Perioperative blood transfusion decreases long-term survival in pediatric living donor liver transplantation
Karina Gordon, Estela Regina Ramos Figueira, Joel Avancini Rocha-Filho, Luiz Antonio Mondadori, Eduardo Henrique Giroud Joaquim, Joao Seda-Neto, Eduardo Antunes da Fonseca, Renata Pereira Sustovitch Pugliese, Agustin Moscoso Vintimilla, Jose Otavio Costa Auler Jr, Maria Jose Carvalho Carmona, Luiz Augusto Carneiro D'Alburquerque
Abstract
BACKGROUND The impact of perioperative blood transfusion on short- and long-term outcomes in pediatric living donor liver transplantation (PLDLT) must still be ascertained,mainly among young children. Clinical and surgical postoperative complications related to perioperative blood transfusion are well described up to three months after adult liver transplantation.
AIM To determine whether transfusion is associated with early and late postoperative complications and mortality in small patients undergoing PLDLT.
METHODS We evaluated the effects of perioperative transfusion on postoperative complications in recipients up to 20 kg of body weight, submitted to PLDLT. A total of 240 patients were retrospectively allocated into two groups according to postoperative complications: Minor complications (n = 109 ) and major complications (n = 131 ). Multiple logistic regression analysis identified the volume of perioperative packed red blood cells (RBC) transfusion as the only independent risk factor for major postoperative complications. The receiver operating characteristic curve was drawn to identify the optimal volume of the perioperative RBC transfusion related to the presence of major postoperative complications, defining a cutoff point of 27 .5 mL/kg. Subsequently, patients were reallocated to a low-volume transfusion group (LTr; n = 103 , RBC ≤ 27 .5 mL/kg)and a high-volume transfusion group (HTr; n = 137 , RBC > 27 .5 mL/kg) so that the outcome could be analyzed.
RESULTS High-volume transfusion was associated with an increased number of major complications and mortality during hospitalization up to a 10 -year follow-up period. During a short-term period, the HTr showed an increase in major infectious, cardiovascular, respiratory, and bleeding complications, with a decrease in rejection complications compared to the LTr. Over a long-term period,the HTr showed an increase in major infectious, cardiovascular, respiratory, and minor neoplastic complications, with a decrease in rejection complications.Additionally, Cox hazard regression found that high-volume RBC transfusion increased the mortality risk by 3 .031 -fold compared to low-volume transfusion.The Kaplan-Meier survival curves of the studied groups were compared using log-rank tests and the analysis showed significantly decreased graft survival, but with no impact in patient survival related to major complications. On the other hand, there was a significant decrease in both graft and patient survival, with high-volume RBC transfusion.
CONCLUSION Transfusion of RBC volume higher than 27 .5 mL/kg during the perioperative period is associated with a significant increase in short- and long-term postoperative morbidity and mortality after PLDLT.
Institutional review board statement: The study was reviewed and approved by the ACCamargo Cancer Center Institutional Review Board, No.103 .402 ; the University of Sao Paulo School of Medicine Institutional Review Board, No.243 /12 .
Informed consent statement:Informed consent statements are not required.
Conflict-of-interest statement: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to World Journal of Gastroenterology.
Data sharing statement: No additional data are available.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4 .0 )license, which permits others to distribute, remix, adapt, build upon this work non-commercially,and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: htt p://creativecommons.org/License s/by-nc/4 .0 /
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Key Words: Liver transplantation; Child; Blood transfusion; Outcome; Liver cirrhosis;Mortality
INTRODUCTION
Blood transfusion has been associated with increased morbimortality rates in major surgical procedures such as hepatic resection[1,2 ], cardiac[3]and non-cardiac major thoracoabdominal surgeries[4], and adult liver transplantation (LT)[5 ,6]. Regarding adult LT, Boyd et al[7]showed that intraoperative red blood cell (RBC) transfusion volume, a positive history of anti-RBC alloantibodies, and the immunosuppressive regimen used are associated with patient mortality. De Boer et al[5]reported that the indication for LT and the number of platelets or RBC units transfused during surgery are risk factors for 1 -year graft survival; additionally, the number of RBC or platelet units transfused during surgery, cold ischemia time, and surgical team experience are risk factors for 5 -year graft survival. Also, RBC transfusion has been associated with an increased rate of cancer recurrence. Indeed, the relative risk of digestive cancer recurrence has been reported to increase by 2 .1 -fold after the administration of ≥ 3 units[8]. Storage time and the timing of the transfusion related to surgery may also play a role[8]. In patients undergoing adult LT, all blood products (BP) are related to increased postoperative complications[9,10 ]; consequently, there is a trend towards reducing their use[11 -13].
The harmful effects of perioperative transfusion in small pediatric patients are unclear pediatric LT. López Santamaría et al[11]suggested decreased graft survival associated with massive intraoperative blood transfusion, which was defined in their study as a loss greater than four volemias. They found four independent risk factors for mortality: Recipient’s age < 3 years, retransplantation, severity of the underlying disease, and the transplant team’s experience. Blood transfusion was not among them,however. To date, few reports have addressed the risks of transfusion in pediatric LT.In 2012 , Nacoti et al[14]found that perioperative transfusion of fresh frozen plasma(FFP) RBC are independent risk factors for decreasing 1 -year patient and graft survival. Nacoti et al[15]reported that intraoperative platelet and RBC transfusion are independent risk factors for developing major complications in the first year after pediatric LT.
Fanna et al[16]showed that high intraoperative bleeding is associated with pre-LT abdominal surgeries, factor V level ≤ 30 %, and ex-situ parenchymal transection of the liver graft. Jin et al[17]identified high white blood cell count, low platelet count, and a deceased donor as independent risk factors for massive transfusion, which was defined as the administration of RBC volume ≥ 100 % of the total blood volume (TBV).Although the graft failure incidence was higher in the massive group compared to the non-massive group, they found no difference in survival between the groups. Huang et al[18]evaluated pediatric living donor liver transplantation (LDLT) procedures and observed that younger patients with a lower weight, shorter stature, and preoperative prolonged international normalized ratio (INR) required larger blood transfusion volumes. Notwithstanding, preoperative INR was the only risk factor for massive blood transfusion. Kloesel et al[19]identified predictors of massive intraoperative bleeding (estimated blood loss of > TBV within a 24 h period): Preoperative hemoglobin (Hb) < 8 .5 g/dL, INR > 1 .5 , platelet count < 100 .000 /mm3 , and surgery length > 10 h. Except for a longer intensive care unit (ICU) stay, there was no other correlation between massive transfusion and morbimortality.
Pediatric LT includes patients less than 18 years of age with either chronic or acute liver diseases involving deceased and living donors. Underlying pathologies may vary, and even the severity score is different under 12 years old [Pediatric end-stage liver disease (PELD) score]. Importantly, previous studies did not exclusively include young children. In addition, the grafts came from split and whole organs from deceased donors in most samples. Massive transfusion definitions vary across pediatric studies as BP transfusion from one[18 ] to four[11]volemias. There is still much controversy regarding the type, volume, and timing of BP transfusion and its association with postoperative morbimortality. Thus, this study assessed whether perioperative transfusion is associated with early and late postoperative complications and mortality in small patients undergoing pediatric LDLT (PLDLT).
MATERIALS AND METHODS
The Institutional Research Ethics Committees of ACCamargo Cancer Center and the University of São Paulo School of Medicine approved this observational, retrospective,and analytical cohort study according to the Helsinki Statement. All data were completely anonymized before they were accessed, and both committees waived the requirement for informed consent.
We investigated 254 pediatric patients weighing up to 20 kg with non-acute liver diseases who underwent first LDLT performed at the ACCamargo Cancer Center over 10 years. Fourteen patients were excluded: Five had fulminant hepatitis, three had their first transplant performed in other center, three were lost to follow-up, and three had missing data. All 240 enrolled patients underwent standardized procedures and techniques (total intravenous general anesthesia, piggyback inferior vena cava clamping, and exclusive use of hepatic left lateral segment grafts).
Study groups
Based on the severity of postoperative complications (graded according to the Clavien-Dindo classification during hospital stay), a total of 240 patients were initially allocated into two groups (Figure 1 A): The minor complications (MiC) group (n = 109 ,either with no complications or with grade I-IIIa complications) and the major complications (MaC) group (n = 131 , with at least one grade IIIb-V complication).Subsequently, all patients were reallocated into two further groups according to the RBC volume transfused during the perioperative period from 24 h before to 48 h after LT: The low-volume transfusion group (LTr n = 103 , RBC ≤ 27 .5 mL/kg) and the highvolume transfusion group (HTr n = 137 , RBC > 27 .5 mL/kg) (Figure 1 B).
Classification of postoperative complications and postoperative follow-up
The Clavien-Dindo classification was applied to assess the relationship between blood transfusion and postoperative complications. In this pediatric population, general anesthesia was ostensibly required to ensure stillness and safety for diagnostic and treatment procedures. Thus, the original grade III was modified and subdivided into IIIa and IIIb according to the complexity of the procedures as low and high,respectively (Table 1 ). Data on mortality and complications were collected over a 10 -year period post-LT. Patients who failed to attend outpatient follow-up after hospital discharge were considered lost to follow-up.
Complications were categorized by clinical presentation in 14 types: (1 ) Bleeding:Epistaxis, gastrointestinal, surgical wound, drains, and systemic hemorrhages due to portal hypertension or coagulopathy with or without need for surgery; (2 )Cardiovascular: Systemic arterial hypertension, mismatches of cardiac rhythm, heart failure, hemodynamic instability, and cardiorespiratory arrest; (3 ) Dermatologic: Skin manifestations of drugs, food, and environmental factors; (4 ) Gastrointestinal:Malnutrition with weight-for-age z-score, height-for-age z-score[20], weight-for-height z-score, or body-mass-index z-score (BMIZ) ≤ -2 standard deviation, need for enteral or parenteral diet, persistent vomiting or diarrhea ≥ 3 wk, gastroesophageal reflux disease with or without brochoaspiration, visceromegaly, or ascites caused by maintained portal hypertension; (5 ) Infectious: Positive cultures with clinical or laboratory manifestations except from respiratory infections; (6 ) Malignancy: Posttransplant lymphoprolipherative disease (PTLD), lymphomas, skin tumors, and relapse of tumors; (7 ) Metabolic: Hydro-electrolytic serum changes such as hyponatremia (sodium < 133 mEq/L), hypernatremia (sodium > 147 mEq/L),hypokalemia (potassium < 3 .0 mEq/L), hyperkalemia (potassium > 5 .4 mEq/L),hypocalcemia (ionic calcium < 1 .17 mmol/Lol/L), hypomagnesemia (magnesium < 1 .8 mg/dL), hypophosphatemia (phosphorus < 2 .5 mg/dL), arterial blood gases with pH< 7 .2 , acidosis and pH > 7 .5 , alkalosis, hyperlactatemia (lactate > 22 mg/dL), oliguria(diuresis < 0 .5 mL/kg/h), adrenal insufficiency, diabetes mellitus, obesity (BMIZ > 2 standard deviations), or dyslipidemia (total cholesterol > 170 mg/dL, LDL fraction >130 mg/dL, and triglycerides > 130 mg/dL); (8 ) Miscellaneous: Accidental injuries linked to LT procedure or postoperative follow-up; (9 ) Neuropsychiatric: Headache,vertigo, seizures, sedation withdrawal syndrome, delayed neuropsychomotor development, school learning difficulties, behavioral changes with psychomotor agitation, attention deficit, mood lability, anxiety, or depression; (10 ) Primary nonfunction (PNF) of the graft; (11 ) Rejection: Clinical and laboratory responsiveness to pulse therapy with methylprednisolone or anatomopathological documentation of acute or chronic rejection; (12 ) Renal: Renal failure was considered a decay of at least 50 % of the estimated glomerular filtration rate (eGFR) applying the simplified revised Schwartz formula[21 ]; (13 ) Respiratory: Upper airway infections (rhinitis, sinusitis,otitis, tonsillitis, epiglottitis, pharyngolaryngitis), lower airway infections (tracheitis,bronchopneumonia, pneumonia), prolonged intubations (over 48 h), bronchospasm,atelectasis, effusions, pleural fistulas, hemothorax, pneumothorax, pneumomediastinum, non-cardiogenic edema, or acute respiratory failure; and (14 ) Surgical:LT specific complications (vascular thrombosis, biliary stenosis and fistulas,reoperation or retransplantation), hernias, dehiscence of anastomoses, or need for exploratory laparotomy except if caused by bleeding.

Table 1 Modified Clavien-Dindo classification for pediatric liver transplantation

Figure 1 Patient flowchart. A: Minor complication group (grade I-IIIa) and major complication group (grade IIIb-V); B: Low red blood cell (RBC) volume transfusion group (RBC ≤ 27 .5 mL/kg) and high RBC volume transfusion group (RBC > 27 .5 mL/kg). LT: Liver transplantation; RBC: Red blood cell; MiC: Minor complication; MaC: Major complication; LTr: Low-volume transfusion; HTr: High-volume transfusion.
Statistical analysis
Multiple logistic regression analysis was performed to identify risk factors for major postoperative complications. The stepwise method was used for the selection of the variables. A perioperative RBC transfusion volume was identified as a single risk factor. A receiver operating characteristic curve was constructed using the perioperative RBC transfusion volume, and the occurrence of major complications were input parameters. A cutoff point of 27 .5 mL/kg was identified using Youden’s index (Figure 2 ).
Student’s t-test or the Mann-Whitney test was used for quantitative variables while the chi-square test or Fisher’s exact test was used for qualitative variables.Independent risk factors for mortality were identified using simple and multiple Cox regression analyses. Overall patient and graft survival analyses were performed using Kaplan–Meier survival curves, which were compared using the log-rank test. A P value level < 0 .05 was used to define statistical significance. Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, United States) and R program version 3 .2 .1 (R Foundation for Statistical Computing, Vienna, Austria).

Figure 2 Receiver operating characteristic curve. A Receiver operation characteristic curve determined the optimal volume of perioperative red blood cells transfusion related to the presence of major postoperative complication. (Area under the curve = 0 .648 , P < 0 .0001 . Sensitivity = 68 .7 % and specificity = 56 .9 %.Cutoff point = 27 .5 mL/kg; 95 %CI: 0 .578 -0 .717 ).
RESULTS
Patient characteristics
Of the 240 patients included in the study, 136 (56 .7 %) were females. The median patient age, weight, and stature were 12 .4 mo, 8 .07 kg, and 70 cm, respectively. Biliary atresia was found in 151 patients (62 .9 % of the underlying diseases) in our cohort. The Kasai procedure was previously performed in 111 cases (46 .3 %). The average pre-LT PELD score was 16 (± 7 .7 ).
Perioperative risk factors for major postoperative complications
The overall incidence of major complications was 54 .6 %. In the MaC, all anthropometric measures, eGFR, Hb, sodium, and albumin levels were significantly lower. The INR, graft-to-body-weight ratio, and transfused BP volume were significantly higher than in the MiC. However, the only independent risk factor for major complications was perioperative RBC transfusion volume (Table 2 ).
Intraoperative and intensive care unit data
The HTr had lower Hb and sodium levels but significantly higher INR during the intraoperative period than the LTr. Additionally, the HTr had longer anesthetic and surgery time, a higher volume of crystalloids and colloids, higher diuresis rates, a lower incidence of extubation in the operating room, a longer intubation time, and a longer ICU and in-hospital stay than the LTr (Table 3 ).
Early postoperative complications
During hospitalization, the incidence of major complications per patient and the proportion of major complications were significantly higher in the HTr compared to the LTr (Table 4 ). Metabolic complications accounted for 28 .2 % of the complications during hospitalization. Additionally, complications such as gastrointestinal,malignancy, miscellany, neuropsychiatric, PNF, renal, and surgical were observed but with no significant difference between transfusion groups. The HTr had significantly more bleeding, respiratory, major cardiovascular, and major infectious complications but less dermatologic complications and rejections than the LTr (Table 5 ). Early LTspecific complications include PNF (2 .1 %), biliary fistula (6 .2 %), hepatic artery thrombosis (HAT) (3 .3 %), portal venous thrombosis (PVT) (9 .2 %), and retransplantation (1 .2 %); these were not related to a higher perioperative transfusion volume(Table 6 ). In terms of RBC transfusion volume, there was a significantly higher rate of30 d reoperation (26 .3 % × 8 .7 %, P < 0 .001 ) and 30 d mortality rate (6 .6 % × 0 .0 %, P <0 .001 ) in the HTr vs LTr, respectively (Table 6 ).
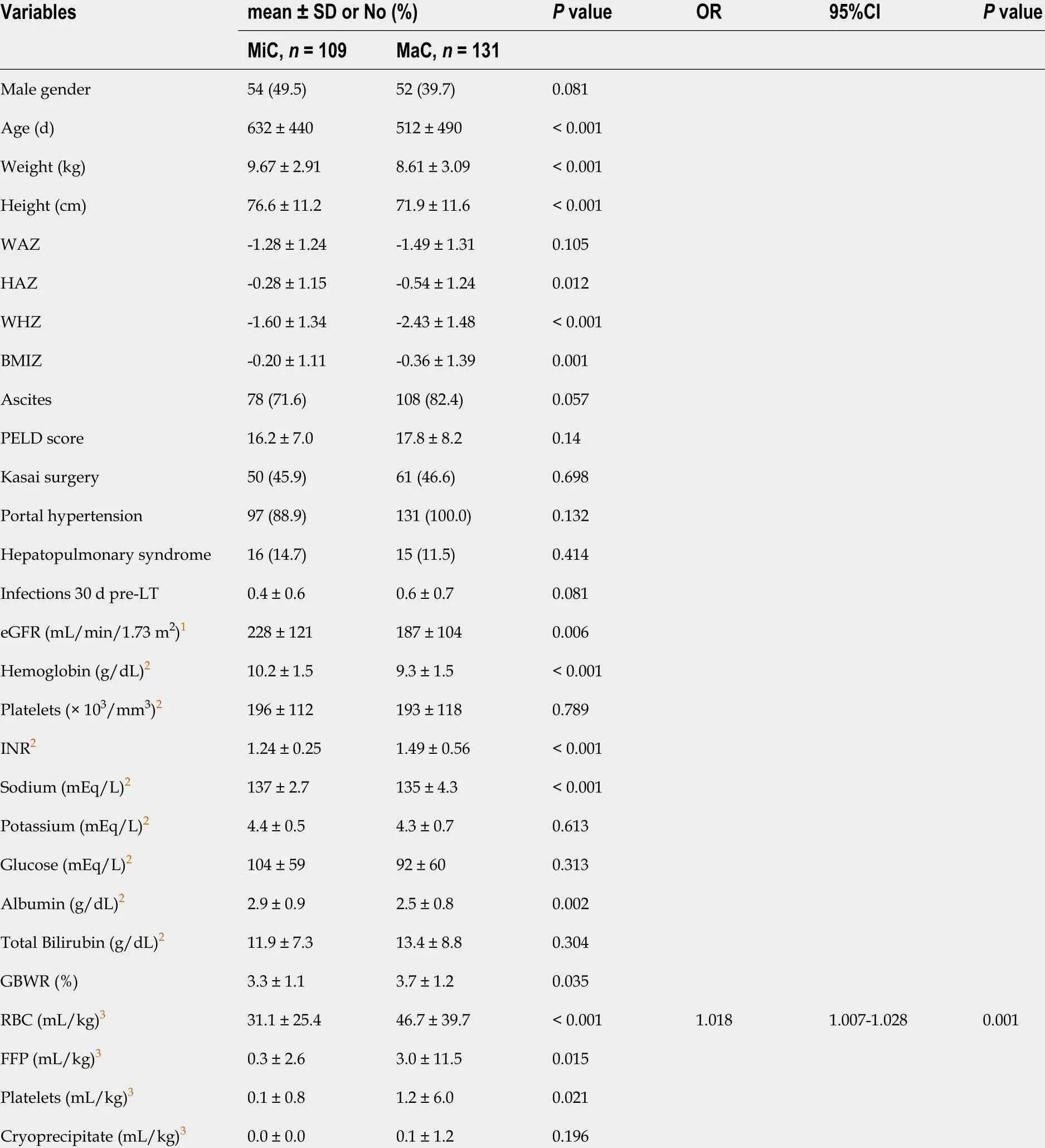
Table 2 Univariate and multiple logistic regression analyses of perioperative data stratified by the severity of complications
Late postoperative complications
Major cardiac complications were more frequent at 1 , 5 , and 10 years post-LT; major respiratory complications were more frequent at 5 and 10 years post-LT in the HTr than in the LTr. Dermatologic complications were less frequent up to 1 year in the HTr than in the LTr. Major infectious complications were more frequent, and rejections were less frequent in the HTr than in the LTr from 1 to 10 years post-LT. Minorneoplastic complications were more frequent at later points in follow-up in the HTr than in the LTr (Table 5 ).

Table 3 Univariate analysis of preoperative, intraoperative and early postoperative data according to perioperative red blood cell volume transfused

1 Estimated glomerular filtration rate, preoperative calculated by Schwartz's formula.2 Blood samples collected up to 72 h before liver transplantation (LT) anesthetic induction.3 Blood samples collected up to 2 h after the onset of LT anesthetic induction.4 Intraoperative volume indexed by body's weight. Extra-hepatic cholestasis, extra-hepatic biliary atresia, coledocus cyst; intrahepatic cholestasis, Alagille's syndrome, non-syndromic biliary hypoplasia, primary sclerosing cholangitis, progressive intrahepatic familial cholestasis; cirrhosis, idiopathic, autoimune and cryptogenic; metabolic diseases, glycogenesis, Cligger-Najar's disease, tyrosinemia, cystic fibrosis, alpha-1 anti trypsin deficiency, urea cycle defects,type 1 oxaluria; malignant diseases, hepatoblastoma and hepatocarcinoma and hepatic miscellany diseases, Budd-Chiari syndrome, Caroli's disease and unclarified fibrosis. SD: Standard deviation; LTr: Low-volume transfusion group; HTr: High-volume transfusion group; WAZ: Weight-for-age z-score;HAZ: Height-for-age z-score; WHZ: Weight-for-height z-score; BMIZ: Body-mass-index-for-age z-score; PELD: Pediatric end-stage liver disease; LT: Liver transplantation; INR: International normalized ratio; GBWR: Graft-to-body-weight ratio; OR: Operating room; ICU: Intensive care unit; eGFR: Estimated glomerular filtration rate.

Table 4 Comparative analysis of postoperative complications during the hospitalization period using the modified Clavien–Dindo classification
Late LT-specific complications were observed after 30 d over 10 years of follow-up,as biliary stenosis (12 .1 %), HAT (1 .2 %), PVT (3 .7 %), reoperation (7 .1 %) and retransplantation (2 .5 %), none of these were related to perioperative transfusion(Table 6 ). Overall, 10 -years mortality rate, with respect to RBC transfusion volume,was significantly higher (25 .5 % vs 7 .8 %, P < 0 .001 ), respectively, in the HTr compared to the LTr (Table 6 ).
Independent risk factors for death
Simple and multiple Cox regression analysis identified perioperative RBC volume >27 .5 mL/kg and preoperative eGFR as independent risk factors for mortality over 10 years of follow-up after LT (Table 7 ).
Patient and graft survival according to outcome and blood transfusion
The overall patient survival rates were 87 .1 %, 81 .5 %, and 80 .3 %, whereas the overall graft survival rates were 87 .1 %, 77 .7 %, and 75 .6 % at 1 , 5 , and 10 years post-LT,respectively. The graft survival rates were significantly lower in the MaC than in the MiC at 1 , 5 , and 10 years post-LT (81 .5 % vs 94 .4 %, 73 .8 % vs 84 .6 , and 72 .2 % vs 81 %,respectively); however, no significant difference was seen in the patient survival rates between the MaC and the MiC. The patient survival rates were significantly lower in the HTr than in the LTr at 1 , 5 , and 10 years post-LT: 82 .7 % vs 97 .7 %, 73 .9 % vs 93 .8 %,and 72 .6 % vs 90 .9 %, respectively. Likewise, the graft survival rates were significantly lower in the HTr than in the LTr at 1 , 5 , and 10 years post-LT: 79 .5 % vs 97 .7 %, 67 .2 % vs 92 .3 %, and 67 .2 % vs 87 %, respectively (Figure 3 ).

HTr: High-volume transfusion group; LTr: Low-volume transfusion group; PNF: Primary non-function.

Table 6 Patients with early and late liver transplantation specific complications and 30 d and 10 years mortality rate
DISCUSSION
Determining predictive factors for complications of pediatric LT may be hindered by patient heterogeneity and a lack of standardization in the definition of complications.This study is the first retrospective study to assess short- and long-term transfusionassociated postoperative complications in a large number of small pediatric patients with chronic liver diseases who received the same type of graft from living donors.
The Clavien-Dindo classification[22 ] was first used by Clavien et al[23]to assess postoperative complications in adult LT patients and has been increasingly used in most pediatric surgical areas with some adaptations[24 ]. Beck-Schimmer et al[25]classified post-LT complications as minor and major providing the basis for the use of this modified classification primarily because general anesthesia was induced for almost all procedures in this pediatric population (e.g., imaging exams, biopsies,catheter insertions, and other minor invasive procedures).
We defined the interval from 24 h pre- to 48 h post-LT as the perioperative period.Over this period, patients had a greater need for transfusion. Our transfusion goals throughout this period were to keep Hb > 8 and < 10 g/dL, INR < 3 .5 , platelet count >45 × 103 /mm3 , and fibrinogen level > 80 mg/dL. Viscoelastic methods were unavailable at our center at this time. Only 16 study participants were not transfused during the perioperative period. Of the 224 transfused patients, 7 .1 %, 98 .8 %, and 37 .5 % received RBC before, during, and after surgery, respectively. Some patients remained within a suitable range during the intraoperative period yet this level dropped during the early postoperative period requiring subsequent transfusion. In this study, the average volume of transfusion of other BP was minimal compared to RBC.
Massive bleeding is usually defined as the loss of 100 % or more of circulating TBV within 24 h[26 ]. Massive transfusion can also be defined as the transfusion of over 10 %of TBV per minute or 50 % in 3 h[27]. Nevertheless, TBV in pediatrics depends on the child’s age and weight ranging from 65 to 100 mL/kg. In our study, the child’s average age was 567 d (1 .5 years) corresponding to a TBV of 75 mL/kg. The mean perioperative RBC volume transfused in the HTr was 57 .7 mL/kg. A cutoff point of 27 .5 mL/kg was used when the perioperative RBC volume represented less than 37 %of TBV transfused within 96 h. Therefore, most of our patients did not meet the definition for massive bleeding or transfusion. Though there was no massive transfusion in the perioperative period in most patients; this lower volume has already been associated with major postoperative complications in PLDLT.
This study identified a perioperative transfusion volume of RBC > 27 .5 mL/kg and preoperative eGFR as independent risk factors for mortality in PLDLT patients. A perioperative RBC transfusion volume higher than 27 .5 mL/kg was a strong independent risk factor for mortality and increased the risk by 3 .031 -fold vs lower or equal volumes. This volume of RBC is definitely below that reported in other studies,which analyzed risk factors for survival in LT.
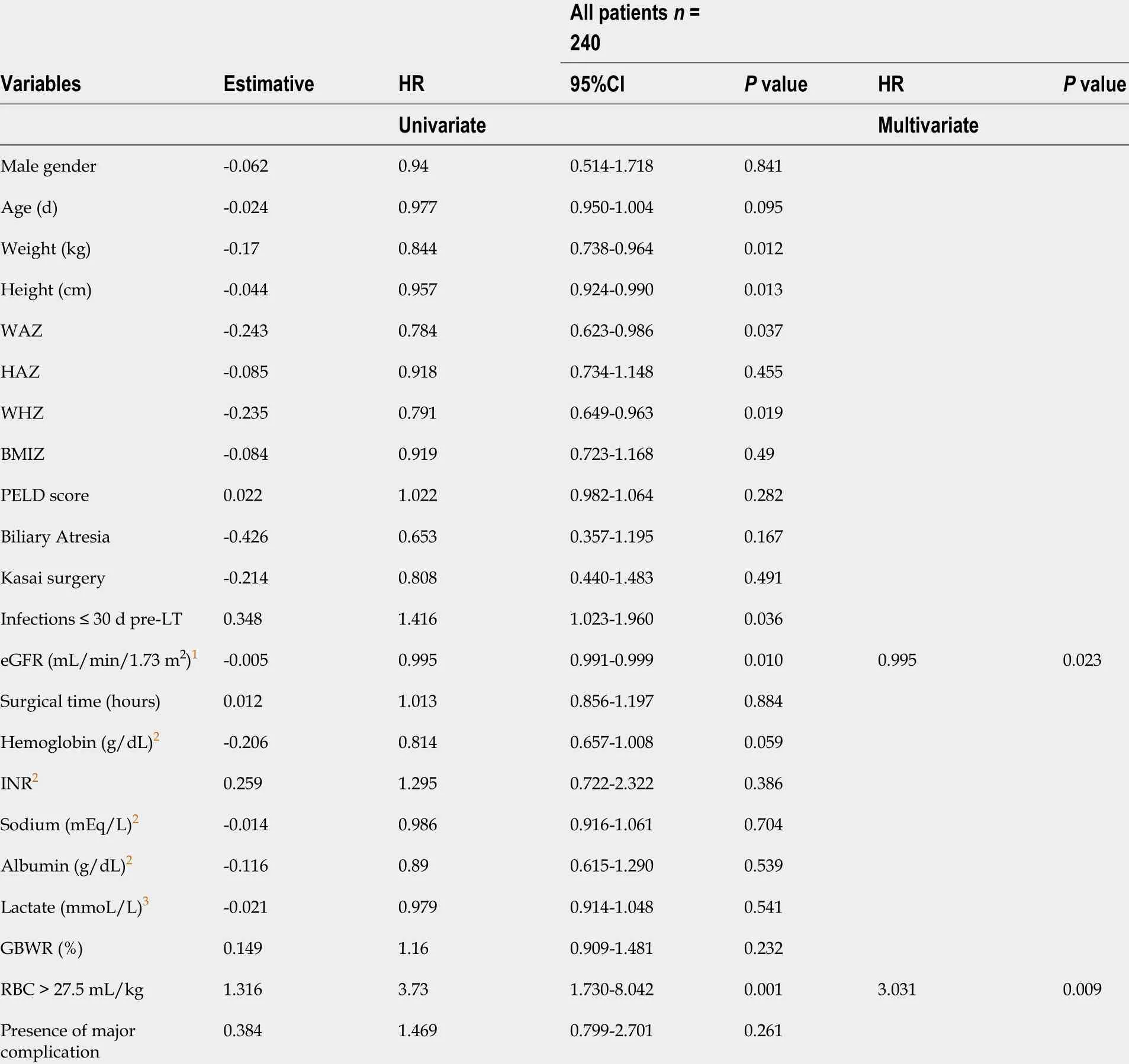
Table 7 Independent risk factors for death identified by simple and multiple Cox regression
Matinlauri et al[28 ] identified transfusion of RBC > 20 units as a risk factor for graft survival post-LT in adults. Nacoti et al[14 ] evaluated the effect of transfusion on 1 -year patient and graft survival in pediatric LT and estimated a mortality risk of 3 .15 for ≥ 3 units of RBC. Preoperative eGFR was a weak risk factor for mortality in our study probably because malnourished children tend to have underestimated serum creatinine values, and the simplified revised formula [eGFR = 0 .413 × Height(cm)/serum creatinine (mL/min/1 .73 m2 )] increases the error of the results at higher GFR values[29]. In the meantime, serum creatinine is known to be an independent risk factor for mortality in adult LT[30 ] and in pediatric LT with deceased donor[15].
Though LDLT is currently a standard treatment with good outcomes for young children with end-stage liver diseases, the postoperative complication rate can lead to a high morbimortality[31 ]. Among the 240 children, early postoperative complications were observed in 94 .6 % of patients of whom 54 .6 % had major complications associated with higher rates of graft loss. Although, the 1 -year survival rate of 87 .1 was slightly lower than in other centers that reported overall survival higher than 90 %, the 5 and 10 -year survival rates of 81 .5 % and 80 .3 %, respectively, are comparable to rates higher than 80 %, reported by others[32 -34]. This could be attributed to the fact that we studied exclusively small children, with inherent risks of early childhood, in comparison to other studies that include patients aged up to 18 years. In this study, the only risk factor for major postoperative complications after PLDLT was the volume of perioperative RBC transfused.
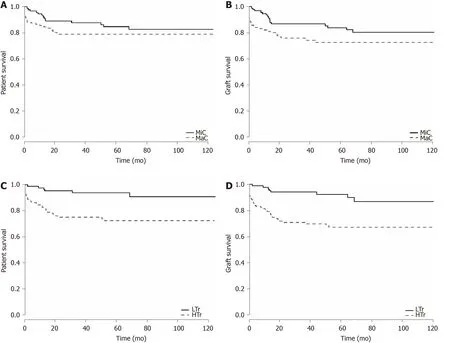
Figure 3 Patient and graft survival curves. A: Patient survival analysis comparing the minor complication (MiC) group (grade I-IIIa, n = 109 ) and the major complication (MaC) group (grade IIIb-V, n = 131 ) with number of patients at risk. Log-rank test: Chi-square = 12 .6 , degrees of freedom (df) = 1 , P = 0 .12 ; B: Graft survival analysis comparing the MiC and MaC groups. Log-rank test: Chi-square = 14 .6 , df = 1 , P = 0 .03 ; C: Patient survival analysis comparing the low-volume transfusion (LTr) group (n = 103 ) and the high-volume transfusion (HTr) group (n = 137 ). Log-rank test: Chi-square = 12 .6 , df = 1 , P < 0 .001 ; D: Graft survival analysis comparing the LTr (n = 103 ) and HTr (n = 137 ) groups. Log-rank test: Chi-square = 14 .6 , df = 1 , P < 0 .001 . MiC: Minor complications group (I-IIIa); MaC: Major complications group (IIIb-V); LTr: Low-volume transfusion group; HTr: High-volume transfusion group.
Transfusion was more frequently employed for younger and undernourished patients with extrahepatic cholestasis, previous Kasai procedure, increased rates of ascites, higher preoperative INR, lower Hb and sodium levels, and worse pulmonary and renal function. These findings are consistent with many reports on adult LT[35 -37].
BP transfusion has often been reported to be associated with increased rates of both early and late LT complications[38 ,39 ]. Our sample consisted of 234 (97 .5 %) outpatients and 6 (2 .5 %) patients previously admitted to the ICU. During hospitalization, the total complication rates were similar between patients of the LTr and HTr groups (92 .2 % vs 96 .4 %). The HTr had more patients with severe complications (65 .7 % vs 39 .8 %), a higher average of complications per patient (3 .8 ± 3 .6 vs 2 .5 ± 1 .7 ), and a higher median number of major complications (IIIa vs IVa). Although the median grade of complications per patient was equal in the groups (II), there was a greater variability (IV vs I-IIIb) in the HTr than in the LTr. Higher transfusion volumes were associated with longer anesthetic and surgery time, prolonged intubation, and longer ICU and inhospital stays; these conclusions are corroborated by the findings of Massicotte et al[39]and Ramos et al[40]in adult LT patient studies.
Other specific complications might be related to perioperative BP transfusion in LT patients. Feltracco et al[41]reported that intraoperative BP transfusion was a risk factor for early postoperative pulmonary complications. Li et al[9]observed an increase in infectious complications during in-hospital and ICU stays among LDLT adults in the early postoperative period. Furthermore, Pereboom et al[12]reported a higher incidence and mortality associated with intraoperative platelet transfusion and acute lung injury related to transfusion. Benson et al[42]also reported similar findings with plasma containing BP and a higher incidence of early postoperative infections with dosedependent RBC transfusion in adult LT.
During hospitalization occurred 30 .8 % of the total complications observed within the 10 years of follow-up. Minor metabolic complications (e.g., hydro electrolytic disorders, hypo- or hyper-glycemia, acidosis) were the most common. Nevertheless,no association between this type of complication and RBC transfusion volume was observed. Renal complications were not significantly different between groups, and dialysis was performed in seven patients during this period. HTr patients exhibited a higher frequency of major infectious complications (15 .7 % of complications, e.g., severe sepsis, with hemodynamic instability), general respiratory complications (11 .5 % of complications, e.g., pulmonary edema, pleural effusion, bronchospasm, pneumonia,and acute respiratory distress syndrome), general bleeding (2 .8 % of complications, e.g.,oozing and draining with a need for reoperation), and major cardiovascular complications (2 .1 % of complications, e.g., hemodynamic instability requiring vasoactive drugs, severe arrhythmias, and cardiorespiratory arrest) than LTr patients.HTr patients had a lower frequency of rejections (6 .3 % of complications, e.g., mild acute cellular reaction) and dermatologic complications (1 .2 % of complications, e.g.,oral ulceration and dermatitis.
Kloesel et al[19]did not observe significant differences in perioperative complications between the major and minor transfusion groups within the hospitalization period in pediatric LT patients despite an incidence rate of 43 % for massive bleeding. In this last study, conflicting results might be attributed to the fact that all patients received an RBC transfusion and 88 % of them received FFP transfusion thus creating a bias in the comparison between groups. Aside from increasing the risk of complications, RBC transfusion is a predictive factor for survival in adult[28 ] and pediatric LT[43]. In our study, patients with MaC presented reduced graft survival but patient survival was not affected relative to patients with no or MiC. Meanwhile, patients who underwent a HTr showed both worse graft and patient survival relative to patients of LTr.
The late period had 69 .2 % of the total complications with 55 .3 % up to the first year.Among the late postoperative complications of LT, infections are the leading cause of death[44]. In line with this, the frequency of major complications in HTr patients was higher at 1 up to 10 years post-LT. The most frequent major complications were infectious (15 .8 %-8 .7 % of complications, e.g., severe sepsis), pulmonary (5 .0 %-4 .7 % of complications, e.g., severe pneumonia requiring mechanical ventilation), and cardiovascular (2 .0 %-1 .2 % of complications, e.g., cardiorespiratory arrest). In the HTr patients, the frequency of minor neoplastic complications was significantly higher at 10 years (2 .3 % of complications, e.g., PTLD) than in LTr patients. The frequency of rejections was lower in the HTr at 1 to 10 years post-LT (8 .0 %-8 .7 % of complications,e.g., mild acute cellular reaction) than in the LTr. Renal complications were still not different between groups, and dialysis was necessary in 14 patients up to 10 years.Dermatological complications are common among LT patients and are related to genetics, allergic factors, and the side effects of immunosuppressive drugs.Surprisingly, patients with HTr had fewer dermatological complications than patients with LTr up to 1 year after LT (1 .9 %). This could be attributed to a lower frequency of rejections and, consequently, less of a need for immunosuppressive drugs. However,this would fail to explain why the rate of dermatological complications does not differ significantly afterward while rejections remained lower in HTr throughout the study.A higher-volume transfusion of RBC might have had some influence. Notwithstanding the fact that such complications are usually related to LT[45], there is no strong evidence of the association of perioperative BP transfusion and long-term outcomes in pediatric LT. We found a significant difference in 30 d and 10 -year postoperative mortality rates between the LTr and the HTr (0 vs 6 .6 % and 7 .8 vs 25 .5 %), respectively, confirming that HTr patients generally experienced more severe complications.
Concerning LT-specific complications, the incidence of PNF corresponded to 2 .1 %and was not related to transfusion. This is consistent with others who reported an incidence of 0 .9 %-8 .5 %[46]. Hypercoagulability is a risk factor for vascular thrombosis in LT, mainly in children, as determined by rebalanced hemostasis in cirrhosis, technical vascular issues, inflammatory response to trauma, and massive transfusion[47 ,48].Incidences of HAT have been reported to be 4 %-8 % and PVT of 5 %-10 %[49 -51]. In our study, the overall incidence of HAT and PVT was 4 .6 % and 13 .3 %, respectively.Nevertheless, no relation between transfusion volume and an increase in arterial or venous thrombosis were observed, in both, short- and long-term periods. The lower incidence of HAT is likely related to the microsurgical anastomosis technique whereas the higher incidence of PVT may be attributed to previous portal hypoplasia frequently observed in patients with biliary atresia-this was the most prevalent underlying disease in our cohort. Portal vein graft interposition was employed in 37 (15 .4 %) patients of the total cohort, and only three cases evolved with PVT. Biliary complications accounted for 18 .3 % of the total cases, and neither fistulas nor stenosis were related to RBC transfusion. The incidence rate of biliary complications in PLDLT has been reported to be 10 %-20 %[52], depending on the size of the graft and subsequent technical difficulties. Reoperations corresponded to 25 .8 %, which is consistent with others who reported an incidence of 8 %-29 %[33]in PLDLT. In the short-term period,reoperation (18 .7 %) was three-fold higher in the patients who received an RBC volume higher than 27 .5 mL/kg (HTr), due mostly to bleeding and intestinal injuries. In the long-term period, reoperations were not related to perioperative transfusion.Retransplantation corresponded to 3 .8 % and was not related to RBC transfusion. The incidence was below the historical average of 9 %-29 %[53], probably because of the lower incidence of total hip arthroplasty and the good quality of the grafts. Although the HTr had fewer minor rejection episodes, such as a mild acute cellular reaction over 10 years, this fact did not impact the retransplantation rate in both, short- and longterm periods.
Immunosuppression associated with blood transfusion occurs via a decrease in the number and function of natural killer cells, a decrease in cytotoxic T-cell function, an increase in the number of suppressor T-cells, and a reduction in macrophage and monocyte function[54]. Blood transfusion may induce immunomodulatory effects (both proinflammatory and immunosuppressive) that are of variable intensity and longterm duration. These antagonistic effects are associated with a decrease in rejection episodes[55 ] as well as an increase in the frequency of infection[56 ], neoplasia[57], and tumor recurrence[8]. We essentially found that HTr patients displayed more major infections and fewer rejections during early and late postoperative periods and more minor neoplastic complications in the late postoperative period than LTr patients.
Detailing the type, severity, and chronology of postoperative complications is of paramount importance for a better understanding of the clinical evolution. It can assist in the implementation of preventive measures that may positively impact the outcome of PLDLT.
Several strategies have been adopted to decrease perioperative transfusion in adult LT patients. They can be classified into three groups of measures: (1 ) Prophylactic such as the recognition of patients at risk for bleeding and the previous suspension of drugs that interfere with coagulation; (2 ) Technical such as maintenance of low central venous pressure, controlled hypotension, use of vascular clamping, ultrasonic or argon scalpels, and capture and reuse of blood lost; guided fluid therapy by multiparametric data, reduction of transfusion trigger values, and viscoelastic tests; and (3 )Pharmacological such as erythropoietin, desmopressin, vasopressin, antifibrinolytics,prothrombin complex, lyophilized fibrinogen, recombinant factor VIIa, fibrin sealants,and vasoactive drugs[58 ,59]. Certainly, not all of them apply to this population, that remain to be a challenge in conducting LT. Fluid management in small children undergoing LT cannot be guided by minimally invasive multiparametric monitors.These are of limited use, once their softwares are designed for adult patients.Nonetheless, the analysis of the trend curves can assist in decision-making, there is a lack of accuracy in the assessment of volemia, hemodynamic state and effect of vasoactive drugs during the perioperative period of pediatric LT. Besides, techniques as hemodilution and controlled hypotension are not validated in this group.Hemodilution increases hydrostatic pressure in the portal vein and inferior vena cava system and worsens the coagulopathy, exacerbating surgical bleeding. Controlled hypotension is a debatable issue and might be of potential risk for target organ damage. It is crucial to recognize that small children with chronic liver disease have a tenuous rebalance of the hemostatic system, not entirely understood, which might be easily disrupted by hasted interventions, pushing the patient towards hemorrhage and/or thrombosis. Prophylactic use of FFP is not advised, because it can increase intravascular pressure and increase RBC transfusion. Routine prophilatic use of antifibrinolytic drugs is no longer recommended, tranexamic acid and aminocaproic acid are possibly useful for patients in hyperfibrinolysis, demonstrated by microvascular oozing or viscoelastic tests. Prophylactic use of recombinant factor VIIa,should be avoided in all, except for highest-risk procedures[60]. Although, preoperative blood transfusion has been demonstraded to be independently associated to morbidity up to 30 d of postoperative period and harmful in neonates undergoing general pediatric surgery, neurosurgery, otolaringology, cardiothoracic, plastics and urology surgery[61], no strong evidence is found in pediatric LT in the long-term period.Concurrent transfusion of “red” and “yellow” BP, in adult liver resection with compromised function, was associated with a significantly higher risk of postoperative morbidity compared to only RBC or only FFP transfusion, what might be attributed to synergistic effects[2]. Though, no similar study was conducted in pediatric LT. If there is an absence of universal definition of massive bleeding or massive transfusion and a scarcity of studies relating survival to specifc BP dosages, ratios, timing and guidance even in adult trauma victims[62], let alone pediatric LT in small children. Specific transfusion trigger thresholds in pediatric LT have not been validated and need to be determined by prospective controlled studies that seek to standardize patient samples,according to age or weight, underlying diseases, type of donor and type of graft.
This study does have some limitations. First, this was a retrospective study performed in a single center. Second, the collection of data was refined on an ongoing basis since the implementation of the LT program; the learning curve may have influenced the results. Third, and most importantly, complications due to increased transfusion volume may be an epiphenomenon related to a sicker patient and of higher technical difficulty, or, indeed, a risk factor for postoperative morbimortality.Nonetheless, this study has several strengths, such as the size and homogeneity of the sample as well as the standardization of the anesthetic/surgical approaches and the immunosuppression regimen. The follow-up was conducted in the same center, which included facilities for patients and their families to remain close during local treatment thus improving patient recruitment and reducing loss to follow-up throughout the study period.
CONCLUSION
In this study, blood transfusion volumes less than one total blood volume, though not considered massive transfusions, were already associated with a higher incidence of more serious complications and mortality as assessed by hospitalization up to 10 years after PLDLT. A perioperative RBC transfusion volume higher than 27 .5 mL/kg is associated with not only increased rates of infectious, cardiovascular, respiratory, and neoplastic complications but also decreased frequency of rejection episodes.Furthermore, a perioperative volume of RBC transfusion higher than 27 .5 mL/kg is an independent risk factor for mortality and is directly related to reduced patient and graft survival in PLDLT. These results underscore the need for more restrictive criteria to guide the use of blood transfusion in PLDLT patients to prevent potentially related postoperative complications.
Appropriate protocols should be tailored to each center according to the infrastructure, clinical staff experience, and patient’s profile. Indeed, some strategies to reduce blood consumption should be implemented. An accurate nutritional assessment with specific dietary support and early supplementation is mandatory.Treatment with iron and vitamins should be considered. Prophylaxis of digestive bleeding and treatment of renal dysfunction and infection can decrease the incidence of preoperative anemia. The use of recombinant human erythropoietin therapy is controversial. Reduce blood tests and perform microsampling. During surgery, a more restrictive fluid management and a reduction of Hb trigger values to less than 8 .0 g/dL, could reduce blood transfusion especially when combined with low doses of continuous infusion of norepinephrine. This could mitigate fluid overload, reduce portal hypertension, restore splanchnic and central circulatory imbalances and optimize tissue oxygenation. Assessment of coagulation with viscoelastic tests to improve blood management in pediatric surgery is feasible, but specific algorithms must be developed. The goals are to optimize the erythrocyte mass, minimize blood loss, increase tolerance to anemia and maintain hemostatic balance. As demonstrated,a small reduction in perioperative RBC transfusion volume may determine a better outcome in the short- and long-term postoperative periods. The evaluation of risk,effectiveness, and cost-benefit assessment of these strategies in young children with liver diseases is outside the scope of the present study and should be carried out in future research.
ARTICLE HIGHLIGHTS


Research motivation
To study in depth the short- and long-term evolution of this specific group of highly fragile pediatric patients, in order to improve the proficiency acquired in 20 years of working with PLDLT, and to be able to share knowledge.
Research objectives
This study assessed whether perioperative transfusion is associated with early and late postoperative complications and mortality in small patients undergoing PLDLT.
Research methods
Postoperative complications along 10 years of follow up were graduated with Clavien-Dindo modified classification in order to assess relationship between blood transfusion and postoperative complications. Multiple logistic regression analysis identified risk factors for major postoperative complications. Perioperative red blood cells volume was identified as a single risk factor and a receiver operating characteristic curve identified a cutoff point of 27 .5 mL/kg. Cox regression analyses identified independent risk factors for mortality. Overall patient and graft survival analyses was performed using Kaplan–Meier survival curves, which were compared using the logrank test and a P < 0 .05 was considered statistically significant.
Research results
In terms of red blood cells (RBC) transfusion volume, there was a significantly higher rate of 30 d reoperation (26 .3 % × 8 .7 %, P < 0 .001 ) and 30 d mortality rate (6 .6 % × 0 .0 %,P < 0 .001 ) in the high-volume transfusion (HTr) vs low-volume transfusion (LTr),respectively. Early liver transplantation (LT)-specific complications include primary non-function, biliary complications, vascular thrombosis, and retransplantation that were not related to a higher perioperative transfusion volume. Over 10 years of followup, with respect to RBC transfusion volume, there was a significantly higher rate of reoperation (36 .5 % × 12 .6 %, P < 0 .001 ) and mortality (25 .5 % × 7 .8 %, P < 0 .001 ),respectively, in the HTr compared to the LTr. Perioperative RBC volume > 27 .5 mL/kg and preoperative estimated glomerular filtration rate were identified as independent risk factors for mortality over 10 years of follow-up after LT. The patient survival rates were significantly lower in the HTr than in the LTr at 1 , 5 , and 10 years post-LT: 82 .7 %vs 97 .7 %, 73 .9 % vs 93 .8 %, and 72 .6 % vs 90 .9 %, respectively. Likewise, the graft survival rates were significantly lower in the HTr than in the LTr at 1 , 5 , and 10 years post-LT:79 .5 % vs 97 .7 %, 67 .2 % vs 92 .3 %, and 67 .2 % vs 87 %, respectively.
Research conclusions
A perioperative RBC transfusion volume > 27 .5 mL/kg is associated with not only increased rates of infectious, cardiovascular, respiratory, and neoplastic complications but also decreased frequency of rejection episodes. Furthermore, a perioperative volume of RBC transfusion higher than 27 .5 mL is an independent risk factor for mortality, and is directly related to reduced patient and graft survival in PLDLT.
Research perspectives
The detailed analysis of this study allows the construction of strategy protocols to reduce the need for transfusion of patients undergoing PLDLT improving short- and long-term outcome.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Vinicius Fernando Calsavara, statistical researcher at the International Research Center of AC Camargo Cancer Center, professor at the postgraduation course of Antonio Prudente Foundation and member of the Research Ethics Committee of AC Camargo Cancer Center for performing the statistical analysis and providing vital technical support.
 World Journal of Gastroenterology2021年12期
World Journal of Gastroenterology2021年12期
- World Journal of Gastroenterology的其它文章
- Cascade of care for children and adolescents with chronic hepatitis C
- R2 * value derived from multi-echo Dixon technique can aid discrimination between benign and malignant focal liver lesions
- Risk perception and knowledge of COVID-19 in patients with celiac disease
- Risk stratification and geographical mapping of Brazilian inflammatory bowel disease patients during the COVID-19 outbreak: Results from a nationwide survey
- Hepatitis E in solid organ transplant recipients: A systematic review and meta-analysis
- Primary localized gastric amyloidosis: A scoping review of the literature from clinical presentations to prognosis
