Expression and role of PTV1 lncRNA in glioma cells progression*
Department of Neurosurgery,Mianyang Central Hospital,Mianyang 621000,China
Abstract Objective The aim of this study was to investigate the expression of PTV1 lncRNA in gliomas and the mechanism of its interaction with miR-203a.Methods U87 and U251 cells were cultured stably and transfected with sh-PTV1 or ov-PTV1,respectively.The proliferative activity of U87 and U251 cells was detected and the transplanted tumor model nude mice were divided into U87 and U251 groups.U87-sh and u251-ov cells were injected into the armpit,then miR-203a mic and miR-203a inhibitors were administered to detect the changes in the expression of tumorrelated proteins.Results The relative expression of PTV1 in gliomas was significantly higher than that in normal brain tissues,while in GBM it was significantly higher than that in low-grade gliomas.Knockdown of PTV1 significantly inhibited the proliferation of U87 cells,resulting in fewer cell clones;overexpression of rPTV1 significantly promoted the proliferation of U251 cells,resulting in more cell colonies.The dual Luciferase Reporter assay showed that SP2 was a potential target of miR-203a.When U87 cells were treated with a miR-203a mimic,the expression of SP2 decreased;and when U251 cells were treated with a miR-203a inhibitor,the expression of SP2 increased significantly.SP2 was overexpressed in u87-sh cells and the proliferation,migration,and invasion of u87-sh cells were significantly enhanced.U251-ov cells showed the opposite trend.Compared with the control group mice,the tumor volume in u87-sh group mice was significantly smaller and the positive rate of SP2 in tumor tissue was significantly lower.After administration of the miR-203a inhibitor,the tumor volume increased gradually and the positive rate of SP2 increased significantly,while u251-ov mice showed the opposite trend.Conclusion lncRNA PTV1 can be used as a molecule to interfere with miR-203a expression in order to downregulate SP2 and to promote the proliferation and invasion of glioma cells.lncRNA PTV1 may be a new biomarker and therapeutic target for glioma.
Key words:glioma;miRNA-203a;long-chain noncoding RNA;transcription factor PTV1 lncRNA
Glioma is the most common brain cancer,accounting for more than 60% of the primary brain tumors in adults.The molecular mechanisms underlying its occurrence and development are still unclear[1].Long noncoding RNAs (lncRNAs) are a class of RNAs (> 200 nucleotides long) that are not translated into protein.They are widely involved in biological processes such as posttranscriptional regulation and chromatin modification[2].A variety of lncRNAs have been proved to play important roles in different types of cancer cells,including colorectal cancer[3],bladder cancer[4],and esophageal cancer[5].MicroRNAs (miRNAs) are also recognized as noncoding RNAs that play important roles in tumor biology.Lncrna can interact with miRNA as a competitive endogenous RNA and participate in the regulation of target gene expression.Through competition,lncRNAs sequester numerous miRNAs in the cell,reducing their ability to interfere with the target gene mRNA,thus affecting downstream gene expression at the post-transcriptional level.Such lncRNAs and mRNAs are referred to as mutually competitive endogenous RNAs (ceRNA)[6-7].PTV1 is an lncRNA,highly expressed in thyroid and renal cancers but has not yet been reported in gliomas.In a previous study,our bioinformatic analysis with TargetScan and other software revealed that PTV1 may have a targeted effect on miR-203a and may affect the expression of the downstream transcription factor SP2.In this study,we verified this conjecture through cell experiments and in vivo experiments in mice,in order to provide more efficient biological targets for the prevention and treatment of glioma.
Materials and methods
Clinical specimens and cell culture
From January 2018 to June 2019,62 patients with glioma and 24 patients with subarachnoid hemorrhage or brain injury who were admitted to neurosurgery of Mianyang Central Hospital (China) were selected as research subjects.Glioma samples and normal brain tissue samples were collected from patients and quickly stored in liquid nitrogen.All glioma patients were diagnosed for the first time and had never received radiotherapy nor chemotherapy before.The human brain glial cell line Heb was purchased from Lonza (Switzerland).Glioma cell lines U87,A172,and ln229 were all purchased from American type culture collection,while the glioma cell line SHG-44 U251 was purchased from the Institute of Biochemistry and Cell Biology,Chinese Academy of Sciences (China).All glioma and Heb cell lines were cultured in Modified Eagle’s Medium containing 10%fetal bovine serum and were incubated at 37°C in an atmosphere containing 5% CO2.
Plasmid construction and cell transfection
Short hairpin RNAs (shRNAs) for lncRNA PTV1 and SP2 and overexpression plasmids containing PTV1 lncRNA (OV) and SP2 were designed and synthesized by Jikai Gene Chemical Technology Co.,Ltd.(China).hsa-miR-203a mimics (mimic) and inhibitors (inhibitor)were designed and synthesized by Lucky Gene Chemical Technology Co.,Ltd.(China) full-length sequence of PTV1 lncRNA was amplified and cloned into pcDNA3.1 vector (GeneChem,China),and full-length cDNAs of miR-203a and SP2 were transcribed and cloned into pcDNA3.1.According to the manufacturer’s protocol,oligonucleotides and constructs were transfected into the cell lines using Lipofectamine 3000 (Abcam,UK).
RT-PCR and western blot
Total RNA was extracted from clinical samples and cell lines.The expression of PTV1 lncRNA,miR-203a,and SP2 was detected by real-time quantitative polymerase chain reaction (qRT-PCR) and normalized against that of GAPDH and U6.The primer sequence for lncRNA is as follows:lnc PTV1:forward primer 5’-CGACCAGACCACACTGAA-3’,reverse primer 5‘-ACACCTCCAAAGCAGCCCTCAAA-3.Western blotting was performed as follows:clinical tissues and cells were collected,cell samples were lysed using RIPA buffer,and total protein was extracted,then protein concentration was determined with BCA protein analysis kit (Abcam,UK),which included the following steps:sample buffer mixing,boiling (denaturation),electrophoresis,membrane transfer,and sealing.Membranes containing the protein bands were probed overnight with SP2 antibody (1:1000) at 4°C.After washing,the membrane was probed with SP2 antibody and secondary antibody were purchased from Beyotime Biotechnology,and developed using the ECL reagent;GAPDH (1:10000) protein as the internal reference.
Luciferase reporter assay
The full-length sequence and fragment of lncRNA PTV1 containing the indicated miRNA binding sequence were cloned into the pmiR reporter vector.The 3’-untranslated region fragment of SP2 containing a specific miRNA binding sequence was also cloned into the pmiR reporter vector.The corresponding miRNAs and reporter plasmids were transfected into the glioma cell line,while the mutant plasmid was used as control.After 48 h,the cells were collected and the luciferase activity was measured using the dual Luciferase Report Analysis System.
CCK-8 and cell colony formation assay
Cell count kit-8 (CCK-8) and colony forming assay(CFA) were used to detect the proliferation of glioma cells.CCK-8 detection was performed by inoculating a 96 well plate with 1 × 104cells per well,then measuring the absorbance at 450 nm with a micro flat-panel reader.To evaluate colony formation,glioma cells were inoculated in 6-well plates at a density of 1 × 104cells/ well and cultured in DMEM containing 10% fetal bovine serum for 14 days.The cells were then fixed with methanol and stained with 0.5% crystal violet for 10 min.
Analysis of cell migration and invasiveness
The chamber was placed in a 24 well plate;the lower chamber contained 1:8 diluted Matrigel (60 μL per well) and incubated in the incubator for about 5 h.In each experimental group,4 wells were set up,and cell suspension was added into Transwell chamber,each well was about 100 μL.Then 600 μL DMEM containing fetal bovine serum was added to the lower chamber,and the set up was then incubated for 24 h.The medium was discarded,and cells adhering on the upper layer of the membrane were removed with a cotton swab,stained with crystal violet;after counting,5 visual fields were randomly selected and observed under high-power microscope (Abcam,UK).
Immunohistochemistry
Tumor tissue in mice were fixed with formaldehyde,embedded in paraffin,and sectioned.Tissue sections were dewaxed,hydrated,and incubated with H2O2deionized water.The antigen was exposed to 80℃,washed with PBS,and incubated overnight with 100 μL SP2 monoclonal antibody (1:100) at 4°C in a refrigerator.Sections were rinsed with PBS 3 times for 2 min.Then,the sections were incubated with a secondary antibody(added dropwise) at 37°C for 20-30 min,then sections were rinsed again with PBS (3 times,2 min).The sections were stained with DAB,then dehydrated,transparent with xylene and sealed with neutral gum.The appearance of brown particles was taken as the standard of SP2.The appearance of brown particles was used as an indicator of SP2 expression.
Mouse model construction
A total of 36 SPF-grade BALB/c nude mice were purchased from the experimental animal center of the Academy of Military Medical Sciences (China).Singlecell suspension of U87 cells stably transformed with shRNA-PTV1 and U251 cells overexpressing PTV1 was prepared using trypsin,then centrifuged,resuspended,and counted under a microscope.Thirty-six mice were randomly divided into two groups,U87 group and U251 group.The mice in U87 group were randomly divided into the following groups:(1) U87 group,injected with u87-nc glioma cells;(2) u87-sh group,injected with u87-sh glioma cells;and (3) U87 inhibitor group,injected with u87-sh glioma cells and treated with miR-203a inhibitor.U251 group mice were randomly divided into the following groups:(1) U251 group,injected with u251-nc glioma cells;(2) u251-ov group,injected with u251-ov glioma cells;and (3) U251 mimic group,injected simultaneously with miR-203a mimic and u251-sh glioma cells.After injection,all mice were observed every other day,timely provided with drinking water and feed,and checked for temperature and humidity.At the end of the experiment,the nude mice were euthanized and their subcutaneous tumor tissues were collected for observation,imaging,and measurement of tumor size.The breeding and treatment of nude mice were performed in strict accordance with the regulations of the Ethics Committee for experimental animals.The length (L) and weight (W) of the implanted tumors were measured using a vernier caliper at the designated time point and the volume of the tumor was calculated.
Results
Expression of PTV1 lncRNA in glioma
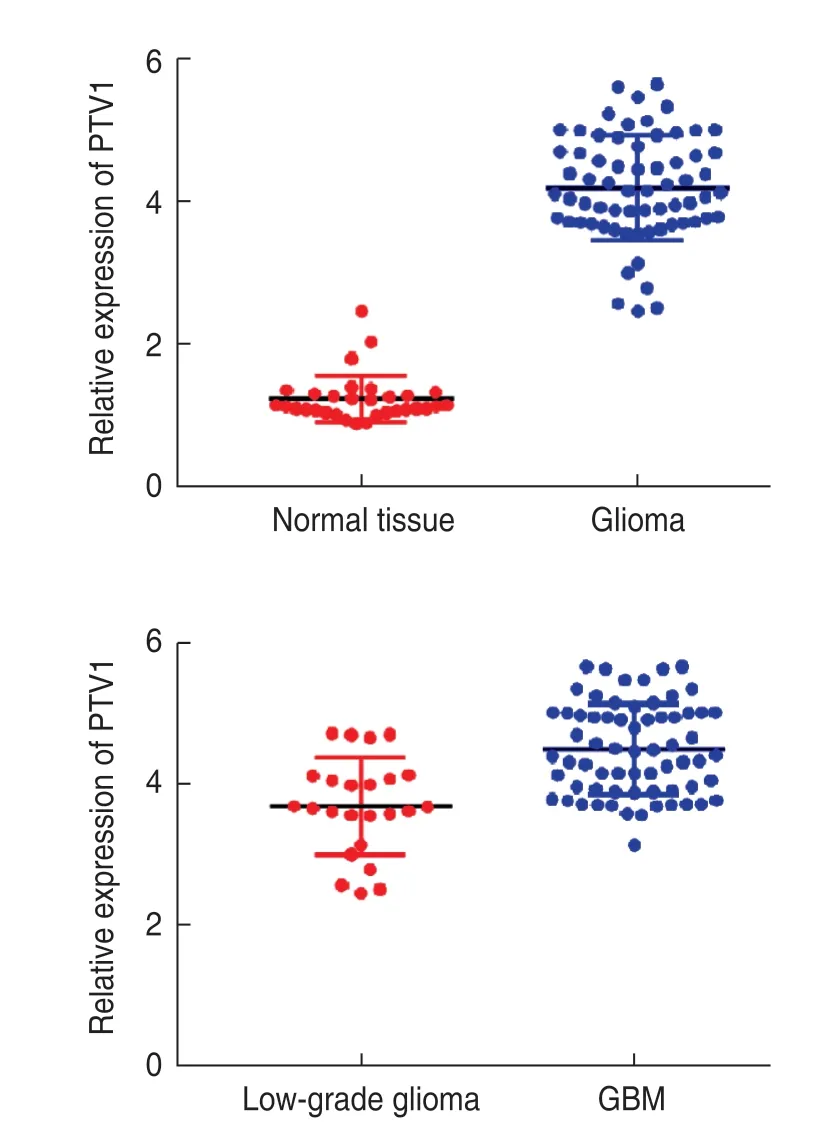
Fig.1 Expression of lncRNA PTV1 in glioma
RT-PCR revealed that the relative expression of PTV1 in the normal brain tissue and glioma tissue was (1.2 ± 0.2)and (4.3 ± 0.8),respectively.The expression of PTV1 in glioma tissue was significantly higher (P< 0.05).Based on the results of pathological examination,62 patients were divided into 24 cases of low-grade glioma and 38 cases of glioblastoma multiforme (GBM).The relative expression of PTV1 was (3.6 ± 0.5) and (4.7 ± 0.9),respectively.The expression of PTV1 in GBM was significantly higher than that in low-grade glioma (Fig.1).
lncRNA PTV1 promotes the proliferation,migration,and invasion of glioma cells
RT-PCR revealed that PTV1 was generally highly expressed in glioma cell lines U87,A172,ln229,and SHG-44;in particular,U87 had the highest expression of PTV1 and U251 had the lowest expression,compared with that in the normal human brain glioma cell line Heb (Figure 2a).Therefore,we transfected U87 cells (u87-sh) with shRNA against lncRNA PTV1,and U251 cells (u251-ov) with functional PTV1-cdna lncRNA.CCK-8 assay and cell cloning experiments revealed that knockdown of rPTV1 significantly inhibited the proliferation of U87 cells,as evidenced by fewer cell clones;OV significantly promoted the proliferation of U251 cells,which produced more colonies (Fig.2b and 2c).Transwell migration and invasion analysis showed that the migration and invasion ability of U87 cells decreased significantly after rPTV1 lncRNA knockdown,while OV significantly promoted the migration and invasion ability of U251 cells (Fig.2d).This indicated that PTV1 could significantly promote the proliferation,migration,and invasion of glioma cells.
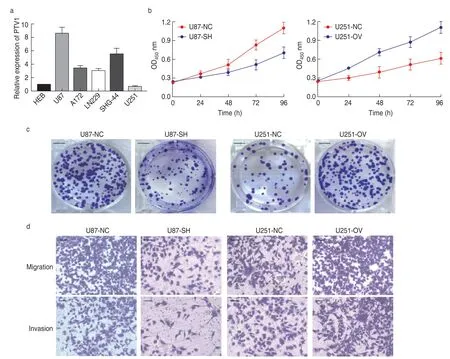
Fig.2 lncRNA PTV1 promotes proliferation,migration,and invasion of glioma cells.(a) Relative levels of PVT1 in different cell lines;(b) lncRNA PTV1 promotes the proliferation ability of U87 and U251;(c) Proliferation experiment of U87 and U251 cell lines;(d) Migration and invasion experiment of U87 and U251 cell lines
PTV1 lncRNA can target miR-203a
Compared with u87-sh cells,the expression of miR-203a in u87-nc cells was significantly higher,while that in u251-ov cells was significantly lower.The potential binding site between miR-203a and lncRNA PTV1 was determined by the bioinformatic software starBase (Fig.3).
miR-203a directly targets SP2 in glioma cells
TargetScan revealed that SP2 may be a potential miR-203a target gene (Fig.4a).Furthermore,upon cotransfection with miR-203a,we found that the dual luciferase reporter activity of sp1-wt in glioma cells was lower than that of SP1-Mut.The negative control group had no such effect on luciferase activity (Fig.4b).Western blotting revealed that the expression of SP2 decreased when U87 cells were treated with miR-203a mimic,and increased significantly when U251 cells were treated with the miR-203a inhibitor (Fig.4c).This indicated that miR-203a could directly target SP2 in glioma cells.
Positive regulation of SP2 expression by lncRNA PTV1 promotes the growth of glioma cells
We overexpressed SP2 in u87-sh cells and downregulated SP2 expression in u251-ov cells.The results showed that the proliferation,migration,and invasion of u87-sh cells were significantly enhanced.The corresponding activity of u251-ov cells was significantly inhibited.This suggests that PTV1 lncRNA can promote glioma progression by positively regulating the expression of SP2 (Fig.5).
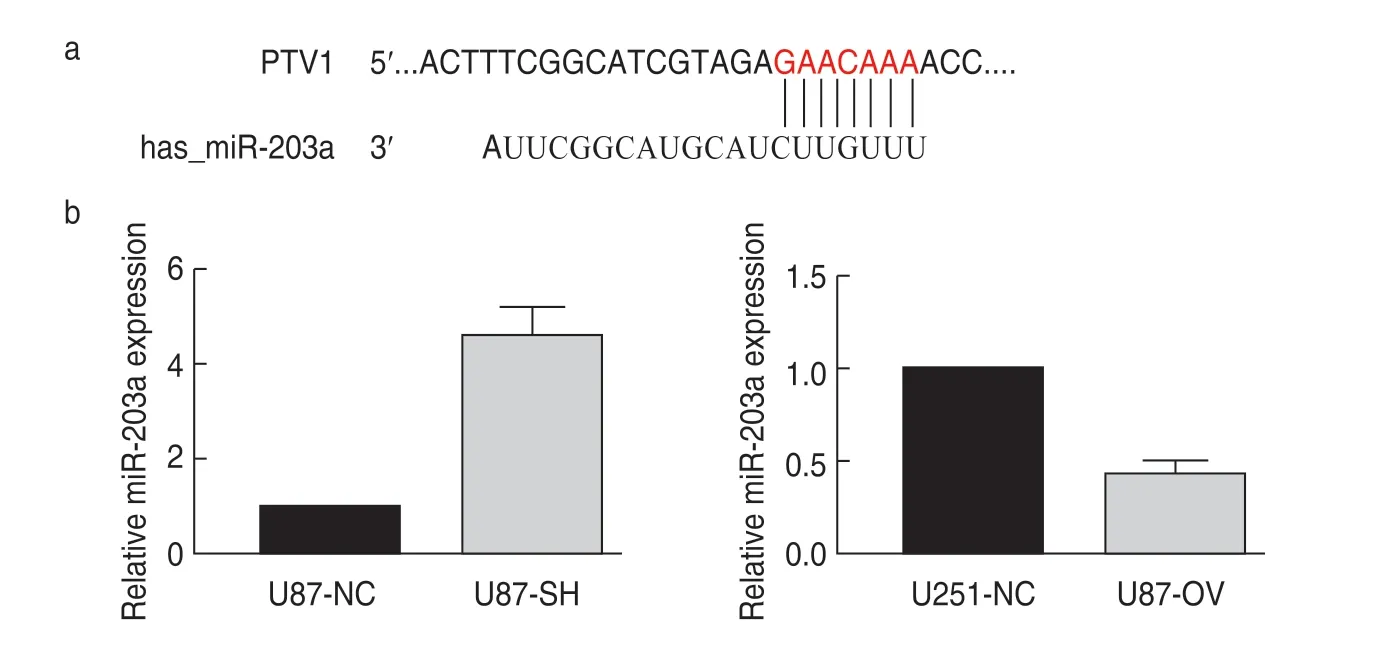
Fig.3 PTV1 lncRNA can target miR-203a.(a) Site of interaction between lncRNA PTV1 and miR-203a;(b) Expression of miR-203a in U87 and U251 cell lines.
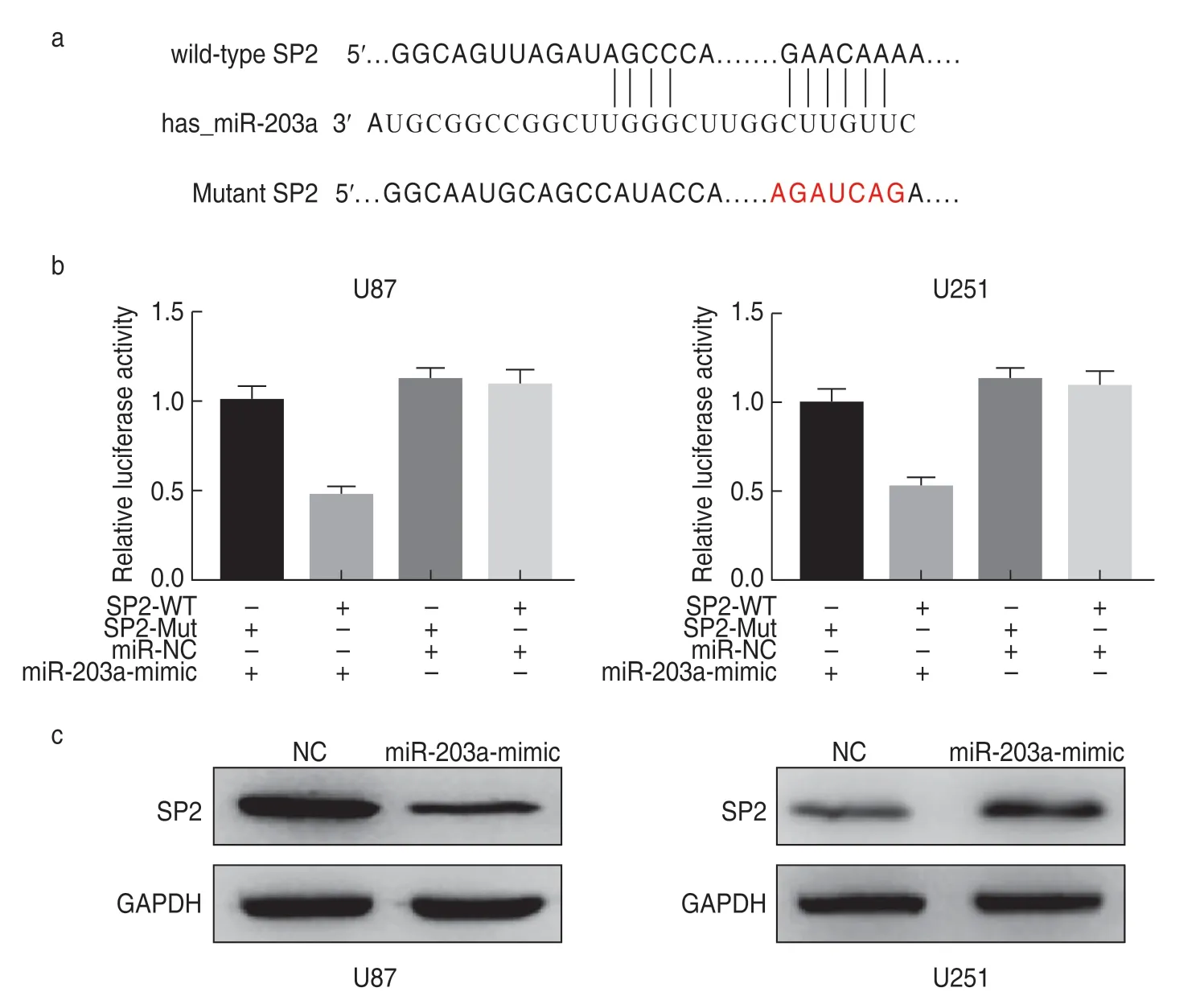
Fig.4 miR-203a directly targets SP2 in glioma cells.(a) Site of interaction between SP2 and miR-203a;(b)Double luciferase reporting experiment;(c)Western blot assays for SP2
miR-203a targeting by lncRNA PTV1 upregulates the expression of SP2 in glioma cells
miR-203a inhibitor was used to treat u87-sh,while miR-203a mimic was used to treat u251-ov.Western blotting showed that the expression of SP2 protein in u87-sh cells was significantly higher than that in the corresponding control group,while the expression of SP2 protein in u251-ov was significantly lower (P< 0.05;Fig.6).
lncRNA PTV1 targeting miR-203a upregulates SP2 expression in transplanted tumor
Compared with the control group,the tumor volume of mice in u87-sh group was significantly smaller and the positive rate of SP2 in tumor tissue was significantly lower.After treatment with the miR-203a inhibitor,the tumor volume increased gradually and the positive rate of SP2 increased significantly.Compared with mice in the control group,the mice in u251-ov group exhibited significantly larger tumor volumes and the positive rate of SP2 in tumor tissue was significantly higher.Tumor volume as well as the positive rate of SP2 were significantly reduced after miR-203a mimic treatment.This indicated that PTV1 lncRNA could promote the growth of glioma through the miR-203a/SP1 axis (Fig.7).
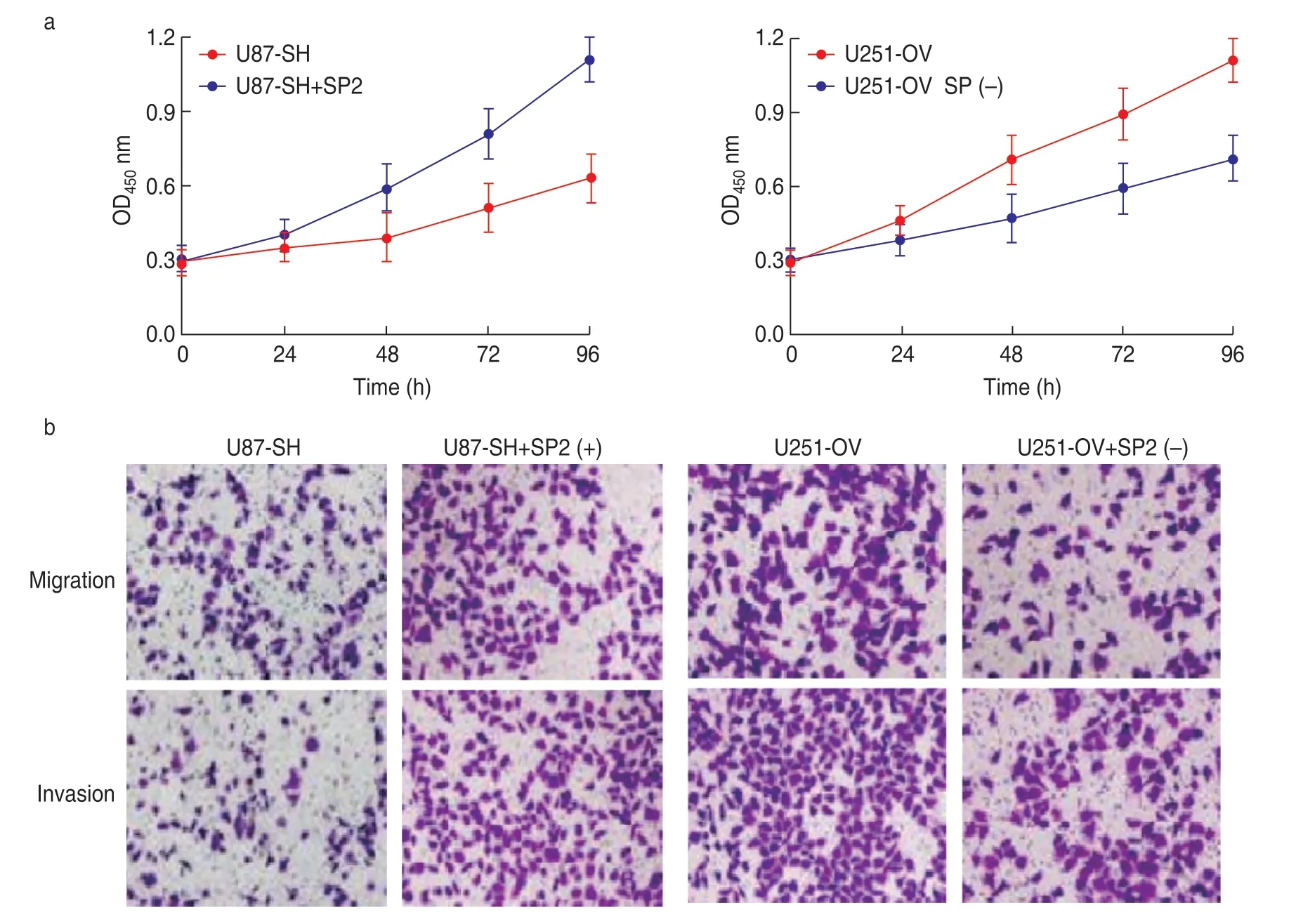
Fig.5 Positive regulation of SP2 expression by lncRNA PTV1 promotes glioma cell growth.(a) SP2 promotes the proliferation ability of U87 and U251.(b) Migration and invasion experiment of U87 and U251 cell lines
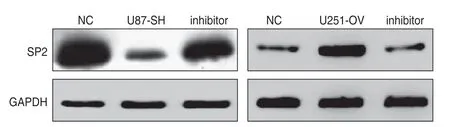
Fig.6 Effect of miR-203a inhibitor and mimics on the expression of SP2 protein in glioma cell lines.
Discussion
Glioma results from the interaction between congenital genetic high-risk factors and environmental carcinogenic factors.Some known genetic diseases,such as neurofibromatosis and tuberculous sclerosis,predispose individuals to glioma.The risk of glioma in patients with such basic diseases is significantly higher than in the general population[8-9].In addition,some environmental factors may also be related to the occurrence of glioma,such as mobile phone electromagnetic radiation,but there is no evidence of a clear causal relationship between the two.Glioma,especially glioblastoma,usually has a poor prognosis,with a median survival time of less than 12 months[10].Due to the high invasion and rapid proliferation of glioma and despite the progress in its diagnosis and even treatment,low cure rate and frequent recurrence are still the main challenges in clinical practice.
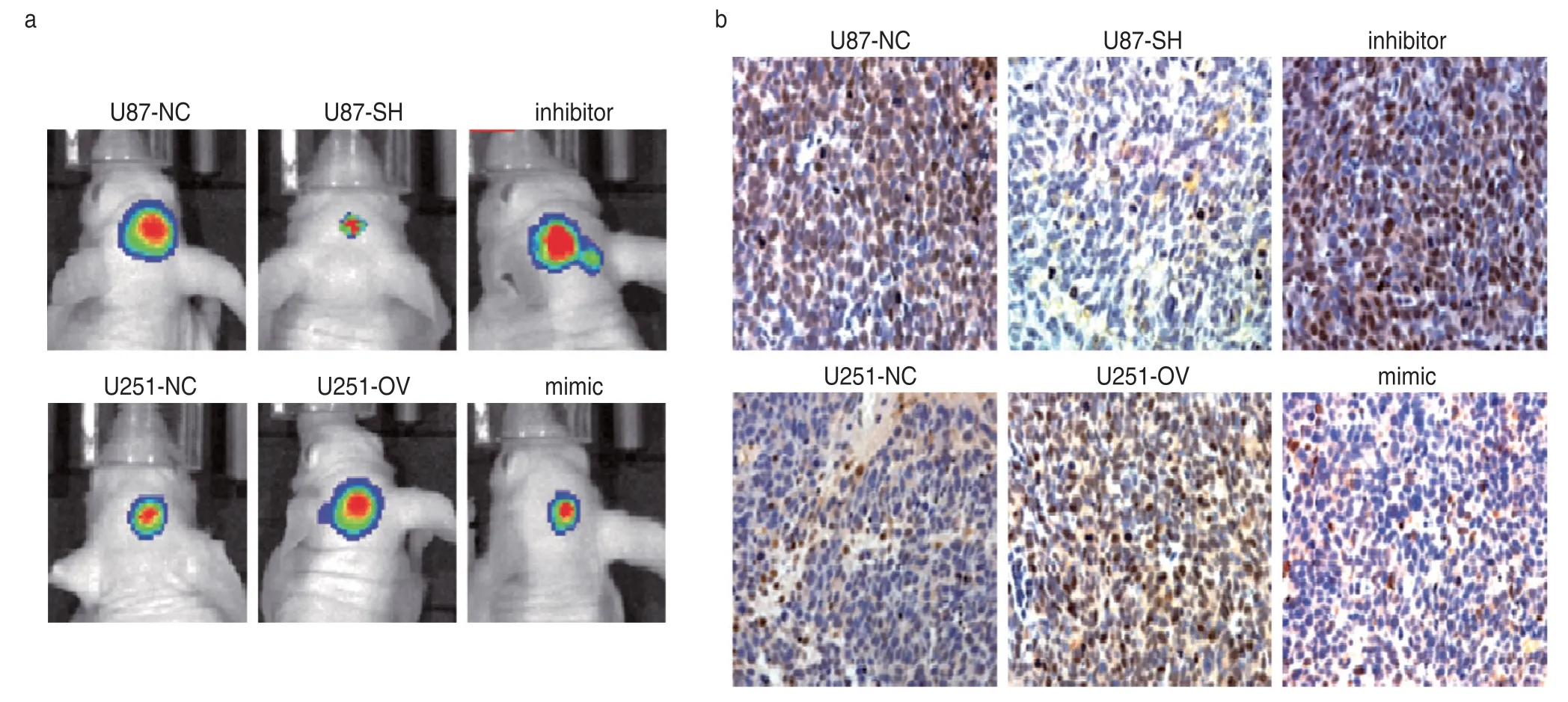
Fig.7 lncRNA PTV1 targeting miR-203a upregulates SP2 expression in transplanted tumor.(a) Tumorigenesis of mice treated with miR-203a;(b)Immunohistochemical detection of SP2 expression in mice tumor
Recent studies have shown that lncRNAs can regulate the expression of downstream genes at the posttranscriptional level,thereby affecting tumor progression.In order to improve the rate of early diagnosis in glioma patients and the efficacy of targeted therapy,more biomarkers and therapeutic targets are urgently needed.lncRNA PTV1 is the first lncRNA reported to be related to tumors.It is a proto-oncogene and can stabilize the protooncoprotein myc,which also plays a role in promoting cancer[11].In this study,by detecting the expression of PTV1 lncRNA in gliomas and normal brain tissues,we found that the expression of PTV1 in gliomas was significantly higher,and that the expression of PTV1 in glioblastomas was higher than that in low-grade gliomas.This indicates that PTV1 also plays an oncogenic role in glioma.In order to explore the function of PTV1 lncRNA in glioma cells,we performed a series of experiments to detect the effect of lncRNA PTV1 on the proliferation,migration,and invasion of glioma cells.The results showed that PTV1 lncRNA promoted the proliferation,migration,and invasion of glioma cells.However,the specific mechanism by which PTV1 lncRNA contributes to glioma development is not clear.lncRNA can target miRNAs to regulate mRNA expression.For example,it has been found that hcg11 lncRNA can target miR-421,which inhibits the proliferation,invasion and migration of cervical cancer cells,and induces their apoptosis by regulating the expression of matrix metalloproteinase-2(MMP2) and E-cadherin[12].
Previous studies have shown that miR-203a can directly target fatty acid transporter 4 (FABP4) to inhibit the proliferation and invasion of lung cancer cells[13].Through TargetScan,we found that SP2 may also be a downstream target of miR-203a.Therefore,we speculated that PTV1 lncRNA might act by sequestering miRNA-203a to regulate the proliferation and invasion of SP2-regulated glioma cells.In order to test this hypothesis,we have verified the relationship between PTV1 lncRNA and miR-203a by RT-PCR and luciferase reporter experiments.The results show that PTV1 lncRNA targets miR-375 and competitively modulates the expression of SP2.In vivoexperiments in mice revealed that PTV1 lncRNA can promote the growth of glioma through the miR-203a/SP1 axis.This suggests that PTV1 lncRNA can promote the proliferation and invasion of glioma cells and play the role of ceRNA by targeting miR-375 to upregulate the expression of SP2.In addition,downregulating the expression of PTV1 lncRNA can decrease SP2 expression and slow down the growth of glioma cells.Based on the findings of this study,we hypothesize that PTV1 abnormality can be used as a biomarker for identifying glioma patients with poor prognosis,and that downregulation of PTV1 lncRNA may be a new means of inhibiting the progression of glioma.
In conclusion,in vitroandin vivoexperiments show that PTV1 lncRNA can be used to interfere with miR-203a expression in order to downregulate SP2 and promote the proliferation and invasion of glioma cells.PTV1 lncRNA may be a new biomarker and therapeutic target for glioma.
Conflicts of interest
The authors indicated no potential conflicts of interest.
 Oncology and Translational Medicine2021年2期
Oncology and Translational Medicine2021年2期
- Oncology and Translational Medicine的其它文章
- A case report of iodine-125 seed placement during operation for the treatment of advanced gallbladder carcinoma with septic shock*
- Analysis of the adverse reactions of atezolizumab:A real-world study based on FAERS database
- Analyses of the clinical characteristics of 49 cases of malignancy with multiple bone lesions as the first manifestation
- Application of endoscopic nasobiliary cutting in the treatment of hilar cholangiocarcinoma
- KIF15 expression characteristics:Relevance toneo-adjuvant chemotherapy efficacy in breast cancer*
- Study on the antitumor effects of autologous and allogeneic CIK cells in patients with breast cancer*
