花青素代谢对陆地棉叶片和纤维色泽呈现的影响
袁景丽,郑红丽,梁先利,梅俊,余东亮,孙玉强,柯丽萍
花青素代谢对陆地棉叶片和纤维色泽呈现的影响
袁景丽,郑红丽,梁先利,梅俊,余东亮,孙玉强,柯丽萍
浙江理工大学生命科学与医药学院/植物基因组与彩色纤维分子改良实验室,杭州 310018
【】棉花作为一种重要的经济作物和油料作物,其叶片和纤维均可积累色素物质,呈现不同颜色。叶绿素、类胡萝卜素和花青素的含量及其比例是棉花叶片呈色的主要原因,而棕色纤维中主要色素成分为花青素单体氧化聚合而成的原花青素及其衍生物。通过分析陆地棉不同的叶色突变体叶片和纤维中的花青素含量,花青素和原花青素合成途径中关键基因的表达,探究棉花叶片和纤维颜色呈现与花青素合成的关系,为叶色突变体的利用和彩色棉纤维色泽的改良奠定基础。通过测定21个陆地棉叶色突变体的叶片花青素含量,根据叶色突变体叶片、纤维颜色和花青素含量差异,筛选了其中6个典型的棉花叶色突变体作为研究材料,比较叶片和纤维(开花后15 d)中的花青素含量,分析花青素含量与叶片、纤维颜色呈现的关系;同时检测叶片及不同发育时期纤维(开花后5、10、15和20 d)中花青素合成关键基因和原花青素合成途径关键基因和的表达水平,分析目标基因对叶片和纤维颜色呈现的影响。21个陆地棉叶色突变体叶片中的花青素含量差异显著,呈现紫红色或紫色的叶片花青素含量高。在筛选的6个陆地棉叶色突变体及其对照叶片和不同发育时期纤维中,叶片花青素含量显著高于纤维,棕色纤维的花青素含量显著高于白色纤维。叶片中,表达量较高,而和表达量较低,花青素积累与颜色呈现与其表达量没有显著的相关性;而在纤维中,和在棕色纤维的表达量极显著高于白色纤维中,且主要集中在纤维发育的5—15 DPA高表达。陆地棉叶片和纤维的颜色呈现均与花青素含量有关,紫色及紫红色叶片以及棕色纤维中花青素含量高,但纤维颜色的形成与棉花叶片颜色呈现没有显著的相关性,其花青素含量与原花青素合成途径的关键基因和表达水平直接相关,表明棉花叶片和纤维中的呈色机制不一致,原花青素主要在纤维中积累显色。
陆地棉;叶色突变体;纤维色泽;花青素;基因表达
0 引言
【研究意义】棉花是中国重要经济作物和油料作物之一,也是人们生活必需品的重要纺织原料。因为天然彩色棉纤维腔中富含天然色素而呈现色彩,所以可以直接纺纱成衣,无需化学印染,而且制品无污染、纯天然,和皮肤亲和性好,呵护皮肤健康,符合大众对于健康、环保的要求,具有广阔的发展前景。但是彩色棉的纤维品质较差、颜色种类少(只有绿色和棕色)、色牢度不稳等因素限制了彩色棉产品的开发。为了满足消费市场需求,培育出更多色系的彩色棉显得尤为重要。因此,解析陆地棉叶片和纤维中花青素生物合成调控及呈色机理,对进一步培育出更多颜色类型彩色棉品种具有重要意义。【前人研究进展】叶绿素、类胡萝卜素和花青素广泛存在于植物的叶片、花朵、茎秆、根以及果实中[1-3],三者之间的比例和含量是植物呈现色彩的主要原因。花青素是一种水溶性色素,主要以糖苷形式存在,能形成红色和蓝色等多种颜色,在植物的生长、发育和防御中起重要作用[4-7]。花青素生物合成途径及调控网络一直是研究的重点和热点,目前,已对拟南芥()、芜菁(L.)、葡萄(L.)、菊花()和苹果(Mill)等[8-14]多种植物的花青素合成途径进行了深入研究,在不同植物中,花青素代谢基因可分为结构基因和调节基因。根据其在花青素合成途径中的位置和作用,结构基因又可分为上游合成基因(包括(phenylalanine ammonialyase)、(chalcone synthase)、(chalconesynthase)、(flavanone-3’ -hydroxylase)、(flavanone-3’5’-hydroxylase)等)和下游合成基因(包括(dihydroflavonol 4 reductase)、(anthocyanidin synthase)、(flavonoid- 3-O-glycosyltransferase)、(O-methyltransferase)等)。其中,是类黄酮合成途径中的第一个关键酶,它催化丙二酰辅酶A的3个乙酸基和对羟基苯丙烯酰辅酶A的一个乙酸基缩合生成柚苷配基查尔酮,参与植物逆境反应和植物花色等诸多过程[15-16]。位于下游的原花青素特异性途径涉及2条支路和2种酶,即无色花青素还原酶(leucoanthocyanin reductase,LAR)参与的LAR途径和花青素还原酶(anthocyanidin reductase,ANR)参与的ANR途径。最早在银叶山蚂蝗((Jacs) DC)中被克隆获得,LAR是植物类黄酮化合物合成路径中一个关键酶,它可以催化无色花青素转化成为儿茶素[17-18]。ANR负责将有色花青素还原成表儿茶素,参与调控植物组织中花青素的水平及原花青素的形成,在花青素积累过程中具有重要的调节作用[19-20]。虽然天然彩色棉纤维色素的具体组分还未明确,但LIU等[21]研究证实天然彩棉纤维色素成分与植物类黄酮合成途径密切相关,并且类黄酮生物合成途径调控棕色棉色素沉积过程。Xiao等[22]和郭帅等[23]从棕色棉中克隆了花青素合成相关结构基因,并发现基因的表达量在棕色棉纤维中明显高于白色棉纤维;Feng等[24]研究证实棕色棉中的色素类物质为原花青素,纤维颜色的呈现与原花青素的氧化聚合有关。而绿色棉纤维色素最早被认为其中含有有机酸、甾醇、香豆素和黄酮类物质,随后发现苯乙烯酸及其衍生物是绿色棉纤维产生颜色的重要因素[25-28]。【本研究切入点】花青素生物合成途径及调控因子是彩色叶片和果蔬色泽形成研究的重点和热点,在陆地棉中叶片和纤维的颜色呈现都与植物类黄酮合成途径密切相关,但棉纤维色素物质的合成代谢及其转运机理研究甚少。【拟解决的关键问题】本研究利用收集到的陆地棉21种叶色突变体及白色纤维棉和棕色纤维棉为材料,通过比较不同颜色叶片和纤维中花青素含量,分析花青素和原花青素合成通路关键酶基因、、的表达水平对叶色和纤维色泽的影响,探究陆地棉叶片和纤维呈色与花青素积累的关系及其分子调控水平的差异,为培育出更多颜色类型彩色棉品种奠定基础。
1 材料与方法
1.1 试验材料
所用20份陆地棉叶色突变体由国家棉花种质资源中期库和国家棉花种质资源平台提供(表1)。紫化突变体、C312和棕絮1号(ZX1)由浙江理工大学植物基因组与彩色纤维分子改良实验室保存。试验材料于2017—2020年种植在浙江理工大学棉花试验地(浙江杭州下沙校区),按照大田常规栽培管理。采集幼叶及5、10、15和20 DPA(day post anthesis,开花后天数)纤维,3个生物学重复,液氮速冻,-80℃保存备用。
1.2 花青素的提取
采集陆地棉叶色突变体的新生叶片和纤维(15 DPA)于液氮中速冻,放于-80℃冰箱中保存备用。采用酸化甲醇法[29]测定花青素含量。

表1 试验材料
绿/紫(红):表示夏季叶片呈绿色,秋天呈紫(红)色;黄/绿:表示苗期叶片呈黄色,后期叶片呈绿色
Green/Purple (Red): indicates leaves showed green in summer and purple (red) in autumn; Yellow/Green: indicates leaves in young seedlings showed yellow and turned green in autumn
1.3 RNA提取及cDNA的合成
采集叶色突变体幼叶及5、10、15和20 DPA(day post anthesis,开花后天数)纤维,按照多糖多酚植物RNA提取试剂盒说明书进行RNA提取,并将RNA浓度稀释到200 ng·μL-1,参照反转录试剂盒说明书反转录成cDNA,以泛素延伸蛋白7(ubiquitin extension protein 7,GhUBQ7)基因为内参基因,进行PCR扩增,检测其纯度。最终将cDNA稀释10倍后于-20℃保存。
1.4 实时荧光定量PCR
根据前期工作中在陆地棉叶片和纤维中优势表达的基因序列[30],在NCBI和COTTONGEN中搜索、和的CDS序列,用Primer Express 5.0设计目的基因、和所需引物(表2)。
以陆地棉叶色突变体幼叶、不同发育时期纤维cDNA为模板,以陆地棉泛素延伸蛋白7基因()为内参基因,利用QuantStudio 3 applied biosystem,按照Solarbio SYBR green Mix说明书进行实时荧光定量PCR扩增。反应条件为95℃2 min;95℃15 s,58℃30 s,40个循环。熔解曲线95℃15 s,60℃15 s。生物学重复和技术重复各3次。按照2-ΔΔCt方法计算相对表达量,用Graphpad Prism 5绘图。

表2 引物序列及用途
:查尔酮合成酶;:无色花青素还原酶;:花青素还原酶;:泛素延伸蛋白。下同
: Chalcone synthase;: Leucoanthocyanidin reductase;: Anthocyanidin reductase;: Ubiquitin extension protein. The same as below
2 结果
2.1 陆地棉叶色突变体叶片花青素含量分析
通过对陆地棉不同叶色突变体花青素的提取,发现提取液颜色差异显著(图1),黄色叶片的芽黄棉1号提取液呈浅黄色;红紫色叶片的红叶白絮、矮红株、红鸡脚柳苞棉、贵池红叶、以及红色叶片的送兴红叶B、红叶鸡脚、安徽红桃棉、抗红叶提取液呈现出不同深浅的红色、褐色、红褐色。陆地棉叶色突变体叶色呈红色或红紫色,其叶片花青素提取液色泽较深。
通过对陆地棉叶色突变体花青素提取液进行500—700 nm的波段扫描(图2),不同陆地棉叶色突变体花青素提取液均在530、620和650 nm处呈现吸收高峰。530 nm是花青素酸性溶液的吸收高峰,620 nm是可溶性糖的吸收高峰,650 nm是叶绿素的吸收高峰,表明不同陆地棉叶色突变体在500—700 nm所含的物质相同,但不同陆地棉叶色突变体之间的吸光值呈现差异。
通过比较不同陆地棉叶色突变体叶片的花青素含量(图3),以陆地棉C312为对照,安徽红桃棉的花青素含量略低;石河子822、锦9-70、紫花棉、小红叶等的花青素含量无显著差异;送兴红叶B、红鸡脚柳苞棉、抗红叶、红叶花苞棉、花斑叶、红鸡脚柳苞、贵池红叶、等的花青素含量均显著高于陆地棉C312。
2.2 陆地棉叶色突变体纤维花青素含量分析
为了研究花青素合成相关基因表达与花青素含量及叶片和纤维颜色呈现的关系,根据叶色突变体叶片、纤维颜色和花青素含量选取了6个突变体,包括叶片呈紫色且花青素含量较高的贵池红叶和矮红株,叶片为绿色且花青素含量与对照C312持平的红槿矮和棕色纤维的紫花棉,叶色为紫红但花青素含量较低的安徽红桃棉,及花青素大量累积的紫叶突变株,测定其纤维(15 DPA)中的花青素含量以及叶片和纤维中、和的相对表达量。6个叶色突变体的叶片和纤维颜色如图4所示。

样本编号同表1。下同 The sample ID is the same as table 1. The same as below

依据光波长530 nm处吸收峰值自上向下的顺序为:20、21、17、13、7、1、4、15、10、19、11、3、2、18、12、14、6、16、5、9、8
测定6个陆地棉叶色突变体和白絮对照C312 及棕絮对照棕絮1号(ZX1)15 DPA纤维中的花青素含量(图5),结果显示,所有材料中,叶片花青素含量在0.5—40 nmol·g-1,而纤维花青素含量只有0.1—0.8 nmol·g-1,纤维中花青素含量都明显低于叶片。叶片中,呈紫红色的贵池红叶、矮红株和中花青素含量略高,显著高于C312;纤维中,棕色纤维的紫花棉和棕絮1号的花青素含量极显著高于白色纤维的C312和其他叶色突变体;但不同叶色突变体中叶片与纤维中的花青素含量没有显著相关性,如叶片中花青素含量较高的贵池红叶,其纤维花青素含量极低,显著低于棕絮1号和紫花棉;而叶片花青素较低的紫花棉,其纤维花青素含量极显著高于C312。
2.3 陆地棉叶色突变体叶片GhCHS、GhLAR和GhANR的表达分析
通过分析3个花青素通路关键酶基因查尔酮合成酶()、花青素还原酶基因()和无色花青素还原酶基因()在6个陆地棉叶色突变体中的表达,发现3个基因的表达水平各不相同(图6)。在红槿矮和中表达水平显著高于C312;和在不同叶色突变体的叶片中表达量普遍较低,除安徽红桃棉、矮红株和贵池红叶中表达量显著低于C312外,在其他突变体中,这两个基因的表达变化不显著。而且,在各棉花叶色突变体的叶片中,的表达水平显著高于和。

显著性分析均以C312为对照,星号表示具有显著性差异(*P<0.05,**P<0.01)。下同

A:叶色突变体的叶片表型;B:叶色突变体的纤维表型
2.4 陆地棉叶色突变体不同发育时期纤维GhCHS、GhLAR和GhANR的表达分析
通过对6个陆地棉叶色突变体不同发育时期纤维中的3个基因进行表达分析,发现、和均在发育5、10和15 DPA的纤维中表达量较高,棕色纤维与白色纤维之间的表达差异较大(图7)。其中,在叶色突变体和棕絮1号5—10 DPA纤维中的表达均显著高于对照C312,在、HT和ZX1的15 DPA纤维中仍保持较高表达量;而在20 DPA左右纤维中,所有叶色突变体和对照中表达量都降至极低水平。在白色纤维的叶色突变体中,和在10 DPA纤维中的表达量均显著高于C312;棕色纤维的棕絮1号在5—15 DPA纤维中,和的表达量均显著高于C312,而紫花棉中3个基因都集中在5 DPA纤维中高表达。

A:叶色突变体的叶片花青素含量;B:叶色突变体的纤维花青素含量

图6 GhCHS、GhLAR和GhANR在陆地棉叶色突变体叶片中的表达
3 讨论
叶片是植物主要的光合作用器官,含有大量叶绿素使其呈绿色,叶片随色素种类及比例的变化而呈现不同色彩。植物中除了叶绿素外,还含有花青素、类胡萝卜素等,使植物茎、叶、花、果实及种子等组织呈现五彩缤纷的色彩[31]。自然界中常见的花青素有6种,分别为天竺葵色素(pelargonidin,Pg)、矢车菊色素(cyanidin,Cy)、飞燕草色素(delphindin,Dp)、芍药色素(peonidin,Pn)、锦葵色素(malvidin,Mv)和矮牵牛色素(petunidin,Pt)[32-33],而棕色棉纤维中主要色素成分为原矢车菊素和原飞燕草色素[24]。为了探究花青素代谢与陆地棉叶色突变体表型之间的关系,本研究提取21种陆地棉叶色突变体幼叶花青素进行分析。不同陆地棉叶色突变体的花青素提取液呈现出色泽差异。黄色叶片的芽黄棉1号花青素提取液色泽呈现黄色,紫色或红色叶色的陆地棉叶色突变体叶片花青素提取液色泽呈现深红色、红褐色,表明花青素提取液色泽与叶片颜色基本一致。测定叶片花青素含量,发现叶片为紫色或红色的陆地棉叶色突变体花青素含量高,与吴华玲等发现茶树芽叶花青素含量较高时能使茶树嫩叶呈现紫色、红色或紫红色结果相一致[34]。叶色突变体中,花青素含量差异较大,除斑叶棉、安徽红桃棉、锦9-70、石河子822、芽黄棉1号外,其他突变体中花青素含量都显著高于绿叶的陆地棉C312。其中,芽黄棉1号叶绿素和花青素含量都较低,其叶片颜色应该是由类胡萝卜素呈色所致。矮红株、红叶鸡脚、红槿矮夏叶呈绿色,秋叶呈紫色,可能是由于光照、温度等刺激花青素合成,也有可能是秋季叶绿素开始降解,导致植物内色素比例发生改变[35]。
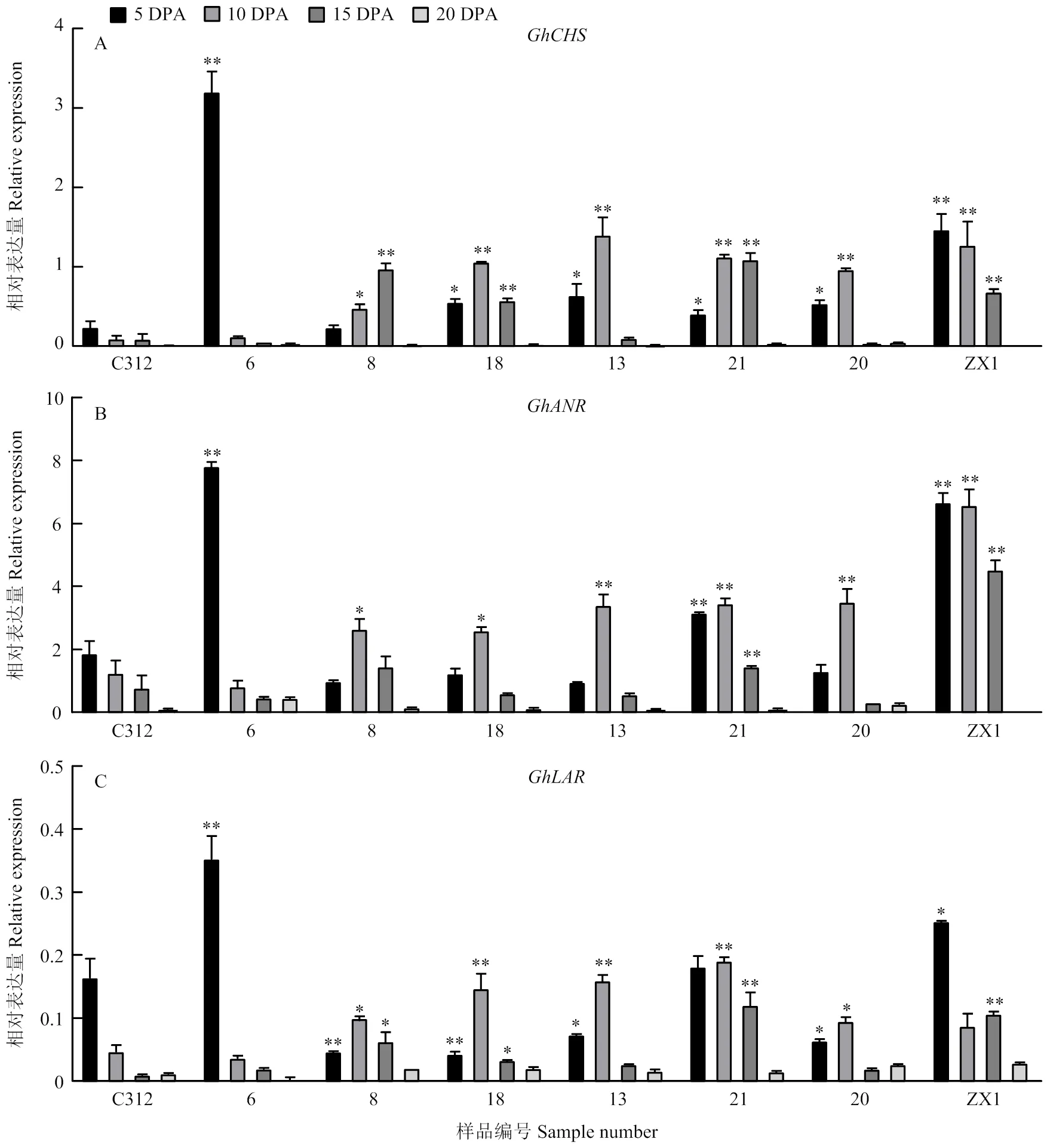
图7 不同陆地棉叶色突变体不同发育时期纤维中GhCHS、GhLAR和GhANR的表达
通过对6个突变体15 DPA纤维的花青素含量分析显示,纤维中花青素含量显著低于叶片中;白色纤维中虽然没有颜色呈现,但仍然能检测到花青素存在,只是含量显著低于棕色纤维,与Feng等[36]研究棕色棉纤维中柑橘素、槲皮素、山奈酚和杨梅素4种黄酮类化合物的积累量明显高于白色棉纤维中这一结果相一致。
为了进一步研究花青素代谢对陆地棉叶片和纤维呈色的影响,本研究分析了6个陆地棉叶色突变体中、和在叶片和纤维中的表达变化。在叶片中,表达量较高,而和在叶片中表达量较低,花青素积累与颜色呈现与其表达量没有显著的相关性。而在纤维中,和在棕色纤维的表达量极显著高于白色纤维中,且主要集中在纤维发育的5—15 DPA高表达,表明二者可以促进纤维花青素的合成,且纤维发育早期是色泽形成的重要时期。前期对、和的病毒诱导的基因沉默(virus induced gene silencing,VIGS)干涉,已经证实3个基因表达量的降低可以不同程度地使棕色棉纤维色泽变浅[30],本研究进一步证实,、和主要参与纤维颜色的形成,对叶片花青素含量和叶片颜色呈现影响不大,由此推测叶片颜色和纤维色泽的形成之间没有显著的相关性。
4 结论
陆地棉叶片和纤维中颜色呈现都与花青素含量相关,紫色及紫红色叶片以及棕色纤维中花青素含量高,但纤维颜色的形成与棉花叶片颜色呈现没有显著的相关性,其花青素含量与原花青素合成途径的关键基因和表达水平直接相关,表明棉花叶片和纤维中的呈色机制不完全一致,原花青素主要在纤维中积累显色。
[1] Tian J, Shen H, Zhang J, Song T, Yao Y. Characteristics of chalcone synthase promoters from different leaf-color malus crabapple cultivars. Scientia Horticulturae, 2011, 129: 449-458.
[2] Lin Q H, Zhong Q Z, Zhang Z H. Comparative transcriptome analysis of genes involved in anthocyanin biosynthesis in the pink-white and red fruits of Chinese bayberry (). Scientia Horticulturae, 2019, 250: 278-286.
[3] Bradshaw H D, Schemske D W. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature, 2003, 426(6963): 176-178.
[4] Silva V O, Freitas A A, Ma A, Quina F H. Chemistry and photochemistry of natural plant pigments: The anthocyanins. Journal of Physical Organic Chemistry, 2016, 29: 594-599.
[5] Bueno J M, Saez-Plaza P, Ramos-Escudero F, Muñoz A M, Navas M J, Asuero G A. Analysis and antioxidant capacity of anthocyanin pigments. Part I: General considerations concerning polyphenols and flavonoids. Critical Reviews in Analytical Chemistry, 2012, 42: 126-151.
[6] Heldt H W, Piechulla B. Phenylpropanoids comprise a multitude of plant secondary metabolites and cell wall components// Plant Biochemistry. 4th ed. ELSEVIER: Academic Press, 2011: 431-449.
[7] 彭祖茂, 邓梦雅, 严虞虞, 朱丽, 张协光. 植物中花青素含量测定及种类分布研究. 食品研究与开发, 2018, 39(17): 100-104.
Peng Z M, Deng M Y, Yan Y Y, Zhu L, Zhang X G. Study on the determination of anthocyanin content and its species distribution in plants. Journal of food research and development,2018, 39(17): 100-104. (in Chinese)
[8] Ahmed N U, Park J I, Jung H J, Hur Y, Nou I S. Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in. Functional & Integrative Genomics, 2015, 15: 383-394.
[9] Ahmed N U, Park J I, Jung H J, Yang T J, Hur Y, Nou I S. Characterization of dihydro flavonol 4-reductase () genes and their association with cold and freezing stress in. Gene, 2014, 550(1): 46-55.
[10] Azuma A, Yakushiji H, Koshita Y, Kobayashi S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta, 2012, 236: 1067-1080.
[11] Li Z, Wang J, Zhang X, Xu L. Comparative transcriptome analysis of“Albama” and its anthocyanin-loss mutant. PLoS One, 2015, 10(3): e0119027-1-e0119027-20.
[12] Hong Y, Tang X, Huang H, Zhang Y, Dai S. Transcriptomic analyses reveal species-specific light-induced anthocyanin biosynthesis in. BMC Genomics, 2015, 16: 202.
[13] 韩科厅, 赵莉, 唐杏姣,胡可, 戴思兰. 菊花花青素苷合成关键基因表达与花色表型的关系. 园艺学报, 2012, 39(3): 516-524.
Han K T, Zhao L, Tang X J, Hu K, Dai S L. The relationship between the expression of key genes in anthocyanin biosynthesis and the color of. Acta Horticulturae Sinica, 2012, 39(3): 516-524. (in Chinese)
[14] Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiology, 2007, 48(7): 958-970.
[15] Burbulis I E, Winkel-Shirley B. Interactions among enzymes of theflavonoid biosynthetic pathway. Poceedings of the National Academy of Sciences of the United States of America, 1999, 96(22): 12929-12934.
[16] Koes R E, Quattrocchio F, Mol J N. The flavonoid biosynthetic pathway in plants: Function and evolution. Bioessays, 1994, 16(2): 123-132.
[17] Devic M, Guilleminot J, Debeaujon I, Bechtold N, Bensaude E, Koornneef M, Pelletier G, Delseny M. Thegene encodes a‐like protein and is a marker of early seed coat development. The Plant Journal, 1999, 19(4): 387-398.
[18] Furukawa T, Eshima A, Kouya M, Takio S, Takano H, Ono K. Coordinate expression of genes involved in catechin biosynthesis incells. Plant Cell Reports, 2002, 21(4): 385-389.
[19] Xie D Y, Sharma S B, Dixon R A. Anthocyanidin reductases fromand. Archives of Biochemistry and Biophysics, 2004, 422(1): 100-102.
[20] Han Y, Vimolmangkang S, Soria-Guerra R E, Korban S. Introduction of applegenes into tobacco inhibits expression of bothandgenes in flowersleading to loss of anthocyanin. Journal of Experimental Botany, 2012, 63(7): 2437-2447.
[21] Liu H F, Luo C, Song W, Shen H T, Li G L, He Z G, Chen W G, Cao Y Y, Huang F, Tang S W, Hong P, Zhao E F, Zhu J B, He D J, Wang S M, Huo G Y, Liu H L. Flavonoid biosynthesis controls fiber color in naturally colored cotton. PeerJ, 2018, 6(10): e4537.
[22] Xiao Y H, Zhang Z S, Yin M H, Luo M, Li X B, Hou L, Pei Y. Cotton flavonoid structural genes related to the pigmentation in brown fibers. Biochemical and biophysical research communications, 2007, 358(1): 73-78.
[23] 郭帅, 郭倩瑜, 郭红彦. 五种绿叶和彩叶树种光合色素含量的动态变化. 安徽农学通报, 2011, 17: 38-44.
Guo S, Guo Q Y, Guo H Y. Dynamic changes of photosynthetic pigment contents in five green and colored leaf trees. Anhui Agricultural Science Bulletin, 2011, 17: 38-44. (In Chinese)
[24] Feng H, Li Y, Wang S, Zhang L, Liu Y, Xue F, Sun Y, Wang Y, Sun J. Molecular analysis of proanthocyanins related to pigmentation in brown cotton fiber (L.). Journal of Experimental Botany, 2014, 65(20): 5759-5769.
[25] Yatsu L Y, Espelie K E, Kolattukudy P E. Ultrastructural and chemical evidence that the cell wall of green cotton fiber is suberized. Plant Physiology, 1983, 73(2): 521-524.
[26] Schmutz A, Jenny T, Ryser U. A caffeoyl-fatty acid-glycerol ester from wax associated with green cotton fiber suberin. Phytochemistry, 1994, 36(6): 1343-1346.
[27] Schmutz A, Buchala A J, Ryser U. Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongates. Plant Physiology, 1996, 110(2): 403-411.
[28] Feng H, Yang Y, Sun S, Li Y, Zhang L, Tian J, Zhu Q, Feng Z, Zhu H, Sun J. Molecular analysis of caffeoyl residues related to pigmentation in green cotton fibers. Journal of Experimental Botany, 2017, 68(16): 4559-4569.
[29] GIUSTI M M, WROLSTAD R E. Characterization and measurement of anthocyanins by UV-Visible spectroscopy//GIUSTI M M, WROLSTAD R E, eds., Current protocols in food analytical chemistry, John Wiley and Sons, Inc., Hoboken, 2001, F1.2.1. -F1.2.13.
[30] Gao J F, Shen L, Yuan J L, Zheng H L. Su Q S, Yang W G, Zhang L Q, Nnaemeka E V, Sun J, Ke L P, Sun Y Q. Functional analysis ofandin colored fiber formation ofL.. BMC Plant Biology, 2019, 19(1): 455.
[31] Ma D, Sun D, Wang C, Li Y, Guo T. Expression of flavonoid biosynthesis genes andaccumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiology and Biochemistry, 2014, 80: 60-66.
[32] Zhao C L, Chen Z J, Bai X S, Ding C, Long T J, Wei F G, Miao K RStructure-activity relationships of anthocyanidin glycosylationMolecular Diversity,2014, 18(3): 687-700
[33] Kong J M, Chia L S, Goh N K,Chia T F,Brouillard R. Analysis and biological activities of anthocyaninsPhytochemistry,2003, 64(5): 923-933
[34] 吴华玲, 何玉媚, 李家贤, 陈栋, 黄华林, 乔小燕, 刘军. 11个红紫芽茶树新品系的芽叶特性和生化成分研究. 植物遗传资源学报, 2012, 13(1): 42-47.
Wu H L, He Y M, Li J X, Chen D, Huang H L, Qiao X Y, Liu J. Shoot traits and biological compositions among eleven new tea germplasms with reddish violet shoots.Journal of Plant Genetic Resources, 2012, 13(1): 42-47. (in Chinese)
[35] Saure M C. External control of anthocyanin formation in apple. Scientia Horticulturae, 1990, 42(3): 181-218.
[36] Feng H, Tian X, Liu Y, Li Y, Zhang X, Jones B J, Sun Y, Sun J. Analysis of flavonoids and the flavonoid structural genes in brown fiber of upland cotton. PLoS ONE, 2013, 8(3): e58820.
Influence of anthocyanin biosynthesis on leaf and fiber color ofL.
Yuan Jingli, Zheng Hongli, Liang Xianli, Mei Jun, Yu Dongliang, Sun Yuqiang, Ke Liping
School of Life Sciences and Medicine, Zhejiang Sci-Tech University/Plant Genomics & Molecular Improvement of Colored Fiber Lab, Hangzhou 310018
【】Cotton is an important economic and oil crop. Both its leaves and fibers can accumulate pigments and present different colors. Studies have confirmed that chlorophyll, carotenoids, and anthocyanin are the main pigments in cotton leaves and their relative proportion changes leaf colors. While proanthocyanidins and their derivatives, which are oligomeric and polymeric products from anthocyanidins, are thought to be responsible for the color formation of brown fibers. This article intends to explore the relationship between the color of leaves and fibers in upland cotton through analyzing the anthocyanidin content and gene expression level in the anthocyanin biosynthesis pathway in different leaf color mutants. The result will help to lay the foundation for the utilization of leaf color mutants and the improvement of the color of colored cotton fibers.【】In this experiment, the anthocyanidin contents in leaves of 21 upland cotton leaf color mutants were detected. According to the leaf and fiber color as well as the anthocyanidin content level, 6 cotton leaf color mutants were selected as materials to measure the anthocyanidin level in leaves and fibers at 15 days post anthesis to analyze the relationship between anthocyanidin content and the leaf or fiber color. Then the expression levels of,andin leaves and fibers at different developmental stages (5, 10, 15, 20 DPA) were measured to analyze the influence of target genes on the color formation of leaves and fibers.【】The anthocyanidin content in the leaves of 21 leaf color mutants ofwas significantly different, and the purple or fuchsia leaves had higher anthocyanidin content. In the selected six leaf color mutants, the anthocyanidin content in leaves was significantly higher than that in fibers, and brown fibers accumulated more anthocyanidins than white fibers. Compared to,andexpressed at a very low level in leaves, and no significant correlation was found between leaf color and their expression level. While in fibers, the expressions ofandwere obviously higher in brown fibers than in white fibers, especially in fibers of 5 DPA to 15 DPA.【】Anthocyanins played important roles in color formation of both leaves and fibers in upland cotton. Purple-red or purple leaves and brown fibers accumulated more anthocyanidins, while the formation of fiber color did not directly correlate with leaf color. In fibers, the contents of anthocyanidins directly related to the expression levels ofand, indicating that coloration mechanism of cotton leaves and fibers was not exactly the same, and proanthocyanidins mainly accumulated in fibers.
L.; leaf color mutant; fiber color; anthocyanin; gene expression

10.3864/j.issn.0578-1752.2021.09.003
2020-10-04;
2020-12-25
国家自然科学基金(U1903204,31671738)、浙江省自然科学基金(LZ21C130004)
袁景丽,E-mail:yuanjingli2017@126.com。通信作者柯丽萍,Tel:0571-86843335;E-mail:keliping@zstu.edu.cn
(责任编辑 李莉)

