The role of radiation therapy and particle therapy in renal cell carcinoma: current evidence and future perspectives
Charlien Berghen, Maarten Albersen, Robin De Roover, Kato Rans, Benoit Beuselinck, Karel Decaestecker, Kenneth Poels, Francois-Xavier Otte, Steven Joniau, Karin Haustermans, Gert De Meerleer
1Department of Radiation Oncology, University Hospitals Leuven, Leuven 3000, Belgium.
2Department of Urology, University Hospitals Leuven, Leuven 3000, Belgium.
3Department of General Medical Oncology, University Hospitals Leuven, Leuven Cancer Institute, and Laboratory of experimental Oncology, Department of Oncology, Catholic University Leuven, Leuven 3000, Belgium.
Abstract For both primary and metastatic renal cell carcinoma (RCC), treatment with stereotactic body radiotherapy (SBRT)has found its way into clinical practice. Being a non-invasive outpatient procedure, SBRT requires only a few visits to the radiation department and may be of interest for the elderly or, in the case of primary RCC, for patients who are not considered surgical candidates due to technical limitations, medical comorbidities, or in the event that the maintenance of kidney function is compromised. In the treatment landscape of oligometastatic RCC, SBRT shows promise in eradicating metastatic disease and delaying the initiation of systemic treatment. Technical advancements in the planning and administration of radiation treatment and improvements in movement management allow irradiating the tumor and/or metastatic lesions with very high doses in few fractions while maximally sparing the surrounding organs at risk, thus minimizing toxicity. In that context, the increasing availability of particle therapy, such as proton beam radiotherapy or carbon ion radiotherapy, could further optimize the delivery of radiation treatment in order to reduce toxicity and improve outcome.
Keywords: Particle therapy, proton therapy, carbon ion radiation therapy
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 3% of all cancers worldwide and is the most common solid tumor within the kidney representing approximately 90% of all kidney malignancies[1]. There are different RCC subtypes, of which clear cell carcinoma is the most common histopathology. The majority of the lesions is diagnosed as small tumors, with a notable proportion of locally advanced disease and up to 20% of patients presenting with distant metastases at the time of diagnosis[1]. For non-metastatic renal cell carcinoma(nmRCC), surgery including partial and radical nephrectomy are considered standard of care, with radiofrequency ablation and cryoablation as alternative treatment options for selected patients with small renal masses[2]. About 20%-40% of non-metastatic patients will eventually develop metastases, for which the standard management consists of immune checkpoint inhibitors (ICI) and/or targeted therapy[2,3]. The role of conventional radiotherapy (RT) in palliation of symptoms of metastatic disease is well established.
Stereotactic body radiotherapy (SBRT), targeting oligometastatic disease as well as treating the primary tumor, both with the aim to cure, has more recently become part of the therapeutic armamentarium[3]. Due to the advancements in treatment planning and delivery techniques, and the increasing availability of particle therapy using protons or heavy ions such as carbon ions, the interest for SBRT in the field of RCC has grown substantially. In this review, we summarize the current evidence of (SB)RT as a treatment option for (m)RCC, with a focus on the advantages of particle therapy.
RADIOTHERAPY IN THE TREATMENT OF RCC
Working mechanism
Unlike healthy kidney cells, which are very sensitive to radiation, RCC has traditionally been considered radio-resistant. In addition, surrounding organs at risk such as jejunum, duodenum, and colon are also susceptible to radiation damage. Because of this presumed radio-resistance and the risk of radiationinduced toxicities, RT was considered marginal when it came to treating primary RCC[4]and/or oligometastatic disease. However, preclinical and clinical evidence has shown that RCC is sensitive to ablative radiation doses (typically > 8 Gy per fraction), resulting in tumor control rates of approximately 90%[5]. SBRT provides the method to deliver these ablative doses. In contrast to conventionally fractionated RT, which aims to cause DNA-damage, SBRT also induces endothelial damage and tumor cell killing by stimulation of the ceramide pathway[6,7]. After irradiation, hydrolyzation of sphingomyelin takes place in the cell membrane, and ceramide is generated, which is a proapoptotic messenger. In addition, SBRT has the ability to activate antitumor host immunity, which can induce the so-called abscopal effect[5,8]. First reported in 1953, this phenomenon describes the ability of irradiation to induce tumor regression at non-irradiated,distant tumor sites[9]. Although rarely observed historically, the advent of ICI reopened the research interest in this effect. Certainly the combination of SBRT and ICI is an emerging treatment option for mRCC[10,11].
Photon SBRT in primary RCC
Several recently published reviews extensively describe the role of SBRT in primary RCC[3,5,10].Unfortunately, the number of patients in prospective trials is small, and a comparison with partial or radical nephrectomy, cryotherapy, or radiofrequency ablation (RFA) is lacking. Globally, SBRT is considered a valuable alternative to surgery for elderly patients (> 70 years old), patients who are medically inoperable,and patients with pre-existing comorbidities such as arterial hypertension, diabetes mellitus, chronic kidney disease, and/or cardiovascular disease[5]. Since cryoablation and RFA both result in less local control (LC) in the case of larger tumors, and central/perihilar location could increase the risk of hemorrhage, fistula formation, and ureteral strictures, SBRT is an excellent alternative with LC rates of 97% for tumors >4 cm[12]. SBRT might be of particular interest in patients under anticoagulation.
In a total of 26 trials, 382 tumors in 372 patients were treated with SBRT, resulting in a random-effect estimate for LC of 97.0% (95%CI: 93.9%-99.5%)[5]. LC rates ranged 70%-100% in the eligible studies, with local failures corresponding to an insufficient biological dose (low-dose arm or in the case of compromise to mitigate toxicity). The most prominent toxicity was mild nausea, fatigue, or dermatitis. Grade 3-4 events ranged from 0% to 25%. The wide range should be interpreted with caution, as the number of patients in the trials was low. Most of the trials report low toxicity, while the 25% toxicity is the result of a phase 1 doseescalation trial, where 30 Gy in five fractions resulted in 3 out of 12 patients with Grade 3 fatigue (n= 2) and bone pain (n= 1)[13]. The random effect for the mean estimated glomerular filtration rate (eGFR) difference before and after SBRT was -7.7 mL/min (95%CI: -12.5 to -2.8 mL/min), with the eGFR difference ranging from -16.7 to +6.0[5]. This is consistent with the eGFR decrease after partial or radical nephrectomy of 13 and 24 mL/min, respectively, for a median follow-up of 44 and 57 months[14]. Overlaying SBRT treatment plans with functional imaging scans (51Cr-EDTA or99m-TC-DSMA SPECT-CT), Sivaet al.[15]showed that regional nephropathy and the resulting loss of function occurred clearly in high-dose regions. Fortunately,the contralateral non-irradiated kidney compensated for this loss except in patients with pre-existing nephropathy. Interestingly, there was a clear dose-related decrease in eGFR: for every 10 Gy of physical dose, eGFR decreased by 25%-39%[15]. Of note, late onset (≥ 1 year) of eGFR has been described, implicating the need for long-term follow-up and the interest of using functional imaging[5,15]. SBRT for primary RCC has thus proven to be effective and well-tolerated, and might even lead to more favorable local control rates when compared to thermal ablative treatments, certainly in case of stage Ib tumors[12].
Photon SBRT in metastatic RCC
RCC metastases are usually located in the lymph nodes, lung, liver, bone, and brain[3]. Up to 20% of patients have upfront metastatic disease, and about 20%-40% of nmRCC patients will eventually develop metastases[3]. Oligometastatic disease, the intermediate state between localized and widespread metastatic disease, typically involves 1-5 metastases. In this particular situation, metastasis-directed therapy (MDT) has evolved as a new treatment option in various tumors, with prostate cancer probably being the most studied urological tumor[16]. Both metastasectomy and SBRT are excellent options for performing MDT. Studies reporting on metastasectomy in different organs (lung, bone, brain, liver,etc.)[17-27]have shown excellent LC and improvement in overall survival (OS) (albeit retrospectively, with an important selection bias), with a five-year survival benefit of 45%[28]. SBRT induces LC rates up to 90%-98% while toxicity rates remain very low[3,8,29-40]. To the best of our knowledge, we are aware of only one (retrospective) trial comparing the two treatment modalities. In general, this trial shows that the results of SBRT are similar to those of metastasectomy[31]. Specific to SBRT, there is the theoretical possibility to interfere positively with the immune response elicited by ICI and to decrease the rate of the metastatic spread, as has been shown for other urological malignancies[11,41].
Whether SBRT will improve outcome in oligometastatic RCC patients in combination with ICI or tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor is the subject of several ongoing trials[42].
Particle therapy and RCC
Compared to photons, particle therapy has several advantages, including a favorable dose-depth profile, a higher linear energy transfer (LET), and a higher relative biological effectiveness (RBE)[43], meaning they have the potential to treat “difficult to treat” tumors, in terms of location (deep seated or critically located),radio-resistance, or a highly aggressive nature[43].
A brief overview of the physics rationale
Most particle therapy worldwide is performed with protons (PBT) or carbon ions (CIRT). Both modalities have the potential to deliver a very high radiation dose to the tumor with maximum sparing of the surrounding healthy tissues[44]. Through the radiation beam entrance path, there is only a low-dose deposition, followed by a rapid incline in energy deposition with most of the energy deposited at the end of the ionization track (i.e., the location of the tumor) and limited or no exit dose[45]. This peak of dose deposition at a specific depth is known as the Bragg peak[43]. To adequately cover the target (i.e., tumor lesion), the Bragg peak is spread out to an optimal range, called “spread-out Bragg peak”. The characteristic dose deposition at depth for photon, PBT, and CIRT is depicted in Figure 1. An example of a photon treatment plan compared to a proton treatment plan for primary RCC is presented in Figure 2.
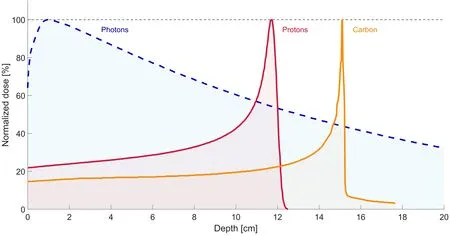
Figure 1. Dose-depth curves of (6 MV FFF) photons (blue dotted line) vs. (130 MeV) proton (red line) vs. (270 MeV) carbon ion (orange line).

Figure 2. An example of a photon treatment plan (A) vs. proton treatment plan (B) of a primary RCC in the upper pole of the right kidney.
Secondly, compared to photons, particle therapy has a higher LET, a quantification of the amount of energy transferred from the ion to the tissue. While photon therapy results in “simple DNA damage”, the “complex DNA damage” caused by the particle therapy is a clustering of multiple DNA lesions in close proximity,making DNA repair more difficult[43]. A higher LET thus correlates with a higher relative RBE and consequently produces more cell killing at equivalent doses[43]. Compared to the RBE of 1 for photon therapy, the RBEs for PBT and CIRT are considered 1.1 and 2.5-3, respectively.
Particle therapy in primary RCC
The initial results of particle therapy in primary RCC are encouraging[3]. The clinical and dosimetric comparative trials that have reported on the use of PBT or CIRT for RCC are listed in Table 1. We briefly describe the trials hereunder.
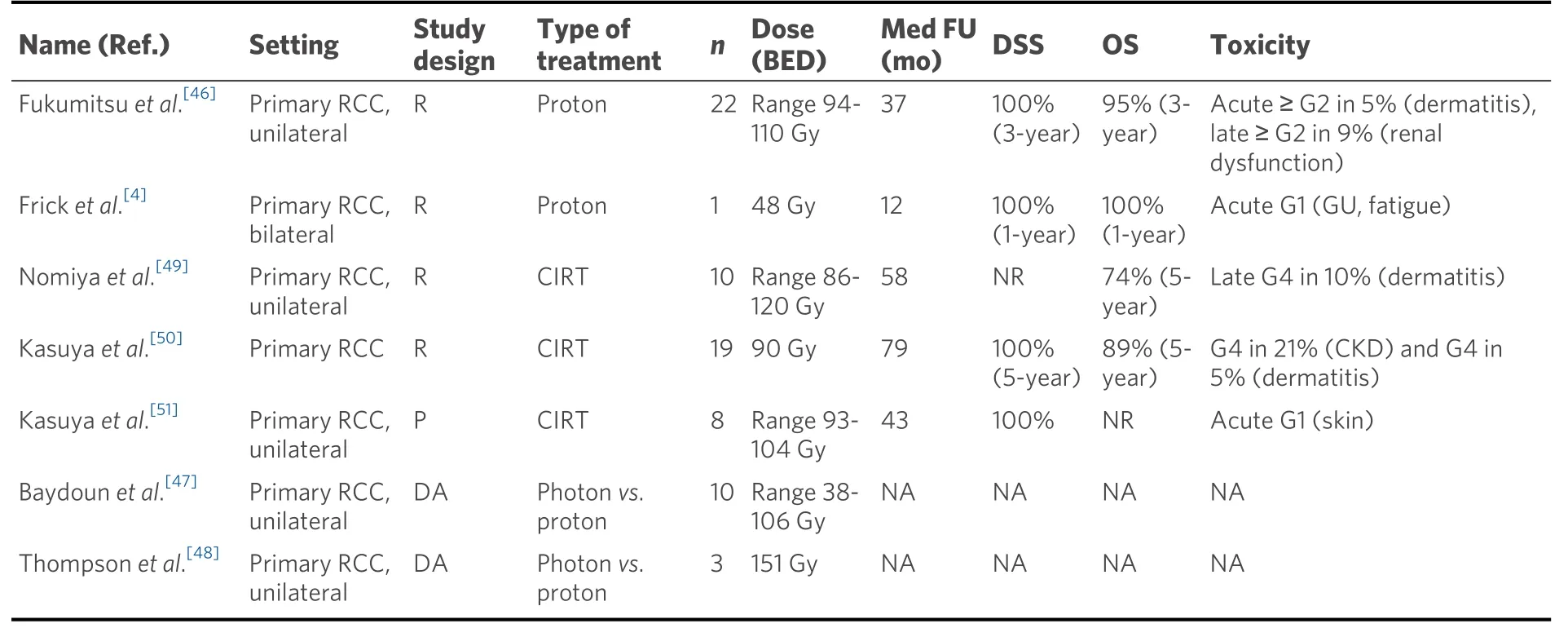
Table 1. Overview of published trials on particle therapy in RCC
In one case report, Fricket al.[4], reported on the use of proton SBRT in a 47-year-old women diagnosed with bilateral Grade 1-2 clear cell RCCs. The patient was inoperable due to multiple comorbidities including stage 2 chronic kidney disease (CKD). A total dose of 30 Gy in five fractions was administered to both lesions. She experienced acute but transient Grade 1 urinary urgency and urinary incontinence, as well as Grade 1 fatigue, which recovered to pre-treatment three months after treatment. Marginal deterioration in renal function was observed (from 34 to 29 mL/min/1.73 m2). The one-year follow-up showed stable tumor findings on the MRI. The authors concluded that proton therapy is feasible and a promising therapeutic approach that can be considered for medically inoperable patients[4].
In a multi-institutional retrospective study, Fukumitsuet al.[46]investigated the efficacy of PBT as a treatment for RCC in 22 patients. The majority of these patients had T1a tumors (77%). The total irradiation dose was 60.0-79.6 Gy (RBE) delivered in 10-36 fractions and the biological equivalent doses ranged from 94 to 110 Gy (median 105 Gy). At a median follow-up of 37 months, the three-year local control, diseasespecific survival, and overall survival rates were 100%, 100%, and 95%. One patient developed Grade 2 acute dermatitis, and two patients developed Grade 2 renal dysfunction at 9 and 28 months, respectively. The mean estimated glomerular filtration rate was reduced by 7.1 ± 11.2 mL/min/1.73 m2, which is comparable to the above-mentioned renal function decline after photon therapy.
We identified two dosimetric analyses of PBT in kidney cancer[47,48]. Baydounet al.[47]evaluated the dosimetric characteristics of Cyberknife®, Volumetric Modulated Arc Therapy (VMAT), and PBT in the stereotactic treatment of RCC. The prescribed dose was 48 Gy to be delivered in four fractions. Compared to Cyberknife®, both VMAT and PBT provided equivalent or superior coverage of the target volume while reducing treatment time per fraction and consequently also intra-fraction motion. Dose to the remaining target kidney, contralateral kidney, liver, spinal cord, and intestine was limited.
Thompsonet al.[48]investigated the nephron-sparing potential of proton SBRT for early stage RCC. In three randomly selected (non-RCC) patients previously treated for pancreatic cancer, they drew spherical contours to mimic renal tumors at four locations in the kidney, for which VMAT plans and PBT plans were generated. The dose prescription was 54 Gy in three fractions. The volume of the kidney receiving 12 Gy(V12 Gy) showed an average improvement of 13% in the case of single-beam PBT. In addition, dose to the duodenum and small bowel was significantly lower, with a mean V20 Gy of 0.39 and 2.31 cc, respectively,for proton plans, compared to 1.54 and 5.54 cc, respectively, for photon plans.
There are two retrospective trials reporting on CIRT in primary RCC. Nomiyaet al.[49]reported on 10 patients treated with CIRT for unilateral RCC. The prescribed dose was 72 GyE in 16 fractions. After a median follow-up of 58 months, the five-year LC rate, progression-free survival, DSS, and OS were 100%,100%, 100%, and 74%, respectively. No acute toxicity above Grade 1 was observed. One patient developed a skin ulcer five years after treatment, which was treated with a skin flap transplantation giving rise to this Grade 4 toxicity. In two patients with diabetic nephropathy, renal function deteriorated significantly after CIRT, but this was not the case in the other patients.
Kasuyaet al.[50]updated the results of 19 CIRT treated patients. Fifteen patients were treated with a 16-fraction scheme, of whom 10 patients received a total dose of 72 Gy (RBE) in 16 fractions. A dose-escalation to 80 Gy was performed in three patients. A total dose of 64 Gy in 16 fractions, used in case of anatomical proximity to the gastrointestinal tract, was given in the other two patients. Four patients received a prescribed fractionation schedule of 66 Gy (RBE) in 12 fractions. For a median follow-up of 6.6 years, the LC rates, DFS, DSS, and OS rates were 94%, 69%, 100%, and 89%, respectively. Seven patients presented with Grade 2 CKD, of whom four progressed to Grade 4 CKD. Notably, all four patients who deteriorated to Grade 4 CKD had definitive renal comorbidities pre-CIRT, such as diabetic nephropathy, renal sclerosis, or a solitary kidney. While caution is advised in patients with pre-existing renal comorbidities, the authors noted that progression to Grade 4 took an average of 5.6 years, so the natural course of renal disease cannot be ruled out. One patient had Grade 4 dermatitis, and one patient had a subcutaneous induration requiring painkillers. Both patients had undergone dose escalation to 80 Gy in 16 fractions.
Kasuyaet al.[51]also reported the results of a prospective clinical trial, in which eight patients were treated with CIRT for unilateral RCC. Five patients received 66 Gy in 16 fractions. Since no dose-limiting toxicity occurred, the following three patients were treated up to 72 Gy in 16 fractions. For a median follow-up of 43 months, the LC and DSS were 100%. No patient developed Grade 3 or higher acute or late toxicity. The average decrease in eGFR at the end of follow-up was 10.8 mL/min/1.73 m2.
For follow-up purposes, it should be noted that there was no volume change or even a transient enlargement during observation in the months following treatment with particle therapy. At long-term follow-up, a very gradually shrinkage pattern was observed[4,46,49]. Therefore, follow-up imaging after CIRT should be used with caution so as not to misinterpret local failure.
PBT and CIRT for metastatic RCC
Nakaoet al.[52](abstract only) reported a case of a 70-year-old woman treated with CIRT for lung and lymph node metastases five years after previous radical nephrectomy. They observed 100% LC in the irradiated lesions at the time of last follow-up. We could not find any other studies with PBT/CIRT for RCC. Planning studies have shown that SBRT plans for spinal metastases for proton and carbon ion RT were feasible[53,54].For equivalent tumoral coverage, the maximum spinal cord dose was lower for PBT/CIRT, and the treatment time was shorter[53]. Future prospective trials will need to elaborate the real benefit of PBT and/or CIRT for metastatic RCC.
Challenges and future prospectsOrgan movements (e.g., by breathing) and variability in setup (positioning of the patient) can cause uncertainties in administrating the correct dose. Knowing that motion management is already a challenge in photon SBRT for primary RCC and metastatic lesions subject to motion, this is even more so for particle therapy[55,56]due to density changes along the beam path that may cause the Bragg peak to occur at a different location than planned, and the interplay between organ motion (especially breathing motion) and beam delivery technique[57]. Range uncertainty, due to patient positioning and movement, is seen as a limiting factor. Robust optimization for treatment planning, four-dimensional planning CT (coping with breathing), and image-guided RT are essential parts of the treatment, to mitigate the potentially deteriorating impact of range uncertainty and inter- and intrafraction motion on the dose distribution[55,58].
Although many other trials are underway regarding SBRT for primary or (oligo)metastatic RCC with photon beam radiotherapy, we are not aware of any ongoing trials with PBT or CIRT for these indications.Prospective trials are badly needed. However, this type of research is limited due to the availability of particle therapy, as well as the higher treatment costs that require appropriate patient selection to ensure a cost-effective implementation of the techniques in daily practice[59].
CONCLUSION
Encouraging results are seen with both photon and particle (SB)RT for the treatment of primary RCC, but prospective trials are needed with a longer follow-up and sufficient patient numbers. PBT and/or CIRT may also be important for the treatment of metastatic lesions adjacent to critical organs. CIRT in particular shows promising results because of its advantages in dose distribution and biological effect.
DECLARATIONS
Acknowledgments
Charlien Berghen is a PhD student at KU Leuven, receiving a grant from “Kom op tegen kanker” (Stand up to Cancer). Kato Rans is a PhD student at KU Leuven, receiving a grant from “Stichting tegen Kanker”.Steven Joniau is a senior clinical researcher of the FWO (research foundation flanders). No writing assistance was utilized in the production of this manuscript.
Authors’ contribution
Literature search, content of the manuscript, data collection, data interpretation, writing of the manuscript:Berghen C
Critical review of the manuscript, advice on surgical items: Albersen M
Creation of figure 1 and 2, critical review of the manuscript, advice on technical parts of radiation therapy:De Roover R
Critical review of the manuscript: Rans K
Critical review of the manuscript, advice on medical oncology: Beuselinck B
Critical review of the manuscript, advice on surgical items: Decaestecker K
Creation figure 1, critical review of the manuscript, advice on technical parts of radiation therapy: Poels K
Critical review of the manuscript: Otte F
Critical review of the manuscript, advice on surgical items: Joniau S
Critical review of the manuscript, advice on proton beam therapy: Haustermans K
Content of the manuscript, data interpretation, writing of the manuscript: De Meerleer G
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.

