemphigoid[14],atezolizumab-related encephalitis[15],and ileal perforation secondary to atezolizumab enterocolitis[16],were also investigated in this study.
Previous studies have shown that approximately 66% of the patients treated with anti-PD-L1 or PD-L1 antibodies experience at least one ADR,whereas approximately 14% of the patients have severe ADRs.The incidence of these ADRs was not significantly different among different types of tumors[17].In addition,the occurrence of ADRs did not affect the efficacy of treatment[18].In a meta-analysis of clinical trials including 8730 patients[19],atezolizumab had the lowest risk of IrAEs.In addition,there was no significant difference in the risk associated with atezolizumab and avelumab.Based on the literature and reports in the FAERS database,the clinical manifestations of common ADEs of ICIs (such as navumab and pamumab) were relatively consistent and mainly concentrated in the gastrointestinal tract,skin,and respiratory system,including diarrhea,heart attack,vomiting,colitis,rash,dyspnea,and pneumonia.These reported ADEs might be considered ICI-induced IrEAs.
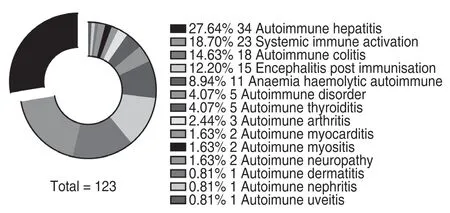
Fig.5 irAEs-related complications of atezolizumab
Conclusion
The ADRs of atezolizumab reported in the FAERS database were consistent with those mentioned in the instructions for its use.This study suggested that physicians need to be more cautious when using atezolizumab clinically.Individuals eligible for atezolizumab treatment should be screened for a personalized treatment,thus promoting the importance of precision medicine.
Compared with cytotoxic T-lymphocyte-associated protein 4-blocking agents,PD-1/PD-L1 inhibitors are generally considered to have minor side effects[20].However,during the course of PD-1/PD-L1 inhibitor treatment,the use of glucocorticoids to treat irAEs can result in opportunistic infections owing to temporary immunosuppression[21].In addition,there are currently reported cases of latent/chronic infection reactivation without irAEs during treatment with PD-1/PD-L1 inhibitors[22].Therefore,it is necessary to fully evaluate the risks that patients with irAEs may be subject to.It should be considered that the irAEs-related complications can reduce the clinical benefit[23].Moreover,some ADEs might occur after treatment;therefore,it is important to provide a timely treatment to reduce the risk of ADEs.In addition,some reported ADEs of ICIs might be specific to some ICIs.
At present,further studies are needed to investigate other aspects of the irAEs,including their mechanism,population characteristics,and effect on tumor treatment,as well as whether immunosuppressive therapy might affect tumor treatment efficacy.It has been suggested that cross-antigens,non-specific immune activation,and T cell diversification contribute to the pathogenesis of irAEs[24].However,there are no prospective studies to support this hypothesis.In addition,it is essential to identify the factors related to irAEs clearly and thus help improve the screening of susceptible patients,thereby reducing the risk of ADRs.Large-scale,multi-center,randomized controlled studies should be encouraged to determine the best medication plan for immunotherapy drugs and provide a basis for the safe and reasonable medication use.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年2期
Oncology and Translational Medicine2021年2期
- Oncology and Translational Medicine的其它文章
- A case report of iodine-125 seed placement during operation for the treatment of advanced gallbladder carcinoma with septic shock*
- Analyses of the clinical characteristics of 49 cases of malignancy with multiple bone lesions as the first manifestation
- Application of endoscopic nasobiliary cutting in the treatment of hilar cholangiocarcinoma
- KIF15 expression characteristics:Relevance toneo-adjuvant chemotherapy efficacy in breast cancer*
- Study on the antitumor effects of autologous and allogeneic CIK cells in patients with breast cancer*
- Differentially expressed genes analysis and target genes prediction of miR-22 in breast cancer*
