The rapid chlorophyll a fluorescence characteristics of different cotton genotypes reflect differences in leaf senescence*
XUE Huiyun, WANG Sufang, ZHANG Xin, ZHANG Zhiyong
The rapid chlorophyll a fluorescence characteristics of different cotton genotypes reflect differences in leaf senescence*
XUE Huiyun, WANG Sufang, ZHANG Xin, ZHANG Zhiyong**
(Henan Institute of Science and Technology / Henan Collaborative Innovation Center of Modern Biological Breeding / Henan Key Laboratory for Molecular Ecology and Germplasm Innovation of Cotton and Wheat, Xinxiang 453003, China)
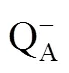
Cotton; Leaf senescence; Chlorophyll a fluorescence; Photosystem Ⅱ; Rapid chlorophyll a fluorescence parameter
Cotton (spp.) has infinite reproductive properties, and is an important cash crop. Appropriate senescence can improve fiber yield and quality of cotton by withstanding adverse weather conditions, efficiently utilizing material resources and energy during the growing season (Chen et al., 2018). Premature senescence generally leads to yield loss of about 10%, even more than 20% (Dong et al., 2005; Wright, 1999), and has been commonly in many cotton-growing countries (Dong et al., 2006; Dong et al., 2005) due to imbalance of source and sink (Wright, 1999) or poor ability to take up nutrients from soil in late season (Brouder and Cassman, 1990) and so on.
Leaf senescence can dramatically affect crop production by reducing photosynthetic capacity and influencing dry matter transfer from senescing leaves to harvestable organs (Gregersen et al., 2008; Rajcan et al., 1999; Rajcan and Tollenaar, 1999a; Rajcan and Tollenaar, 1999b). Research shows that the disassembly of the photosynthetic apparatus (PSA) within chloroplasts of plants and the electron flow that through the light reactions of photosystem Ⅱ (PSⅡ) and photosystem Ⅰ (PSⅠ) are greatly associated with the photosynthetic capacity decline (Grover, 1993; Smart, 1994; Weng et al., 2005; Wingler et al., 2004). Furthermore, it has been proved that PSⅡ is more susceptible to senescence than PSⅠ in senescent leaves (Grover et al., 1986). Kautsky and Hirsch (1931) firstly proposed the possibility using fluorescence methods for PSA analysis, because they founded there was a close relationship between chlorophyll fluorescence and primary reaction of photosynthesis.
Chlorophyll a fluorescence (Chl F) analysis is noninvasive, fast and precise, and has been widely used for assessment of the physiological state of PSA of different species under different environment conditions (Baker and Rosenqvist, 2004; DeEll et al., 1999; Wang et al., 2016). The most commonly and widely used Chl F analyses are performed by the saturation pulse method, which uses the darkness adapted leaf samples to obtain different parameters characterizing the steady-state status of the PSA (Baker, 2008; Lazár, 2015). In the last two decades, because fast Chl F rise kinetics OJIP which showed a typical O-J-I-P phase and OJIP test analysis can capture more detailed information on the structure and function of the PSA, primarily PSⅡplant, it was developed to rapidly evaluate the physiological state of PSA in a high number of field grown plants (Goltsev et al., 2016) based on the energy flux theory in photosynthetic membranes (Strasser and Strasser, 1995). The OJIP test reflects the behavior of PSⅡ function, which includes the energy flow in PSⅡ and electron transport from H2O to the final electron acceptors of PSⅠ (Ivanov et al., 2008; Papageorgiou, 2013). Wang et al (2016) examined the PSⅡ photochemistry by Chl F analysis in high-yield rice () ‘LYPJ’ flag leaves during senescence and found that natural senescence inhibited oxygen-evolving complex (OEC)-PSⅡ electron transport, also significantly limited the PSⅡ-PSⅠ electron flow. Paunov et al. (2018) analyzed the effect of cadmium and zinc on Chl F in durum wheat (var.) and found that both metals disturbed photosynthetic electron transport processes, which led to a 4- to 5-fold suppression of the efficiency of energy transformation in PSⅡ. Urbano Bron et al. (2004) used Chl F as a tool to evaluate the ripening of ‘Golden’ papaya fruit (), finding that maximal chlorophyll fluorescence (M) and minimal chlorophyll fluorescence (O) showed strong correlation with changes in skin color and fruit firmness, which suggested a relationship between the fluorescence and the level of papaya fruit senescence. Although many researches about the Chl F parameters in senescence leaves have been reported, more detailed information describing the status of PSⅡ by the fast induced fluorescence during leaf senescence is still unclear (Lu et al., 2002; Tang et al., 2015). And there is no report about Chl F in different cotton genotypes. In order to capture more detailed information describing the status of PSⅡ during leaf senescence and rapidly screen cotton genotypes with different duration of photosynthetic capacity, we observed the change of PSⅡ status using fast induced Chl F analysis during leaf senescence of different cotton genotypes under the same weather condition.
1 Materials and Methods
1.1 Cotton materials
Three insect-resistant transgenic cotton genotypes were used for this study. They are ‘Baimian1’, bred by Henan Institute of Science and Technology with a performance of early-maturing but slow leaf senescence (Hu et al., 2014; Wang et al., 2020); ‘DP99B’ bred by the Monsanto Company and officially registered in Hebei Province in 2000 with a performance of early-maturing and fast leaf senescence(Zhang et al., 2007; Wang et al., 2020); ‘Baimian5’ bred by Henan Institute of Science and Technology with a performance of early-maturing but middle leaf senescence.
1.2 Experimental design
A field experiment was conducted at the experimental station of Henan Institute of Science and Technology in Xinxiang, Henan Province, China (35°18′N, 113°55′E). A randomized experiment was designed with three cotton genotypes and three replicates in sandy soil with pH 8.5, 6.0 g∙kg−1organic matter, 18.6 mg∙kg−1available nitrogen, 16.2 mg∙kg−1available phosphorus, 158.5 mg∙kg−1available potassium in 2012. The cotton population was 4.5×104plants∙hm−2for three genotypes. Before planting the land was plowed and irrigated. The three different cotton genotypes had a conventional field management, planted in 20 April and topped in 15 July. The weather conditions of the study area were provided in Table 1.

Table 1 The weather conditions of Xinxiang, Henan, China in 2012 and from 1961 to 2011
1.3 Chlorophyll fluorescence measurements
From 20 July (after topping) to 11 October (harvest time), polyphasic Chl F transient (OJIP) of the first leaves counted from the stem top of each cotton genotypes were measured with a portable fluorometer (Handy PEA, Hansatech, UK) by according to methods of Strasser et al. (1995) in the afternoon (16:00–17:00) at approximately 20 days intervals. Each cotton genotypes contained six leaves with the similar growth condition. Before measurements, leaves were dark-adapted for 30 min. Then OJIP transient was induced by 1 second pulses of red light (650 nm, 3500 μmol∙m–2∙s–1). The OJIP test (Strasser et al., 2004) was used to analyze each OJIP transient. The formulae and explanation of the technical data of the OJIP curves, as well as the selected OJIP test parameters used in this study was list blow (Table 2).
1.4 Statistical analysis
Duncan test was used to determine the differences among genotypes at<0.05 with SPSS (version 22.0; IBM). Charts were generated using Excel 2003 and Origin 9.0 software (OriginLab Inc., USA). Data presented in the figures and tables are means of six biological replicates.
2 Results
2.1 Identification of photosynthetic function duration of three cotton genotypes
When the main stem nodes above the uppermost white flower at the first node of fruit branch was equal to 5, it indicates the growth and development of cotton has entered the physiological decline stage. In present study, the physiological decline periods of three cotton genotypes were basically the same between 19 July and 26 July (Table 3). ‘DP99B’ has a significantly less main stem nodes above the uppermost white flower at the first node of fruit branch than that of ‘Baimian1’ between 28 June and 19 July, indicated that the reproductive growth process of ‘DP99B’ was faster than Baimian1 in this stage.

Table 2 Definitions of measured and calculated chlorophyll a fluorescence parameters used in the experiment

Table 3 Main stem nodes number above the uppermost white flower at the first node of fruit branch of different cotton genotypes in 2012
Different lowercase letters in the same column indicated significant differences among different cotton genotypes at<0.05.
The performance index based on light energy absorption (PIABS) is a photosynthesis performance index that reflects the overall photosynthetic activity of PSⅡ. Therefore, the variations of PIABSinthree cotton genotypes were observed every 20 days from 21 July based on the results of the main stem nodes above the uppermost white flower at the first node of fruit branch (Table 4). There was no obvious difference in the value of PIABSin three genotypes of cotton on 21 July. With the duration of leaf senescence, PIABSdeclined gradually inthree cotton genotypes. On 10 August, obvious difference of PIABSappeared in ‘DP99B’ compared with the other cotton genotypes. Then, obvious difference of PIABScontinued in ‘DP99B’ compared with the other cotton genotypes. At last, ‘Baimian1’ decreased 57.13%, ‘DP99B’ decreased 89.66%, ‘Baimian5’ decreased 76.17% compared with the PIABSon 21 July. And obvious difference of PIABSappeared between those cotton genotypes on 11 October. These results indicated that the process of leaf senescence in three cotton genotypes were different. The senescence of leaves in ‘DP99B’ was faster than that in the other cotton genotypes. And the rate of leaf senescence in ‘DP99B’ was the fastest, then was ‘Baimian5’, ‘Baimian1’ was the slowest. Taken together, the functional period of leaf in ‘Baimian1’ was longest, ‘DP99B’ was shortest, ‘Baimian5’ was middle.
Table 4 Trends of the performance index based on light energy absorption (PIABS) with time for different cotton genotypes in 2012
Different lowercase letters in the same line at the same date indicated significant differences among genotypes at0.05.
2.2 Chlorophyll a fluorescence OJIP transient in cotton leaves of three genotypes
The state of the light-dependent photosynthetic processes in three cotton genotypes were analyzed with OJIP transient during the leaf senescence (Fig. 1). Characteristic difference existed at each phase (O−J, J−I, and I−P phase) of the OJIP fluorescence. The O−J phase denotes gradual reduction of QAwhich is the primary electron acceptor in PSⅡ. The K-phase will appear before J point when the donor site of PSⅡ is injured. The J−I phase mainly reflect the reduction of the intersystem electron carriers. The I−P phase reflects the reduction of PSⅠ electron acceptor (Strasser et al., 2004; Yusuf et al., 2010).
Compared with the fluorescence intensity on 21 July,Oincreased by 2.84%, 22.91%, 26.29% andPreduced by 4.98%, 7.15%, 23.86% respectively in ‘Baimian1’, ‘Baimian5’ and ‘DP99B’ on 11 October. These results probably indicated that the components of photosynthetic apparatus of three cotton genotypes were differently injured during the leaf senescence. And this result was accord with the above result of PIABS(Table 4).
2.3 Changes of reaction center, donor and acceptor side of PSⅡ in leaves of three cotton genotypes
More parameters involved the reaction center, electron transport at donor and acceptor sides of PSⅡ were analyzed. The variation of RC/CSOandKdemonstrate the changes in the reaction center and donor side of PSⅡ, respectively, were shown in Fig. 2. Parameters such asO,m,I,J,ando primarily reflect changes in the acceptor site of PSⅡ were shown as a spider plot (Fig. 3).
With the duration of leaf senescence, the RC/CSOof the three cotton genotypes declined greatly before 30 August, then it increased on 20 September, which may be caused by the weather condition during late August. On the whole, the RC/CSOof the three cotton genotypes showed descending trend. It can be seen that those active reaction centers were susceptible to disruption reversibly, but the overall trends of RC/CSOwere decline with the duration of leave senescence. Compared with the highest values of RC/CSO, the RC/CSOvalues of ‘Baimian1’ ‘Baimian5’ and ‘DP99B’ on 11 October decreased by 10.37%, 7.77% and 16.75%, respectively. And different cotton genotypes had similar change on the active reaction centers during leaf senescence. The reduction of active reaction centers number of those cotton genotypes indicated that less energy was used to drive electron transport. With the duration of the leaf senescence, the trends ofKof three cotton genotypes were increasing, the values ofKchanged greatly after 10 August. At last, theKof ‘DP99B’ was significantly greater than that of ‘Baimian1’. And there were evident differences between ‘DP99B’ and ‘Baimian1’, but ‘Baimian5’ had no significantly difference with ‘DP99B’ and ‘Baimian1’ on 11 October. Compared with the value ofKon 21 July, theKon 11 October in ‘Baimian1’ ‘Baimian5’ and ‘DP99B’ increased by 18.40%, 26.95% and 38.53%, respectively. This indicated that the oxygen-evolving complex (OEC) was damaged greatly at the late growth stage, while the damaged extents of the OEC were different in three cotton genotypes even at the same growing environment.
TheJof three cotton genotypes increased during the leaf senescence. TheIof ‘Baimian1’ always increased, while theIof ‘Baimian5’ and ‘DP99B’ first increased then declined during the leaf senescence. TheJof ‘Baimian1’ ‘Baimian5’ and ‘DP99B’ increased by 27.28%, 46.57% and 45.80%, respectively; and theIof ‘Baimian1’, ‘Baimian5’ and ‘DP99B’ increased by 28.74%, 25.78% and 22.18%, respectively, on 11 October, compared with those on 21 July. It indicated that the electron transport had stronger inhibition in ‘Baimian5’ and ‘DP99B’ than that in ‘Baimian1’ at J phase. While the strength of inhibition was ‘Baimian1’ > ‘Baimian5’ > ‘DP99B’ at Ⅰ phase.

2.4 Variations of PSⅡ and PSⅠ efficiency and excitation energy dissipation in cotton leaves of three genotypes
With the duration of the leaf senescence, theΦO, ΦOand ΦOdecreased, while ΦOincreased. On 11 October, there were evident differences among those cotton genotypes in PSⅡ and PSⅠ efficiency. Among those genotypes, the range of change in energy efficiency was ‘DP99B’ > ‘Baimian5’ > ‘Baimian1’ from 20 July to 11 October. This indicated that with the cotton leaf senescence, the functional activity of PSⅡ and PSⅠ could be maintained until 11 October, accompanied the increase of the thermal dissipation quantum yield (Table 5).


Table 5 Mean values of PSⅡ reaction center numbers and energy allocation of three cotton genotypes in different days
Different lowercase letters in the same line at the same date indicated significant differences among genotypes at0.05.
3 Discussion
3.1 Senescence being reflected by PIABS and differences exist among three genotypes
Leaf senescence is an integrated response of leaf cells to age information and other internal and environmental signals, early leaf senescence may decrease yield in crop plants by limiting the growth phase (Lim et al., 2007). Senescence may differ between genotypes because the internal factors, especially the senescence related genes, and the external factors including light, temperature, water availability, carbon dioxide, nutrients and so on (Chen et al., 2018). In this paper, the three cotton genotypes enter into the senescence periods at the time between 19 July and 26 July which were proved by the main stem nodes above the uppermost white flower at the first node of fruit branch under the same environment (Table 3).
The disassembly of the photosynthetic apparatus within chloroplast and the concomitant decrease in photosynthetic activity of PSⅠ and PSⅡ, especially PSⅡ, are the most remarkable events in leaf senescence (Grover and Mohanty, 1992; Grover et al., 1986; Woolhouse, 1987). PIABSis shown to be well correlated with photosynthetic capacity which can be measured as CO2assimilation (Heerden et al., 2003; Ripley et al., 2004). PIABSis more sensitive than commonly used parameter maximum quantum yield of photosystem Ⅱ (V/M) (Živčák et al., 2008), very susceptible to environmental stress, so it has been used to differentiate genotypes by their responses to different abiotic stresses including natural senescence (Boureima et al., 2012; Holland et al., 2014; Kalaji et al., 2014). Chen and Dong (2016) proposed that there were three senescence performance categories including normal, premature and late senility in cotton. In this research, three genotypes showed distinctly leaf senescence differences being expressed by PIABS(Table 4), which could classify ‘Baimian1’ ‘Baimian5’ and ‘DP99B’ into late, middle and early senescence types. Furthermore, the senescence of leaves in ‘DP99B’ not only began earlier, but also expanded faster than that in ‘Baimian1’ and ‘Baimian5’ (Table 4), which was identical to the phenomenon observed at the yield level, and also explained why ‘DP99B’ usually had lower fiber yield than ‘Baimian1’ (Wang et al., 2020).
3.2 Different electron transport exist in different senescence performance
The senescence pattern which affects photosynthesis is variable and differences among cultivars with different genotypes (Falqueto et al., 2009). Anything affecting photosynthesis can affect the intensity of Chl F, so Chl F can be used as a versatile tool to sense environmental and physiological changes of plants (Guo and Tan, 2015). The shape of the fluorescence rise kinetics (OJIP) is highly dependent on the physiological conditions. In the present study, the major difference during natural senescence for different cotton genotypes occurred in the J−P phase, indicating major difference existed at the intersystem electron carriers and PS Ⅰ electron acceptors among different genotypes. Furthermore, during the leaf senescence the PSⅡ−PSⅠ electron flow was also significantly limited. Among those cotton genotypes, the variation of fluorescence intensity about ‘DP99B’ was the biggest, ‘Baimian1’ was the least, ‘Baimian5’ was the middle at the J−P phase on 11 October compared with 21 July (Fig. 1).
In order to further identify the damage site and sensitive functions during the photosynthetic electron transport within senescent leaf, we calculated several OJIP parameters. The OJIP parameters are quantitative analysis of the OJIP transient (Chen et al., 2016), helping account the different steps and phases of the OJIP transient with the redox states of PSⅡ and realize the efficiencies of electron transfer through the intersystem chain to the end electron acceptors at the PSⅠ acceptor side, concomitantly (Pollastrini et al., 2014; Tsimilli-Michael and Strasser, 2008).Kis a relative measure of inactivation of OEC. With the duration of leaf senescence, the overall trends ofKin three cotton genotypes were increasing (Fig. 2). At the same time, the inhibition of the acceptor side of PSⅡ was greater than that of the donor side, which can be proved by the bigger increase ofJandIthanKin three cotton genotypes. Among those genotypes, ‘DP99B’ had the biggest increase ofK, ‘Baimian5’ had the biggest increase ofJ, ‘Baimian1’ had the biggest increase ofI(Fig. 2 and Fig. 3).

4 Conclusion

BAKER N R, ROSENQVIST E. 2004. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities[J]. Journal of Experimental Botany, 55(403): 1607–1621
BAKER N R. 2008. Chlorophyll fluorescence: a probe of photosynthesis[J]. Annual Review of Plant Biology, 59: 89–113
BOUREIMA S, OUKARROUM A, DIOUF M, et al. 2012. Screening for drought tolerance in mutant germplasm of sesame () probing by chlorophyll a fluorescence[J]. Environmental and Experimental Botany, 81: 37–43
BROUDER S M, CASSMAN K G. 1990. Root development of two cotton cultivars in relation to potassium uptake and plant growth in a vermiculitic soil[J]. Field Crops Research, 23(3/4): 187–203
CHEN S G, YANG J, ZHANG M S, et al. 2016. Classification and characteristics of heat tolerance inpopulations using fast chlorophyll a fluorescence rise O-J-I-P[J]. Environmental and Experimental Botany, 122: 126–140
CHEN Y Z, DONG H Z. 2016. Mechanisms and regulation of senescence and maturity performance in cotton[J]. Field Crops Research, 189: 1–9
CHEN Y Z, KONG X Q, DONG H Z. 2018. Removal of early fruiting branches impacts leaf senescence and yield by altering the sink/source ratio of field-grown cotton[J]. Field Crops Research, 216: 10–21
DEELL J R, VAN KOOTEN O, PRANGE R K, et al. 1999. Applications of chlorophyll fluorescence techniques in postharvest physiology[J]. Horticultural Reviews, 23: 69–107
DONG H Z, LI W J, TANG W, et al. 2006. Yield, quality and leaf senescence of cotton grown at varying planting dates and plant densities in the Yellow River Valley of China[J]. Field Crops Research, 98(2/3): 106–115
DONG H Z, LI W J, TANG W, et al. 2005. Research progress in physiological premature senescence in cotton[J]. Acta Gossypii Sinica, 17(1): 56–60
FALQUETO A R, CASSOL D, MAGALH ES JUNIOR A M M, et al. 2009. Physiological analysis of leaf senescence of two rice cultivars with different yield potential[J]. Pesquisa Agropecuária Brasileira, 44: 695–700
GOLTSEV V N, KALAJI H M, PAUNOV M, et al. 2016. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus[J]. Russian Journal of Plant Physiology, 63(6): 869–893
GOVINDJEE, PAPAGEORGIOU G. 1971. Chlorophyll fluorescence and photosynthesis: fluorescence transients[M]//Photophysiology. Amsterdam: Elsevier.
GREGERSEN P L, HOLM P B, KRUPINSKA K. 2008. Leaf senescence and nutrient remobilisation in barley and wheat[J]. Plant Biology: Stuttgart, Germany, 10(1): 37–49
GROVER A, MOHANTY P. 1993. Leaf senescence-induced alterations in structure and function of higher plant chloroplasts[M]//ABROL Y P. Photosynthesis: Photoreactions to Plant Productivity. New Delhi: Springer Science+Business Media Dordrecht, 225–255
GROVER A, SABAT S C, MOHANTY P. 1986. Effect of temperature on photosynthetic activities of senescing detached wheat leaves[J]. Plant and Cell Physiology, 27(1): 117–126
GROVER A. 1993. How do senescing leaves lose photosynthetic activity? [J]. Current Science, 64: 226–234
GUO Y, TAN J L. 2015. Recent advances in the application of chlorophylla fluorescence from photosystem Ⅱ[J]. Photochemistry and Photobiology, 91(1): 1–14
Heerden P D R V, Tsimilli-Michael M, Kruger G H J, et al. 2003. Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation[J]. Physiologia Plantarum, 117: 476–491
HOLLAND V, KOLLER S, BRÜGGEMANN W. 2014. Insight into the photosynthetic apparatus in evergreen and deciduous European oaks during autumn senescence using OJIP fluorescence transient analysis[J]. Plant Biology: Stuttgart, Germany, 16(4): 801–808
HU Z B, WANG S F, ZHANG X, et al. 2014. Differences of potassium efficiency and root responses to potassium deficiency between short-and long-season cotton genotypes[J]. Acta Agriculturae Boreali-Sinica, 29(5): 218–225
IVANOV A G, HURRY V, SANE P V, et al. 2008. Reaction centre quenching of excess light energy and photoprotection of photosystem Ⅱ[J]. Journal of Plant Biology, 51(2): 85–96
KALAJI H M, OUKARROUM A, ALEXANDROV V, et al. 2014. Identification of nutrient deficiency in maize and tomato plants bychlorophyll a fluorescence measurements[J]. Plant Physiology and Biochemistry, 81: 16–25
KAUTSKY H, HIRSCH A. 1931. Neue versuche zur kohlensäureassimilation[J]. Naturwissenschaften, 19(48): 964
LAZÁR D. 2015. Parameters of photosynthetic energy partitioning[J]. Journal of Plant Physiology, 175: 131–147
LIM P O, KIM H J, GIL NAM H. 2007. Leaf senescence[J]. Annual Review of Plant Biology, 58(1): 115–136
LU Q T, LU C M, ZHANG J H, et al. 2002. Photosynthesis and chlorophyllafluorescence during flag leaf senescence of field-grown wheat plants[J]. Journal of Plant Physiology, 159(11): 1173–1178
Papageorgiou G. 2013. Chlorophyll fluorescence and photosynthesis: fluorescence transients[J]. Photophysiology, 1
PAUNOV M, KOLEVA L, VASSILEV A, et al. 2018. Effects of differentmetals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat[J]. International Journal of Molecular Sciences, 19: 787
POLLASTRINI M, HOLLAND V, BRÜGGEMANN W, et al. 2014. Interactions and competition processes among tree species in young experimental mixed forests, assessed with chlorophyll fluorescence and leaf morphology[J]. Plant Biology: Stuttgart, Germany, 16(2): 323–331
RAJCAN I, DWYER L M, TOLLENAAR M. 1999. Note on relationship between leaf soluble carbohydrate and chlorophyll concentrations in maize during leaf senescence[J]. Field Crops Research, 63(1): 13–17
RAJCAN I, TOLLENAAR M. 1999. Source: sink ratio and leaf senescence in maize: Ⅰ. Dry matter accumulation and partitioning during grain filling[J]. Field Crops Research, 60(3): 245–253
RAJCAN I, TOLLENAAR M. 1999. Source: sink ratio and leaf senescence in maize: Ⅱ. Nitrogen metabolism during grain filling[J]. Field Crops Research, 60(3): 255–265
RIPLEY B S, REDFERN S P, DAMES J. 2004. Quantification of the photosynthetic performance of phosphorus-deficientby means of chlorophyll-a fluorescence kinetics[J]. South African Journal of Science, 100(11/12): 615–618
SMART C M. 1994. Gene expression during leaf senescence[J]. New Phytologist, 126(3): 419–448
STRASSER B J, STRASSER R J. 1995. Measuring fast fluorescence transients to address environmental questions: the JIP-test[M]//Photosynthesis: from Light to Biosphere. Dordrecht: Springer Netherlands, 4869–4872
STRASSER R J, TSIMILLI-MICHAEL M, SRIVASTAVA A. 2004. Analysis of the chlorophyll a fluorescence transient[M]// Chlorophyll a Fluorescence. Dordrecht: Springer Netherlands, 321–362
TANG G, LI X, LIN L, et al. 2015. Combined effects of girdling and leaf removal on fluorescence characteristic ofleaf senescence[J]. Plant Biology, 17(5): 980–989
TSIMILLI-MICHAEL M, STRASSER R J. 2008. In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants[M]//Mycorrhiza. Berlin, Heidelberg: Springer Berlin Heidelberg, 679–703
URBANO BRON I, VASCONCELOS RIBEIRO R, AZZOLINI M, et al. 2004. Chlorophyll fluorescence as a tool to evaluate the ripening of ‘Golden’fruit[J]. Postharvest Biology and Technology, 33(2): 163–173
VAN HEERDEN P D, TSIMILLI-MICHAEL M, KRÜGER G H, et al. 2003. Dark chilling effects on soybean genotypes during vegetative development: parallel studies of CO2assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation[J]. Physiologia Plantarum, 117(4): 476–491
WANG S F, XUE H Y, ZHANG Z Y, et al. 2020. Coordination of root growth and leaf senescence in cotton[J]. Acta Agronomica Sinica, 46(1): 93–101
WANG Y W, XU C, LV C F, et al. 2016. Chlorophyll a fluorescence analysis of high-yield rice (L.) LYPJ during leaf senescence[J]. Photosynthetica, 54(3): 422–429
WANG Y W, ZHANG J J, YU J, et al. 2014. Photosynthetic changes of flag leaves during senescence stage in super high-yield hybrid rice LYPJ grown in field condition[J]. Plant Physiology and Biochemistry, 82: 194–201
WENG X Y, XU H X, JIANG D A. 2005. Characteristics of gas exchange, chlorophyll fluorescence and expression of key enzymes in photosynthesis during leaf senescence in rice plant[J]. Journal of Integrative Plant Biology, 47: 560–566
WINGLER A, MARÈS M, POURTAU N. 2004. Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence[J]. New Phytologist, 161(3): 781–789
WOOLHOUSE H. 1987. Leaf senescence[M]//SMITH H, GRIERSON D. The Biology of Plant Development. Oxford: Blackwell Scientific Publications, 256–284
WRIGHT P R. 1999. Premature senescence of cotton (L.) — Predominantly a potassium disorder caused by an imbalance of source and sink[J]. Plant and Soil, 211(2): 231–239
YUSUF M A, KUMAR D, RAJWANSHI R, et al. 2010. Overexpression of gamma-tocopherol methyl transferase gene in transgenicplants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements[J]. Biochimica et Biophysica Acta, 1797(8): 1428–1438
ZHANG Z Y, TIAN X L, DUAN L S, et al. 2007. Differential responses of conventional and bt-transgenic cotton to potassium deficiency[J]. Journal of Plant Nutrition, 30(5): 659–670
ŽIVČÁK M, BRESTIČ M, OLŠOVSKÁ K, et al. 2008. Performance index as a sensitive indicator of water stress inL[J]. Plant, Soil and Environment, 54(4): 133–139
基于快速叶绿素荧光参数的不同基因型棉花叶片衰老研究*
薛惠云, 王素芳, 张 新, 张志勇**
(河南科技学院/河南省现代生物育种协同创新中心/河南省棉麦分子生态和种质创新重点实验室 新乡 453003)
棉花; 叶片衰老; 叶绿素荧光; 光系统Ⅱ(PSⅡ); 快速叶绿素荧光参数
10.13930/j.cnki.cjea.200888
XUE H Y, WANG S F, ZHANG X, ZHANG Z Y. The rapid chlorophyll a fluorescence characteristics of different cotton genotypes reflect differences in leaf senescence[J]. Chinese Journal of Eco-Agriculture, 2021, 29(5): 870−879
S562

*This study was supported by the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (21IRTSTHN023) and the National Natural Science Foundation of China (31571600, 31271648).
, E-mail: z_zy123@126.com
Nov. 17, 2020;
Feb. 20, 2021
* 河南省高校科技创新团队支持计划项目(21IRTSTHN023)和国家自然科学基金项目(31571600, 31271648)资助
张志勇, 主要研究方向为作物栽培生理。E-mail: z_zy123@126.com
薛惠云, 主要研究方向为作物栽培生理。E-mail: xuehy8310@163.com
2020-11-17
2021-02-20

