集约化奶牛场沼液沼灌区类固醇雌激素定量分析
余薇薇,孙尉哲,杜邦昊,杨 威,蒋 晖,唐川东,杨 硕,谢明原,唐竟竞
集约化奶牛场沼液沼灌区类固醇雌激素定量分析
余薇薇1,孙尉哲1,杜邦昊1,杨 威1,蒋 晖1,唐川东2,杨 硕1,谢明原1,唐竟竞1
(1. 重庆交通大学河海学院水利水运工程教育部重点实验室,重庆 400074; 2. 中国城市规划设计研究院西部分院,重庆 401121)
随着集约化奶牛场的发展,其产生的类固醇雌激素已成为新的环境污染源,因此建立沼液及土壤复杂组分中类固醇雌激素定量分析方法,考察不同季节类固醇雌激素含量变化十分必要。该研究对奶牛场沼灌区的沼液及土壤中5种类固醇雌激素(雌酮、17-雌二醇、17-雌二醇、雌三醇、雌酮硫酸钠)建立了固相萃取-液相色谱串联两级质谱(SPE-LC-MS/MS)分析方法。针对沼液及土壤复杂基质特点,分别建立二者样品前处理方法,处理后样品采用电喷雾离子源负离子模式多反应监测分析。结果表明,检测方法线性关系良好,检出限为0.09~0.39 ng/L,定量限为0.24~1.18 ng/L,回收率范围为74.56%~91.84%,该法可有效用于沼液及土壤中痕量雌激素的检测。通过对沼液、沼灌区土壤样品检测发现,雌酮、17-雌二醇与17-雌二醇检出频率及浓度较高,而雌三醇与雌酮硫酸钠检出频率及浓度较低。同时得到沼液出水及土壤纵深雌激素的含量分布,厌氧池出水中各物质浓度雌酮>17-雌二醇>17-雌二醇>雌酮硫酸钠>雌三醇;好氧池出水中17-雌二醇>17-雌二醇>雌酮>雌酮硫酸钠>雌三醇;不同纵深土壤中均为17-雌二醇>雌酮>17-雌二醇>雌酮硫酸钠,未检测出雌三醇;沼液中雌激素浓度随季节温度变化,土壤中随季节性降低。试验结果为研究复杂基质中雌激素检测和分布提供一定理论依据和数据支撑。
沼液; 土壤;液质联用;沼灌区类固醇雌激素;固相萃取;复杂基质
0 引 言
在肉类产品的巨大需求以及政府政策支持的推动下,中国畜牧业已从家庭经营的小型农场转变为大型的集约化养殖场。美国农业部预测,到2025年,中国猪肉和牛肉的消费量将分别达到6 349和838万t[1],消费的增加进一步促进了集约化养殖场的大规模发展。然而,这种大规模发展所带来的潜在环境问题也引起了人们的关注。据报道,类固醇雌激素(Steroid Estrogens,SEs)可由包括牲畜在内的所有哺乳动物排泄,并以40~7 000 ng/L的浓度存在于养殖粪肥和废水中,这些浓度比城市污水(1~80 ng/L)高出100倍,比野生动物内分泌系统紊乱的建议浓度高出1 000倍[2]。中国每年产生的养殖畜牧粪便更是达到40亿t[3],动物粪便中因其含有大量的有机质,常常被用作农业肥料土壤灌溉[4],其中携带的SEs会随之进入到土壤环境中,并不断迁移积累,对周围生态环境造成潜在风险,而现阶段对沼液及沼液灌溉区域土壤中雌激素含量分布尚不明确。因此,建立快速、灵敏、可靠的检测方法对沼液及沼液灌溉的土壤中雌激素含量进行测定很有必要。
在现有测定雌激素的方法中,色谱-质谱联用法(Mass Spectrum, MS)包括气相色谱-质谱联用法(GC(Gas Chromatography)-MS)和液相色谱-质谱联用法(LC (Liquid Chromatography)-MS),因其高灵敏度和高选择性而成为应用最广泛的方法[5-6]。但由于雌激素的低挥发性限制了GC-MS方法的应用,需要复杂的衍生化过程,相比之下LC-MS方法更加灵活和灵敏[7-10]。近年来液相色谱串联两级质谱法(LC-MS/MS)联用技术日趋成熟,该法比LC-MS定性更可靠,定量更灵敏准确。然而,在通过LC-MS/MS进行复杂样品的定量分析时,其准确性经常受到样品基质的影响,且沼液和土壤中含有大量杂质,包括各类氨基酸、维生素、蛋白质以及各种微量元素,均可能会抑制或增强分析物的信号强度。为了减小误差,样品的前处理非常关键,固相萃取(Solid Phase Extraction,SPE)选择性好,富集系数高,是最有效的样品净化和富集方法之一[11-13]。因此,通过建立类固醇雌激素的固相萃取-液相色谱串联两级质谱(SPE-LC-MS/MS)检测方法,能够消除沼液的复杂成分及土壤基质杂质对SEs检测形成的干扰,实现对SEs的定量检测。
当前对雌激素浓度检测的研究多数集中于污水处理厂、自然水体中[14-16],而对浓度较高的集约化奶牛场及周边沼液灌溉土壤研究较少。通过对雌激素浓度含量分布及季节性变化规律的研究,可深入了解SEs污染的环境行为。因此,对这种高风险地区雌激素浓度检测及定性分析,可为今后估算农业生态系统中SEs污染的来源和风险提供理论支撑。
1 材料与方法
1.1 材料、试剂与仪器
材料:Oasis HLB固相萃取柱(6 mL/200 mg,Waters),C18、NH2、Florisil固相萃取柱(6 mL/500 mg,Welchrom),高纯度氮气(纯度≥99.999%),有机系尼龙微孔滤膜(津腾0.8m Φ50 mm)等。
试剂:甲醇、乙腈、乙酸乙酯、正己烷等均为色谱纯,成都科隆;雌酮(E1)、雌二醇(17-E2)、雌二醇(17-E2)、雌三醇(E3)均为色谱纯,北京百灵威(J&K)科技有限公司;雌酮硫酸钠(E1-3S)色谱纯,加拿大Toronto Research Chemicals (TRC)公司;氘代雌酮(E1-d4)、氘代雌酮硫酸盐(E1-3S-d4)均为色谱纯,加拿大CDN同位素公司。
仪器:三重四级杆液质联用仪(Shimadzu LC-MS/MS-8060)、台式低速离心机(JT800)、12位固相萃取装置(Mediwax-12)、水浴氮吹仪(MTN-2800W-12)、涡旋混匀器(VORTEX-5)、纯水仪(摩尔 Millipore)、电热鼓风恒温干燥箱(101A-6)、无油隔膜真空泵(津腾AP-9901S)。
1.2 研究区域概况
样品采集地点位于中国重庆市某集约化奶牛场(N29°19′16″,E106°38′37″),该养殖场占地约200 hm2,主要以饲养奶牛为主,约3 000头。畜禽粪便及尿液主要采用全混合厌氧反应器(Continuous Stirred Tank Reactor,CSTR)工艺进行前处理,部分沼液用于土壤灌溉,部分沼液后续通过好氧池处理排放,日处理能力约480 m3。样品采集时间为6月至12月,采集沼液样品包括厌氧池出水和好氧池出水,选择棕色样品瓶(1 L)收集沼液,同时加入硝酸铜和盐酸抑制微生物;采集土壤样品选自周边沼液灌溉区中性紫色土,沼液还田量为1 094 m3/hm2,还田时长15 d。采集0~2 cm表层土壤和5~10 cm中层土壤,土壤样品放入铝箔袋内避光保存。
1.3 沼液及土壤样品前处理
1)沼液样品处理:采用SPE法进行富集和净化,取100 mL水样经慢速定性滤纸过滤后,用0.8m有机尼龙滤膜进行抽滤,滤液保存在棕色玻璃瓶中,同时加入10 ng/mL的E1-d4和E1-3S-d4内标溶液。用甲醇(5 mL)、乙酸乙酯(5 mL)、超纯水(5 mL)活化Oasis HLB萃取柱后对滤液进行富集,控制流速为2~3 mL/min。连接Oasis HLB柱和NH2柱,NH2柱活化选择甲醇和超纯水溶液,洗脱液选择甲醇/乙酸乙酯(体积比:1/1,5 mL)和5%氨水甲醇溶液(5 mL)在1 mL/min流速下进行洗脱,洗脱液在氮气下吹干并用甲醇重溶解,涡旋混匀。重溶解液利用Florisil柱进行二次洗脱,Florisil萃取柱选择正己烷(5 mL)、正己烷/二氯甲烷(体积比:3/1,5 mL)活化,利用正己烷进行洗脱,氮吹、重溶解、涡旋混匀,1 mL甲醇涡旋混匀,富集倍数为100倍,0.22m有机系尼龙滤膜过滤,4 ℃保存待测。
2)土壤样品处理:采用振荡提取与SPE进行前处理,风干土壤样品研磨后过0.125 mm(120目)筛,称取1.0 g于聚丙烯离心管,选择乙腈/乙酸乙酯(体积比:1/1,5 mL)和正己烷/乙酸乙酯(体积比:1/1,5 mL)作为溶解液,振荡混匀后4 000 r/min离心30 min,上清液进行氮气吹干,并用甲醇重溶解,涡旋混匀,加入10 ng/mL的E1-d4和E1-3S-d4内标溶液。选择NH2柱进行洗脱,甲醇、超纯水作为活化液,甲醇/乙酸乙酯(体积比:1/1,3 mL)和5%氨水甲醇溶液(5 mL)作为洗脱液。洗脱后在氮气下吹干,甲醇重溶解,涡旋混匀,0.22m有机系尼龙滤膜过滤,4 ℃保存待测。
1.4 LC-MS/MS工作条件
色谱条件:进样量为1.0L;柱温:30 ℃;驻留时间20 ms;流动相A为0.1%氨水,流动相B为乙腈;洗脱梯度为0~2.00 min,流动相B体积分数50%;2.00~3.00 min,流动相B体积分数90%;3.00~3.74 min保持流动相比例;3.74~4.19 min,流动相B体积分数10%;4.19~6.00 min保持流动相比例;流速为0.3 mL/min,保留时间为6 min。
质谱条件:离子源模式:电喷雾离子(ESI-);扫描范围:50~600 m/z;雾化气流量:3 L/min;加热气流量:10 L/min;脱溶剂温度:250 ℃;接口温度:300 ℃;加热块温度:400 ℃;干燥气流量:10 L/min;扫描方式:多反应监测(Multiple Reaction Monitoring,MRM)。
2 结果与分析
2.1 微生物抑制剂的选择
环境样品中含有的微生物可对SEs产生一定的降解作用,会对检测结果造成一定误差,特别是针对痕量检测结果[17],因此选择合适的微生物抑制剂可使检测结果更接近真实值。常用的微生物抑制剂有叠氮化钠和盐酸酸化[18-19],但叠氮化钠具有剧毒,一些雌激素降解菌和酶具有广泛的pH适应性[20],均会在取样过程中产生一定的误差。最终选择硝酸铜(0.25 g/L)和盐酸(4 mol/L)联合使用作为微生物抑制剂,可使SEs的损失量小于3%[21],并在低温环境下保存。
2.2 色谱、质谱条件优化
采用乙腈和甲醇作为流动相B的影响,发现乙腈会大大降低系统压力并加快分析物的洗脱速度,因此被选作流动相B。流动相中的添加剂会影响色谱保留时间和信号响应。为了最大限度地提高灵敏度,在具有不同特性的若干常见的添加剂(甲酸、乙酸铵、甲酸铵、氢氧化铵)进行了选择,使用0.1%的氨水溶液可以发现最佳的信号强度,提高化合物的电离强度获得较高的响应值,这与赵超群[22]研究一致,因此选用0.1%氨水-乙腈作为流动相。
在LC-MS/MS电离方式中,用于雌激素分析的负模式ESI已成为电离的首选模式[23],因此,在负离子模式下,对目标物进行全Q1 MS扫描以确定母离子,对母离子进行二次扫描以确定定量子离子和定性子离子,然后选择MRM模式对簇去电压、碰撞能量等质谱参数进行优化。优化条件下目标物的MRM色谱图如图1所示。
2.3 前处理条件优化
为了获得检测样品中痕量雌激素所需的灵敏度和选择性,需要对前处理条件进行优化,优化的参数为萃取柱类型和洗脱溶剂。萃取柱通常有C18柱和Oasis HLB柱,Oasis HLB柱能吸附疏水性强的化合物也能吸附亲水性强的化合物,比C18反向键和硅胶填料更大的容量和吸附能力[24],因此选用Oasis HLB柱作为萃取柱。考虑到样品基质成分较复杂,采用分步净化的方式,分别采用NH2柱和Florisil柱净化2次,雌激素回收率更高。萃取柱根据洗脱强度,沼液样品选择正己烷进行洗脱,土壤样品选择甲醇/乙酸乙酯和5%氨水甲醇溶液进行洗脱。
2.4 方法评价
2.4.1 方法线性范围和检出限
用乙腈配置一定浓度范围的雌激素标准工作液,按照1.4分析条件进行测定,以待测物质的峰面积为纵坐标,雌激素质量浓度(样品溶液:1、2、5、10、20、50、100、200g/L;内标溶液:0.1、0.2、0.5、1、2、5、10、20g/L)为横坐标,进行线性回归分析,分别以3倍、10倍信噪比计算得到方法检出限(Limit of Detection,LOD)和定量限(Limit of Quantitation,LOQ),如表1所示。结果表明峰面积与浓度线性关系高,相关系数2>0.999,检出限在0.09~0.39 ng/L之间,定量限在0.24~1.18 ng/L之间。
2.4.2 回收率
试验中选择E1-d4作为E1、17-E2、17-E2和E3的内标溶液,E1-3S-d4作为E1-3S的内标溶液,进行加标回收率试验,结果表明沼液中平均加标回收率在74.56%~91.84%之间,土壤中平均加标回收率在81.37%~82.13%;沼液中相对标准偏差(Relative Standard Deviation,RDS)为16.94%~17.59%,土壤中RDS为13.23%~14.85%。证明该方法具有良好的准确性和精密度。
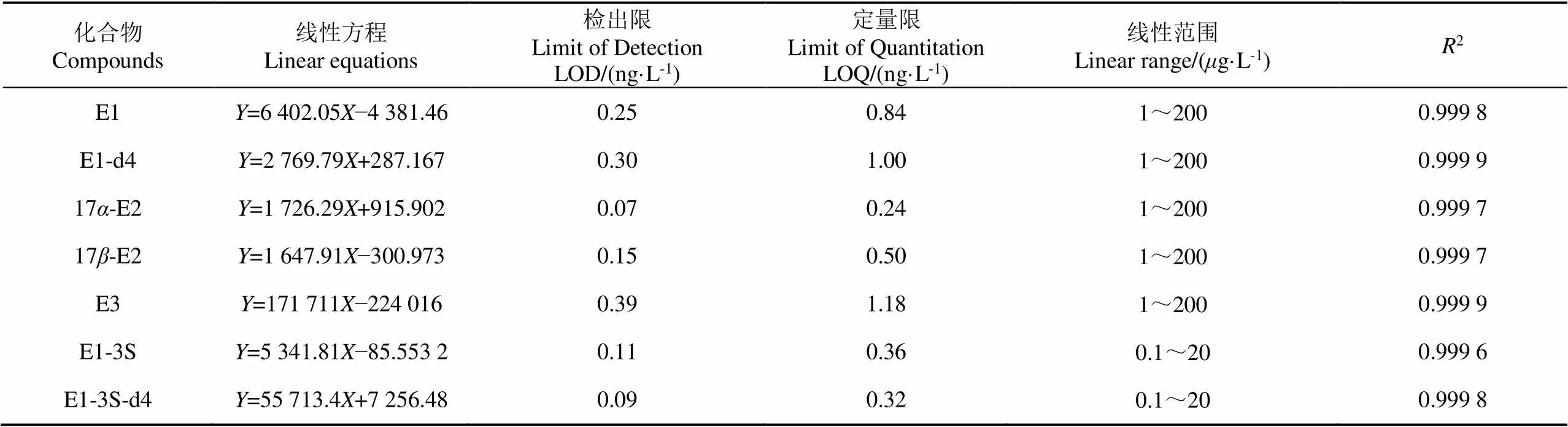
表1 SEs的线性方程、检出限、定量限、线性范围及相关系数
2.5 样品分析
2.5.1 厌氧池出水SEs检测
通过对厌氧池出水中SEs含量进行检测,如表2、图2所示。结果表明畜禽粪便及沼液经过厌氧处理后,5种雌激素仍然存在,其中E1和17-E2的检出频率均为100%,E1检出浓度最大,达709.06 ng/L,且相对丰度均值大于50%,17-E2和17-E2浓度次之,分别为260.31 ng/L和304.92 ng/L,这主要是因为E1、17-E2和17-E2占了奶牛排出的SEs的90%以上[25]。与厌氧池进水中未经厌氧处理的原污水相比,原污水中SEs浓度大小表现为17-E2>E1>17-E2>E3>E1-3S,厌氧反应过后17-E2和17-E2浓度明显降低,使E1在厌氧池出水中浓度最大,相应的E1的相对丰度均值也从40.15%增加至61.20%,原因是厌氧条件下,17-E2和17-E2易于向E1转化[26]。E3和E1-3S浓度均较低,为16.31 ng/L和24.06 ng/L,检出频率也较低,且秋季和冬季均未检测出E3,冬季未检测出E1-3S,是因为E1、17-E2和17-E2是奶牛主要排放SEs之一,而E3主要来自猪和鸡分泌的SEs中[27],E1-3S中的硫酸酯基又很容易被芳基硫酸酯酶分解转化为E1。从夏季到冬季,E1和17-E2的平均浓度有所上升,可能是由于气温较低,厌氧菌活性下降,对雌激素的降解作用减弱。而17-E2的浓度呈下降的趋势,说明在厌氧条件下,17-E2更容易被转化。
2.5.2 好氧池出水SEs检测
图3显示了好氧池出水中SEs浓度变化,畜禽沼液通过好氧处理后,5种雌激素仍然能全部检出,雌激素浓度有所降低,但最大仍能检测到600 ng/L左右的17-E2,若直接排入自然水体,仍然会对人类及动物产生潜在威胁。5种目标SEs平均浓度大小依次为17-E2>17-E2> E1>E1-3S>E3,与厌氧池出水浓度相比,E1浓度降低到80.093 ng/L,表明好氧处理可以有效降低E1的浓度。表3显示检出17-E2浓度最高,其相对丰度均值与好氧池相比增加47.10%,E1和17-E2相对丰度均值均有所降低,E3相对丰度均值增加,可能是由于在好氧条件下E1易于向17-E2和E3转化,17-E2易于向17-E2转化[28-29]。好氧条件下,好氧菌群对硫酸酯基的水解作用增强,E1-3S的去除率增加。冬季均未检测出17-E2和E3,夏季和冬季未检测出E1-3S。除17-E2外,其余SEs在好氧池中浓度均存在高-低-高的季节性变化,原因是秋季温度适宜,SEs降解菌群活性高,而17-E2存在低-高-低的季节性变化,是因为SEs相互转化的结果,也说明传统好氧处理难以有效降解17-E2。
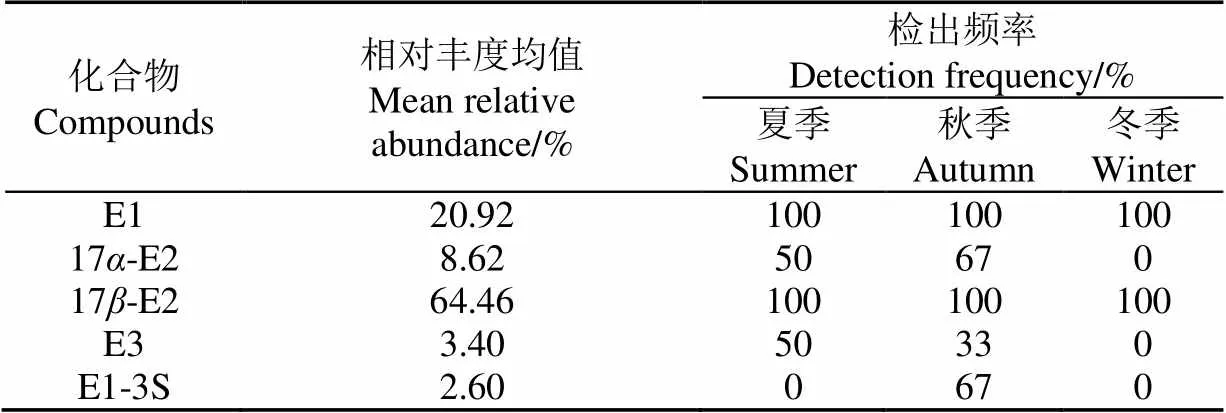
表3 好氧池出水SEs相对丰度均值及检出频率
2.5.3 表层土壤SEs检测
表层土壤样品检测结果如表4、图4所示,结果表明经厌氧池出水灌溉后的土壤中SEs浓度大小表现与沼液有所不同,这是由于SEs运输、转化和降解的协同作用的结果。几种SEs浓度大小表现为17-E2>E1>17-E2> E1-3S,未检测出E3,说明土壤对SEs存在吸附作用。其中17-E2和E1检出最大浓度均大于10 ng/g,两者相对丰度均值之和接近90%,检出频率均为100%,表明土壤中主要存在于17-E2和E1,而17-E2的检出频率和平均浓度较低,说明土壤对17-E2的吸附作用较弱,在土壤中的迁移能力更强。在夏季检出各雌激素浓度均较高,逐渐随季节性雌激素浓度降低,原因是夏季气温较高,农作物生长旺盛,沼液浇灌次数增多,而到冬季气温降低,沼液浇灌次数减少。且夏季到秋季雌激素浓度落差较大,由于夏季的雨水较多,径流作用下表层土壤中雌激素易于流失或纵向迁移。
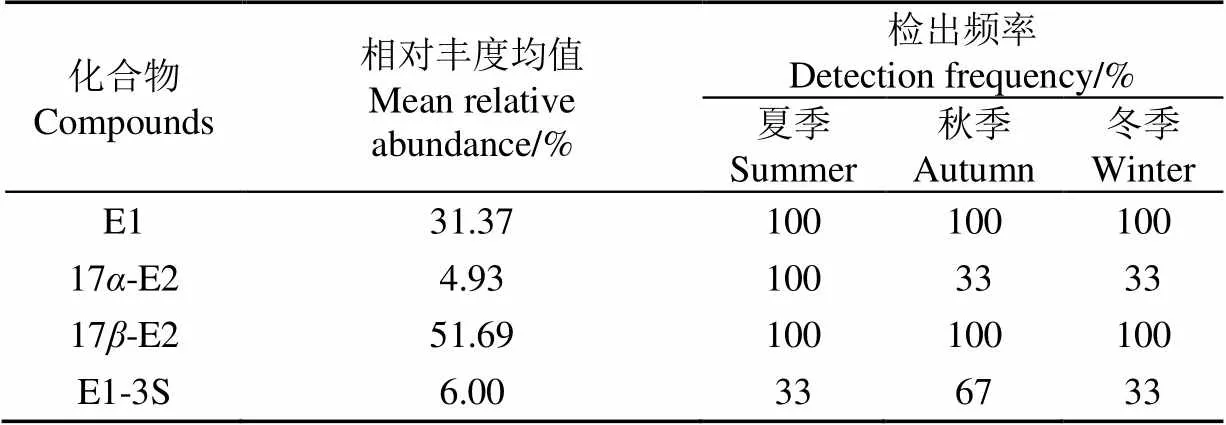
表4 表层土壤SEs相对丰度均值及检出频率
2.5.4 底层土壤SEs检测
在5~10 cm土壤中仍然能够检测出一定浓度的SEs,几种SEs浓度大小表现为17-E2>E1>17-E2>E1-3S,与表层土壤雌激素浓度大小表现相同,原因是雌激素会随降雨淋溶[30-31],仍未检测出E3,说明E3在土壤中易于转化或易于被微生物分解。E1和17-E2检出频率与表层土壤表现相同,而17-E2和E1-3S检出频率和相对丰度均值与表层土壤相比有所增加,表明SEs可以进入土壤环境,甚至渗透到地下含水层。17-E2浓度在秋季表现出反常的增加,可能是由于土壤中微生物的作用使得雌激素的相互转化的结果。底层土壤中雌激素浓度也表现出随季节性降低。
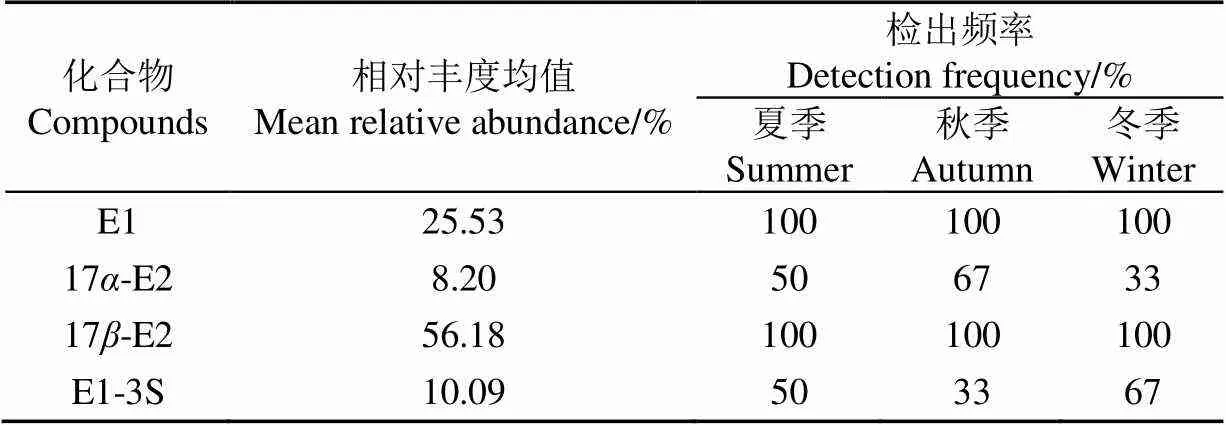
表5 底层土壤SEs相对丰度均值及检出频率
3 结 论
1)针对沼液及土壤中复杂基质的特点,分别建立了沼液及土壤的前处理方法,通过对色谱质谱条件的优化,建立了高效、简便的自由及结合态SEs的SPE-LC-MS/MS检测方法,该方法检出限为0.09~0.39 ng/L,定量限为0.24~1.18 ng/L,回收率范围为74.56%~91.84%,相关系数2>0.999。
2)通过对沼液和沼灌土壤样品进行测定,发现沼液和沼灌土壤样品中主要存在于E1、17-E2与17-E2 3种SEs且浓度较高,而E3与E1-3S检出频率及浓度均较低,厌氧处理和好氧处理均对SEs有一定去除效果,且SEs之间存在相互转化作用。厌氧池出水和好氧池出水中SEs浓度随季节性变化,与温度相关,而土壤中SEs浓度均随季节性降低。借助该方法可对沼液及沼灌区土壤中SEs进行分析检测,探究奶牛养殖场沼灌区SEs含量变化规律,为修复水体及土壤SEs污染提供理论依据和方法技术支撑。
[1] USDA.2016 international long-term projections to 2025 [EB/OL]. (2016-03-22)[2018-03-05].https://www.ers.usda.go/data-products/internatiional-baseline-data.
[2] Tremblay L A, Gadd J B, Northcott G L. Steroid estrogens and estrogenic activity are ubiquitous in dairy farm watersheds regardless of effluent management practices[J]. Agriculture, Ecosystems & Environment, 2018, 253: 48-54.
[3] 刘姝芳,李艳霞,张雪莲,等. 东北三省畜禽养殖类固醇激素排放及其潜在污染风险[J]. 环境科学,2013,34(8):3180-3187.
Liu Shufang, Li Yanxia, Zhang Xuelian, et al. Steroid hormone emissions from livestock and poultry farming in the three northeastern provinces and their potential pollution risks[J]. Environmental Science, 2013, 34(8): 3180-3187. (in Chinese with English abstract)
[4] Duan M, Gu J, Wang X, et al. Factors that affect the occurrence and distribution of antibiotic resistance genes in soils from livestock and poultry farms[J]. Ecotoxicology Environmental Safety, 2019, 180: 114-122.
[5] Vitku J, Chlupacova T, Sosvorova L, et al. Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid[J]. Talanta, 2015, 140: 62-67.
[6] Kolatorova Sosvorova L, Chlupacova T, Vitku J, et al. Determination of selected bisphenols, parabens and estrogens in human plasma using LC-MS/MS[J]. Talanta, 2017, 174: 21-28.
[7] Yuan T F, Le J, Cui Y, et al. An LC-MS/MS analysis for seven sex hormones in serum[J]. Journal of Pharmaceutical and Biomedical Analysis, 2019, 162: 34-40.
[8] Kolatorova Sosvorova L, Sarek J, Vitku J, et al. Synthesis of 3alpha-deuterated 7alpha-hydroxy-DHEA and 7-oxo-DHEA and application in LC-MS/MS plasma analysis[J]. Steroids, 2016, 112: 88-94.
[9] Vitku J, Heracek J, Sosvorova L, et al. Associations of bisphenol A and polychlorinated biphenyls with spermatogenesis and steroidogenesis in two biological fluids from men attending an infertility clinic[J]. Environment International, 2016(89/90): 166-173.
[10] Sosvorova L, Vitku J, Chlupacova T, et al. Determination of seven selected neuro- and immunomodulatory steroids in human cerebrospinal fluid and plasma using LC-MS/MS[J]. Steroids, 2015, 98: 1-8.
[11] Goh S X, Duarah A, Zhang L, et al. Online solid phase extraction with liquid chromatography-tandem mass spectrometry for determination of estrogens and glucocorticoids in water[J]. Journal of Chromatography A, 2016, 1465: 9-19.
[12] Tran N H, Hu J, Ong S L. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC-MS/MS and isotope dilution[J]. Talanta, 2013, 113: 82-92.
[13] Vulliet E, Wiest L, Baudot R, et al. Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry[J]. Journal of Chromatography A, 2008, 1210(1): 84-91.
[14] Nie M, Yan C, Dong W, et al. Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze Estuary[J]. Chemosphere, 2015, 127: 109-116.
[15] Ekpeghere K I, Sim W J, Lee H J, et al. Occurrence and distribution of carbamazepine, nicotine, estrogenic compounds, and their transformation products in wastewater from various treatment plants and the aquatic environment[J]. Science of the Total Environment, 2018, 640/641: 1015-1023.
[16] Zhou X, Lian Z, Wang J, et al. Distribution of estrogens along Licun River in Qingdao, China[J]. Procedia Environmental Sciences, 2011, 10: 1876-1880.
[17] 张肖,丁长青. 粪便类固醇激素检测准确性的影响因素[J].动物学杂志,2012,47(5):143-151.
Zhang Xiao, Ding Changqing. The influence factors on the accuracy of fecal steroid hormone detection[J]. Chinese Journal of Zoology, 2012, 47(5): 143-151. (in Chinese with English abstract)
[18] Zhang H, Shi J, Liu X, et al. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China[J]. Water Research, 2014, 58(7): 248-257.
[19] 宋晓明. 农业土壤中类固醇雌激素的潜在风险与归趋机理研究[D]. 沈阳:沈阳大学,2018.
Song Xiaoming. Study on Potential Risk and Fate and Transport Mechanism of Steroid Estrogens in Agricultural Soil[D]. Shenyang: Shenyang University, 2018. (in Chinese with English abstract)
[20] Xiong W, Yin C, Wang Y, et al. Characterization of an efficient estrogen-degrading bacterium Stenotrophomonas maltophilia SJTH1 in saline-, alkaline-, heavy metal-contained environments or solid soil and identification of four 17-estradiol-oxidizing dehydrogenases[J]. Journal of Hazardous Materials, 2020, 385: 121616.
[21] Butwell A J, Hetheridge M, James H A. Endocrine disrupting chemicals in wastewater: A review of occurrence and removal[M]. UK Water Industry Research Limited, London, 2002.
[22] 赵超群,李樱红,刘柱,等. 超高效液相色谱-串联质谱法同时测定奶粉中8种雌激素残留[J]. 药物分析杂志,2020,40(2):253-259.
Zhao Chaoqun, Li Jianghong, Liu Zhu, et al. Simultaneous determination of 8 estrogen residues in milk powder by ultra performance liquid chromatography-tandem mass spectrometry[J]. Journal of Pharmaceutical Analysis, 2020, 40(2): 253-259. (in Chinese with English abstract)
[23] Tso J, Aga D S. A systematic investigation to optimize simultaneous extraction and liquid chromatography tandem mass spectrometry analysis of estrogens and their conjugated metabolites in milk[J]. Journal of Chromatography A, 2010, 1217(29): 4784-4795.
[24] Zhu B, Ben W, Yuan X, et al. Simultaneous detection of endocrine disrupting chemicals including conjugates in municipal wastewater and sludge with enhanced sample pretreatment and UPLC-MS/MS[J]. Environmental Science: Processes & Impacts, 2015, 17(8): 1377-1385.
[25] Zhang H, Shi J, Liu X, et al. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China[J]. Water Research, 2014, 58: 248-257.
[26] Noguera-Oviedo K, Aga D S. Chemical and biological assessment of endocrine disrupting chemicals in a full scale dairy manure anaerobic digester with thermal pretreatment[J]. Science of the Total Environment, 2016, 550: 827-834.
[27] Katia, Noguera-Oviedo, Diana, et al. Chemical and biological assessment of endocrine disrupting chemicals in a full scale dairy manure anaerobic digester with thermal pretreatment[J]. Science of the Total Environment, 2016.
[28] Mashtare M L, Lee L S, Nies L F, et al. Transformation of 17-Estradiol, 17-Estradiol, and estrone in sediments under nitrate- and sulfate-reducing conditions[J]. Environmental ence & Technology, 2013, 47(13): 7178-7185.
[29] Mashtare M L, Green D A, Lee L S. Biotransformation of 17- and 17-estradiol in aerobic soils[J]. Chemosphere, 2013, 90(2): 647-652.
[30] Gall H E, Sassman S A, Lee L S, et al. Hormone Discharges from a Midwest Tile-Drained Agroecosystem Receiving Animal Wastes[J]. Environmental Science & Technology, 2011, 45(20): 8755-8764.
[31] DeLaune P B, Moore Jr P A. 17-estradiol in runoff as affected by various poultry litter application strategies[J]. Science of the Total Environment, 2013, 444: 26-31.
Detection and variation of steroidal estrogens in intensive dairy farm marsh irrigation areas
Yu Weiwei1, Sun Weizhe1, Du Banghao1, Yang Wei1, Jiang Hui1, Tang Chuandong2, Yang Shuo1, Xie Mingyuan1, Tang Jingjing1
(1.,,,400074,; 2.,401121,)
Steroidal estrogen production has become an emerging source of environmental pollution, due to the huge demand for meat products as the rapid development of intensive farms. Therefore, it is necessary to establish quantitative analysis for steroidal estrogens in complex fractions of digestate and soil, in order to explore the changes of steroidal estrogens in different seasons. In this study, a solid phase extraction-liquid chromatography tandem mass spectrometry (SPE-LC-MS/MS) was developed for the analysis of five steroidal estrogens (estrone, 17-estradiol, 17-estradiol, estriol, and sodium estrone sulfate) in digestate and soil from the methane irrigation area of dairy farms. Sample pretreatments were made on the complex matrix characteristics of methane and soil. The treated samples were analyzed by multi-response monitoring in the negative ion mode with an electrospray ionization source. The results showed that there was good linearity of the assay, where the detection limits were 0.09-0.39 ng/L, the quantification limits were 0.24-1.18 ng/L, and the recoveries ranged from 74.56% to 91.84%. This technology was effectively used for the determination of trace estrogens in methane and soil. The frequency and concentration of estrone, 17-estradiol, and 17-estradiol were higher in the methane irrigation area soil samples from dairy farms, while the frequency and concentration of estriol and sodium estrone sulfate were lower. The average concentrations of estrone and 17-estradiol increased from summer to winter, indicating a weak degradation of estrogens, due to the lower temperature and lower activity of anaerobic bacteria. The concentration of 17-E2 showed a decreasing trend, indicating that 17-E2 was more easily converted under anaerobic conditions. In the anaerobic tank effluent, the concentration of each substance was ranked in order: estrone, 17-estradiol , 17-estradiol , estrone sodium sulfate > estriol in the anaerobic pond effluent, whereas, in the aerobic pond effluent: 17-estradiol ,17-estradiol ,estrone ,estrone sodium sulfate ,estriol. Soil adsorbed estrogens and the concentration of estrogens in soil was lower than that in the digestate, as a result of the synergistic effect of transport, transformation, and degradation of soil Steroid Estrogens (SEs). The surface soil and the subsoil showed the same magnitude of estrogen concentrations, where estrogens migrated with the soil and then accumulated. 17-E2 and E1 mainly presented in the surface soil, while 17-E2 was detected less frequently at a lower mean concentration, indicating a stronger migration capacity in the soil, where the soil was less adsorbed to 17-E2. The concentration of each estrogen was detected higher in summer and gradually decreased with the seasonal change. The reason was that the higher temperature and vigorous crop growth in summer contributed to the increase in the number of methane watering, while reduced, as the temperature decreased in winter. There was a large difference in estrogen concentration from summer to autumn, where the estrogen in the surface soil was easy to lose or migrate longitudinally under the runoff in summer. In the soil at different depths, the concentration of each substance was in order of: 17-estradiol, estrone, 17-estradiol, sodium estrone sulfate, but no estriol was detected. There was a seasonal trend of decline in the concentration of estrogen in the soil. The experimental findings can provide an insightful theoretical basis and data support for the estrogen detection and distribution in complex substrates.
digestate; soil; SPE; steroidal estrogens in wetland irrigation; liquid-mass coupling; complex substrates
余薇薇,孙尉哲,杜邦昊,等. 集约化奶牛场沼液沼灌区类固醇雌激素定量分析[J]. 农业工程学报,2021,37(5):241-247.doi:10.11975/j.issn.1002-6819.2021.05.028 http://www.tcsae.org
Yu Weiwei, Sun Weizhe, Du Banghao, et al. Detection and variation of steroidal estrogens in intensive dairy farm marsh irrigation areas[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(5): 241-247. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.05.028 http://www.tcsae.org
2020-12-03
2020-02-28
国家自然科学基金项目(51608079);重庆市研究生教育教学改革研究项目(yig182028);重庆市大学生创新创业训练(S202010618007);重庆市教委科学技术研究项目(KJ1600541);重庆市基础研究与前沿探索项目(cstc2018jcyjAX0322)
余薇薇,教授,研究方向为水处理。Email:yu11237@cqjtu.edu.cn
10.11975/j.issn.1002-6819.2021.05.028
X713
A
1002-6819(2021)-05-0241-07

