Management scheme influence and nitrogen addition effects on soil CO2,CH4,and N2O fluxes in a Moso bamboo plantation
Junbo Zhang,Quan Li,Jianhua Lv,Changhui Peng,Zhikang Gu,Lianghua Qi,Xuzhong Song and Xinzhang Song*
Abstract
Keywords: Greenhouse gases, Management practices, Nitrogen addition, Phyllostachys edulis, Q10
Introduction
Extensive research on soil greenhouse gases (GHGs) has shown that CO2, CH4, and N2O released from soil significantly contribute to global warming (IPCC 2013; WMO 2019). Atmospheric concentrations of CO2, CH4, and N2O have increased considerably from 278 ppm, 722 ppb, and 270 ppb in 1750 to 408 ppm, 1869 ppb, and 331 ppb in 2018, respectively (WMO 2019). Forest ecosystems, as an important source of atmospheric CO2and N2O and an important sink of CH4(Liu and Greaver 2009),play a key role in regulating GHG fluxes under global climate change.
Recently, atmospheric N deposition has drastically increased in East and South Asia and is expected to continue to increase (Galloway et al. 2008; Reay et al. 2008;Tian et al. 2016), particularly in subtropical China (Liu et al. 2013), where N deposition has reached 30.9 kg N·ha−1·year−1(Xie et al. 2008) and is predicted to reach 50 kg N·ha−1·year−1by 2050 (Galloway et al. 2004).Many studies have shown that N deposition can significantly influence forest soil CO2, CH4, and N2O emissions, including both promotion and inhibition effects(Zhang et al. 2008a; Liu and Greaver 2009; Deng et al.2020). For example, in temperate forests, the effect of N deposition on soil CO2emissions includes promotion(Bowden et al. 2004; Zheng et al. 2018) and inhibition(Geng et al. 2017), as well as no effect (Krause et al.2013; Sun et al. 2014). N deposition reduces (Sitaula et al. 1995; Gulledge et al. 2004; Kim et al. 2012; Krause et al. 2013) or increases (Geng et al. 2017) soil CH4uptake and accelerates soil N2O emissions (Venterea et al.2003; Kim et al. 2012; Krause et al. 2013; Sun et al. 2014;Song et al. 2017a). In tropical forests, N deposition reduces soil CO2emissions (Mo et al. 2008; Cusack et al.2011; Tian et al. 2019); enhances (Zhang et al. 2008b;Wang et al. 2014; Tian et al. 2019) or has no effect(Zhang et al. 2008b; Müller et al. 2015; Tang et al. 2018)on soil N2O emissions; and inhibits or has no effect on soil CH4uptake (Zhang et al. 2012). N deposition was shown to promote soil N2O emissions in an N-saturated forest (Xie et al. 2018), while it increased soil CO2emissions in a bamboo ecosystem (Tu et al. 2013), an evergreen forest (Gao et al. 2014), and a highly P-limited forest (Liu et al. 2019). Additionally, Wang et al. (2015)found that N deposition promoted soil N2O emissions but reduced soil CH4uptake in a slash pine plantation.Li et al. (2015) also measured the effects of N deposition in a slash pine plantation and found that soil CO2and N2O emissions increased, but soil CH4uptake was unaffected. These inconsistent results indicate that the effects of N deposition on GHG emissions strongly depend on forest type. Moreover, most studies have only observed the fluxes of one or two GHGs in forest soil under N deposition (Jassal et al. 2010; Kim et al. 2012;Wang et al. 2015; Tian et al. 2019). Studies that simultaneously measure the fluxes of three GHGs in forest soils under increasing N depositions are scarce(Krause et al. 2013; Song et al. 2017a), especially in subtropical plantations (Li et al. 2015;Song et al. 2020).
An increasing number of plantations have been established in recent years to satisfy the increasing global demand for timber products(FAO 2005).As one of the forestry practices that increases productivity,intensive management(IM)is essential for meeting current and future timber needs (McEwan et al. 2020). Several studies have shown that IM significantly increases CO2emissions(Mori et al.2017;Yang et al.2017)but does not affect CH4or N2O emissions from forest soils (Mori et al. 2017). Moso bamboo (Phyllostachys edulis) plantations are one of the most important types of forests in southern China. Approximately 4.43 million hectares are under Moso bamboo cultivation in this region,comprising 84.02%of the global Moso bamboo forest area(Song et al.2017b).In recent decades,owing to the high economic and ecological benefits of Moso bamboo,an increasing number of farmers have utilized IM practices to replace conventional management (CM) practices in Moso bamboo plantations, including fertilization, plowing, and weeding understory vegetation (Song et al. 2015). In Moso bamboo plantations, IM has been observed to significantly increase soil CO2emissions (Liu et al. 2011; Tang et al. 2016), while its effects on soil N2O and CH4fluxes remain unknown.Therefore, the effect of IM on soil GHG emissions in Moso bamboo plantations is an empirical gap that needs to be addressed.
Furthermore, Moso bamboo plantations are located in subtropical China where N deposition had increased dramatically in recent years (Liu et al. 2013). Our previous study showed that N deposition increased soil CO2emissions (Li et al. 2019) and N2O emissions (Song et al. 2020) but decreased soil CH4uptake(Song et al.2020)in Moso bamboo plantations under IM.However,the comprehensive effects of management scheme combined with N deposition on soil GHG fluxes in Moso bamboo plantations remain unclear.Here, we studied the individual and combined effects of N deposition and management scheme on soil CO2, CH4, and N2O fluxes for one year in a Moso bamboo plantation. We hypothesized that (1) IM increases CO2emissions but does not affect N2O emissions or CH4uptake, because IM can promote soil respiration; (2) N addition promotes CO2and N2O emissions but inhibits CH4uptake under CM, because N addition can promote soil respiration,nitrification and denitrification but inhibit methane oxidation;and(3)IM intensifies the effect of N addition on soil GHG fluxes, because IM can provide more nutriment,especially N.
Materials and methods
Study site
The details of the study site were reported previously(Song et al. 2015). Briefly, the site is located in Lin’an District (30°14′ N, 119°42′ E), Hangzhou City, Zhejiang Province, China, and is characterized by a subtropical monsoon climate with a mean annual temperature of 15.6°C and a mean annual precipitation of 1420 mm.The soil belongs to the Ferrisols derived from granite(Song et al. 2015). The landscape is hilly, with an elevation range of 100 to 300 m a.s.l. The Moso bamboo plantations were initially established in the late 1970s to replace a native evergreen broadleaf forest, with similar soil type and topography (a southwest slope of approximately 6°). Moso bamboo forests in the study area are divided into CM and IM plantations according to the management scheme to which they are subjected. Conventionally managed plantations are selectively and regularly harvested for bamboo stems and shoots according to demand, with no other management practices in place. In IM plantations, additional management practices such as plowing, weeding by herbicide spray, and fertilization are practiced, in addition to bamboo harvesting as per CM plantations. Specifically, every year in September, fertilizers (67.5 kg N·ha−1, 11.8 kg P·ha−1,and 74.7 kg K·ha−1) are evenly spread on the ground and then plowed to mix with the 30-cm topsoil (Song et al.2015). Compared with CM plantations, IM plantations have fewer understory species and lower shrub and herbal biomass. Forest stand and soil characteristics at the study site are shown in Table 1 (Song et al. 2015).
Experimental design and measurements
In November 2012, 24 plots (20 m×20 m) with a 20-mwide buffer zone (to avoid disturbing nearby plots) were set up in the Moso bamboo plantations of the study site.According to the N-deposition simulation method reported by Mo et al. (2007) and background atmospheric N deposition data of the site (30.9 kg N·ha−1·year−1; Xie et al. 2008), the N addition rate was set to equal, double,and triple the local N deposition rate. There were 12 IM plots and 12 CM plots with three replications of four treatment levels: control (ambient N deposition), N30(low N treatment, ambient +30 kg N·ha−1·year−1), N60(medium N treatment, ambient +60 kg N·ha−1·year−1),and N90 (high N treatment, ambient +90 kg N·ha−1·year−1) (Song et al. 2015, 2017c). The N source for Ndeposition simulation was ammonium nitrate (NH4NO3;Song et al. 2015). It has been reported that NH4+and NO3−account for 56.1% and 43.9% of the wet N deposition in China, respectively, and the average NH4+:NO3−ratio was 1.28 (Lei et al. 2016). From January 2013 to December 2015, the amount of NH4NO3(Xilong Chemical Co. Ltd., China) corresponding to each N treatment was dissolved in 10 L of water and uniformly sprayed on the forest floor of each N-treated plot in CM and IM plantations once a month (Song et al. 2015).Each control treatment plot was sprayed with 10 L of Nfree water to balance the effects of added water.
Measurement of soil GHG fluxes
Soil CO2, CH4, and N2O were collected using the static chamber method. The static chamber assembly consists of a permanently mounted base box (40 cm×40 cm×10 cm) with a U-shaped groove (5 cm wide and 5 cm deep)at the top and a removable cover box (40 cm×40 cm×40 cm). During gas sampling, the cover boxes were placed onto the base boxes and the grooves filled with water to serve as an air seal. A small fan was installed in each chamber to mix the air within the chamber during sampling. The base frame was directly inserted 5 cm into the soil in January 2013. Sampling was conducted between 9:00 am and 10:00 am to minimize the influence of variation.Four samples were taken with a 60-mL plastic syringe attached to a 3-way stopcock every 10 min for 30 min (i.e., at 0, 10, 20, and 30 min). Button thermometers (iButton DS1923; Wdsen Electronic Technology Co.Ltd., China) buried at a depth of 5 cm were used to monitor soil temperature at hourly intervals. GHG concentrations were analyzed after Li et al. (2019) using a gas chromatographer (GC-2014 Shimadzu Corp., Japan)within two days. We collected GHG emission data on a clear day once a month from January to December 2015.Gas fluxes were calculated using the following equation(Li et al. 2019):
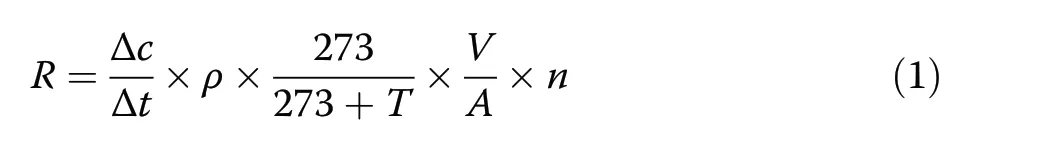
where R is gas flux (mg CO2-C·m−2·h−1for CO2, μg CH4-C·m−2·h−1for CH4, and μg N2O-N·m−2·h−1for N2O), ρ is gas density under normal conditions(mg·m−3), V is the volume of the static chamber (m3), A is the area that the static chamber covered, Δc/Δt is the change in gas concentration (Δc) during a certain time(Δt), T is air temperature (°C), and n is the coefficientfor converting the masses of CO2, CH4, and N2O to the masses of C and N (12/44 for CO2, 12/16 for CH4, and 28/44 for N2O).
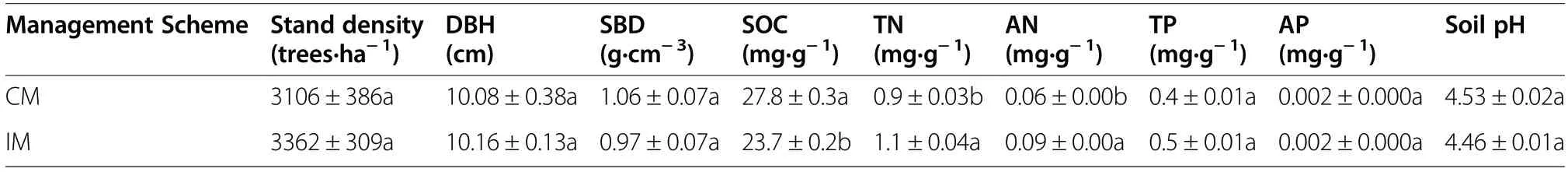
Table 1 Forest stand and soil characteristics in the Moso bamboo forests at the study site
The following equation was used to calculate cumulative soil CO2, CH4,and N2O fluxes (Liu et al. 2011):
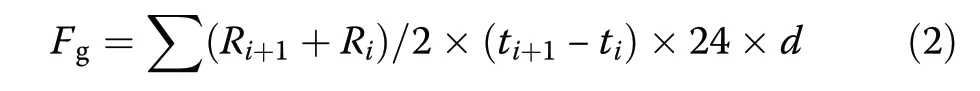
where Fgis cumulative soil CO2(kg CO2-C·ha−1·year−1), CH4(kg CH4-C·ha−1·year−1), or N2O (kg N2ON·ha−1·year−1) flux; R is soil CO2(mg CO2-C·m−2·h−1),CH4(mg CH4-C·m−2·h−1), or N2O (mg N2O-N·m−2·h−1)flux determined at each sampling time;i is the sampling number, t is the sampling time, and d is the number of days in each month.
Based on these measurements, an exponential regression model was used to describe the relationship between soil CO2efflux and soil temperature (Li et al.2019):
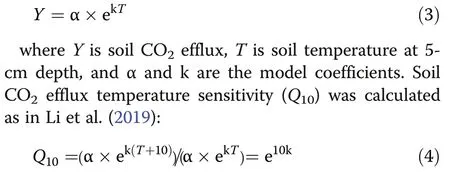
The Global warming potential (GWP) metric was developed to allow comparisons of the global warming impacts of different gases. The GWP of soil GHG emissions was computed by considering the respective GWP coefficients of CH4and N2O using the following equation (Tian et al. 2015):
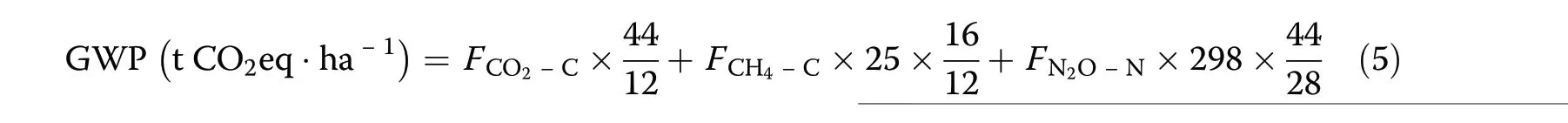
where FCO2−C, FCH4−C, and FN2O−Nare the annual fluxes of CO2, CH4, and N2O respectively based on the masses of C and N; 25 and 298 indicate the radiative forcing of CH4and N2O, respectively, in terms of a CO2eq unit at a 100-year time horizon (Forster et al. 2007).
Soil analysis
Ten soil cores (3.5-cm inner diameter) were collected randomly from the topsoil layer (0-20 cm) in each plot in every quarter of 2015. The soil samples were sieved through a 0.15-mm sieve and divided into two portions. One portion was air-dried for measuring soil pH and conducting soil organic carbon (SOC),soil total N (TN), and soil total phosphorus (TP) assays, and the other portion was stored in a refrigerator for measuring soil microbial biomass carbon(MBC), NH4+, and NO3−. Briefly, soil pH was measured in a soil:water ratio of 1:2.5 using a pH meter(Li et al. 2016). Soil SOC and TN concentrations were determined using an elemental analyzer (Elementar Vario EL III; Germany). Soil TP was extracted with a Bray-2 solution (Bray 1945) and determined using the molybdate blue colorimetric method. Soil MBC was estimated using the chloroform-fumigation extraction method (Bao 2000). Samples were extracted with a 2-mol·L−1KCl solution, and the concentrations of NH4+and NO3−were determined using a Dionex ICS 1500 ion chromatographer (Dionex Corp. Atlanta, GA).
Statistical analysis
Data analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) for Windows. One-way analysis of variance (ANOVA) and least significant difference multiple comparisons were used to identify significant differences in Q10, GWP, and soil CO2, CH4, and N2O fluxes. Two-way ANOVA was used to test the significance of the interaction between N addition and management scheme for the variation in soil CO2, CH4, and N2O fluxes. All data were tested for homogeneity of variance and distribution normality before conducting the ANOVA. In addition, Pearson’s correlation analyses between soil characterization and soil CO2, CH4, and N2O fluxes were conducted.
Results
Soil GHG fluxes
The seasonal variation of soil CO2flux showed the same pattern in all treatments, peaking in summer and reaching a trough in winter (Fig. 1). Compared with CM, IM significantly promoted annual soil CO2emissions by 7.5%. Compared with the control treatment, N30 and N60 significantly promoted annual soil CO2emissions by 31.7% and 22.1% in CM plots, and by 34.0% and 20.9%in IM plots,respectively, while N90 had no significant effect on annual soil CO2emissions in either CM or IM plots (Fig. 1). Management scheme had no significant effect on annual soil CH4uptake. Compared with the control treatment, N30 had no significant effect on annual soil CH4uptake in either CM or IM plots, but N60 and N90 significantly inhibited annual soil CH4uptake by 23.6% and 27.1% in CM plots, respectively(Fig. 2).

Fig.1 Soil CO2 emission rates under different N addition treatments (control,0 kg N·ha−1·year−1;N30,30 kg N·ha−1·year−1; N60,60 kg N·ha−1·year−1;N90,90 kg N·ha−1·year−1)in plots under conventional management (CM,a)or intensive management (IM,b)and annual soil CO2 emissions(c)from a Moso bamboo plantation(mean±standard deviation,n=3).Lowercase letters indicate differences in soil CO2 emissions under different N addition treatments in CM plots(P<0.05).Uppercase letters indicate differences in soil CO2 emissions under different N addition treatments in IM plots(P<0.05).The asterisk indicates differences in soil CO2 emissions between different management schemes under the same N addition treatment(P<0.05)
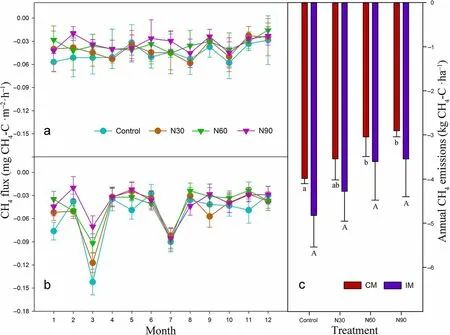
Fig.2 Soil CH4 emission rates of different N addition treatments(control,0 kg N·ha−1·year−1;N30,30 kg N·ha−1·year−1;N60,60 kg N·ha−1·year−1;N90,90 kg N·ha−1·year−1)in plots under conventional management(CM,a)or intensive management(IM,b)and annual soil CH4 emissions(c)from a Moso bamboo plantation(mean±standard deviation,n=3).Lowercase letters indicate differences in soil CH4 emissions under different N addition treatments under CM(P<0.05).Uppercase letters indicate differences in soil CH4 emissions under different N addition treatments under IM(P<0.05)

Fig.3 Soil N2O emission rates from plots under different N addition treatments (control, 0 kg N·ha−1·year−1; N30,30 kg N·ha−1·year−1; N60,60 kg N·ha−1·year−1;N90,90 kg N·ha−1·year−1)under conventional management (CM,a)or under intensive management (IM,b), and annual soil N2O emissions(c)from a Moso bamboo plantation(mean±standard deviation,n=3).Lowercase letters indicate differences in soil N2O emissions from plots under different N addition treatments in CM plots (P<0.05).Uppercase letters indicate differences in soil N2O emissions from plots under different N addition treatments in IM plots (P<0.05).The asterisk indicates a significant difference in soil N2O emissions between management schemes with the same N addition treatment(P<0.05)
The dynamics of annual soil N2O emission rates were not significantly affected by management scheme, but N addition caused N2O emission rates to peak from March to April (Fig. 3). In CM plots, compared with the control treatment, N30 did not significantly affect annual soil N2O emissions, while N60 and N90 significantly promoted annual soil N2O emissions by 52.7% and 47.0%,respectively (Fig. 3). On the contrary, in IM plots, compared with control treatment, N30, N60, and N90 significantly promoted annual soil N2O emissions by 61.3%,69.2%, and 49.3%, respectively. Two-way ANOVA showed that N addition or management scheme independently had significant effects on soil CO2emissions,CH4uptake, and N2O emissions,but the interactions between them did not (Table S1).
The Q10value of soil CO2efflux varied from 1.89 to 2.37 under the different treatments combining management scheme and N addition (Table S2). IM significantly increased the Q10value by 11.3% relative to that in CM when no N was added. N addition had no significant effect on the Q10value in CM plots but significantly decreased the Q10value in IM plots(Table S2). Furthermore, the significantly higher Q10value in IM than in CM plots under no N addition treatments decreased under both N60 and N90 treatments (Table S2).
Soil CO2flux was significantly and positively correlated with soil MBC and TP concentrations but significantly and negatively correlated with soil SOC and TN concentrations, and C/N ratio (P<0.05, Table 2). Soil CH4flux significantly and negatively correlated with soil MBC, TP, and NH4+concentrations (P<0.05, Table 2).Soil N2O flux was significantly and positively correlatedwith soil MBC and NO3−concentrations and negatively correlated with C/N ratio and pH (P<0.05, Table 2).
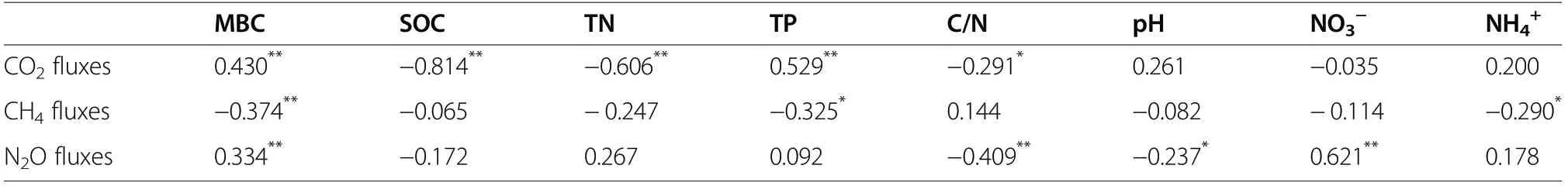
Table 2 Pearson correlation coefficients between soil physicochemical properties and CO2, CH4, and N2O fluxes
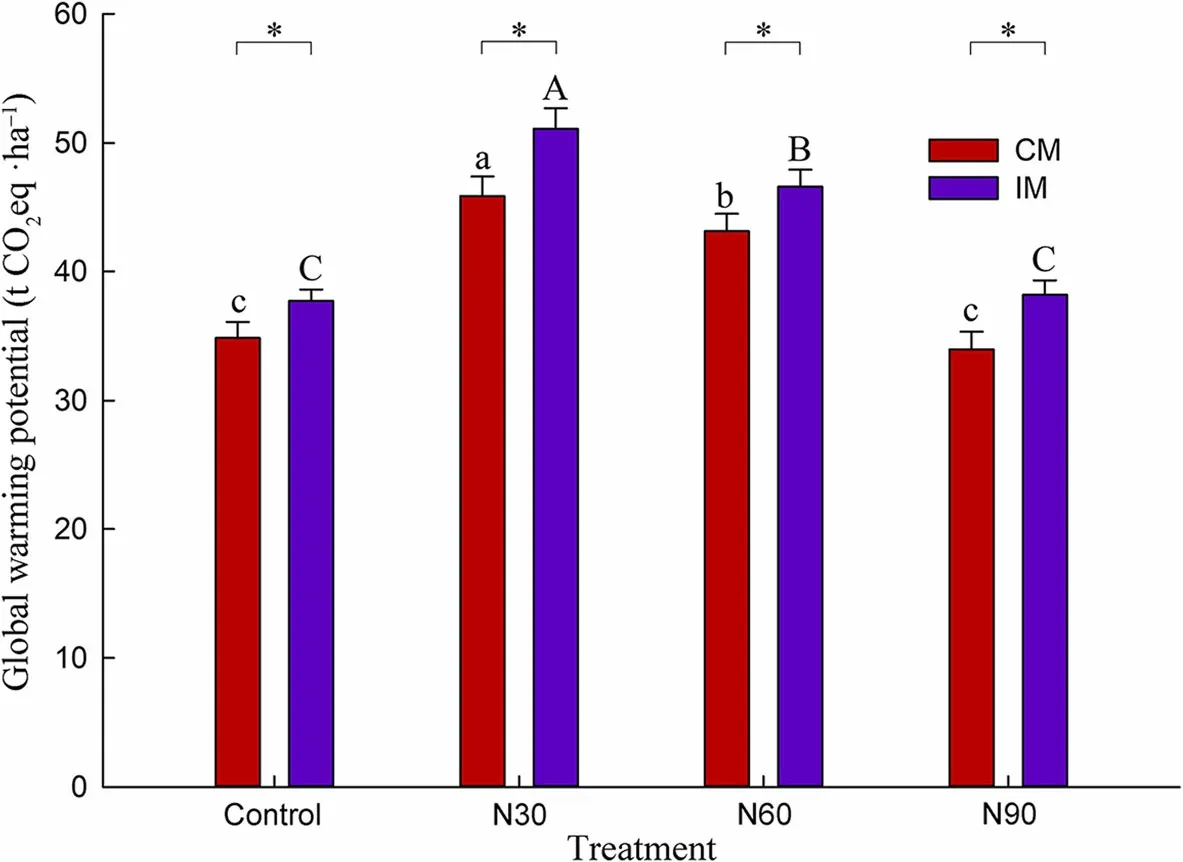
Fig.4 Annual global warming potential(GWP)of soil greenhouse gas emissions(CO2, CH4, and N2O)in Phyllostachys edulis plantations(mean±standard deviation,n=3).Lowercase letters indicate differences in GWP under different N addition treatments in CM plots(P<0.05).Uppercase letters indicate differences in GWP under different N addition treatments in IM plots(P<0.05).The asterisk indicates differences in GWP under different management schemes within the same N addition treatment(P<0.05)
Annual GWP of soil GHG fluxes
Annual GWP owing to soil CO2, CH4, and N2O emissions was 34.88±1.19 t CO2eq·ha−1in the Moso bamboo plantations under CM and without N addition(Fig. 4). Compared with CM, IM significantly increased the annual GWP by 7.98%. Furthermore, compared with the control treatment, N30 and N60 significantly increased the annual GWP by 31.5% and 23.7% in CM plots, and by 35.6% and 23.6% in IM plots, respectively,while N90 had no significant impact on the annual GWP under either CM or IM treatments.
Discussion
Effect of management scheme on soil CO2,CH4, and N2O emissions
We observed that the mean annual soil CO2, CH4, and N2O emission rates in control plots under CM were 9106.09±297.15 kg CO2-C·ha−1, −3.98±0.12 kg CH4-C·ha−1, and 3.48±0.46 kg N2O-N·ha−1, respectively(Table S3). Our study showed that the mean annual soil CO2emission rate in the Moso bamboo forest was higher than that of boreal forests (3220±310 kg CO2-C·ha−1·year−1), temperate coniferous forests (6810±950 kg CO2-C·ha−1·year−1), deciduous forests (6470±510 kg CO2-C·ha−1·year−1; Raich and Schlesinger 1992), subtropical bitter bamboo (Pleioblastus amarus) (4280±110 kg CO2-C·ha−1·year−1; Tu et al. 2013), and Chinese fir (Cunninghamia lanceolata) forests (6637.36±581.24 kg CO2-C·ha−1·year−1; Wang et al. 2018); however, it was lower than the corresponding rate in subtropical evergreen broad-leaved (11,509.09±463.64 kg CO2-C·ha−1·year−1; Liu et al. 2011) and tropical moist forests(12,600±570 kg CO2-C·ha−1·year−1; Raich and Schlesinger 1992). The mean annual soil CH4emission rate in this study was similar to that in mid-subtropical nature forests (−4.13±0.44 kg CH4-C·ha−1·year−1; Chen et al.2014) but lower than that in typical tropical montane rainforests (−1.93±0.15 kg CH4-C·ha−1·year−1; Yang et al. 2018), evergreen broad-leaved forests (−1.90 kg CH4-C·ha−1·year−1; Fang et al. 2009), larch plantations(−0.54 kg CH4-C·ha−1·year−1; Kim et al. 2012), and Korean pine forests (−0.05 kg CH4-C·ha−1·year−1; Song et al. 2017a).The mean annual soil N2O emission rate in this study was higher than that of typical tropical montane rainforests (1.67±0.04 kg N2O-N·ha−1·year−1; Yang et al. 2018), larch (Larix kaempferi) plantations (1.13 kg N2O-N·ha−1·year−1; Kim et al. 2012), and Korean pine(Pinus koraiensis) forests (1.11 kg N2O-N·ha−1·year−1;Song et al. 2017a) but was lower than that of evergreen broad-leaved forests (6.00 kg N2O-N·ha−1·year−1; Fang et al. 2009) and three subtropical forests (6.40±2.41 kg N2O-N·ha−1·year−1;Tang et al.2006).Overall,compared with other subtropical forests, bamboo forest soils under CM showed lower CO2, CH4, and N2O emission rates,which have a significant positive effect in decreasing the GWP of soil GHG emissions.
We found that IM significantly increased annual CO2emissions, which partially supports our first hypothesis and was consistent with the results of Liu et al. (2011) in Moso bamboo forests. However, some studies indicate that the state box method using linear fitting will cause an underestimation of soil CO2efflux (Wang 2005; Luo and Zhou, 2006). Therefore, the real soil CO2efflux may be greater in the Moso bamboo plantation. Soil CO2efflux mainly comprises root respiration and microbial respiration (Coleman 1973). IM practices, such as fertilization, stimulate root respiration (Jassal et al. 2010;Mori et al. 2013; Tang et al. 2016). Concomitantly,ploughing disaggregates soil and releases protected SOC(Sainju et al. 2008; Li et al. 2013; Tivet et al. 2013),which provides more substrate for microbial respiration,and the decomposition of protected SOC can increase soil CO2emissions. Meanwhile, fertilization increases soil MBC by providing abundant nutrients for microbial growth (Li et al. 2016) and accelerates the decomposition of organic matter by heterotrophic microorganisms(Cleveland et al. 2002; Ilstedt et al. 2003), resulting in a decrease in SOC concentration (Ma et al. 2011) and promotion of soil CO2emissions (Tu et al. 2013). We observed that soil CO2flux was significantly and positively correlated with soil MBC and significantly and negatively correlated with SOC (Table 2). IM had no significant effect on annual CH4uptake or N2O emissions, which partially supports our first hypothesis regarding the fluxes of these two gases, i.e., IM does not affect soil N2O emissions or CH4uptake. Previous studies have also shown that management does not affect soil CH4uptake or N2O emissions (Whalen and Reeburgh 2000;Jassal et al. 2010;Zhang et al.2015).The response of soil N2O emissions to external environmental factors and the influence on soil CH4uptake were determined to be the major reasons for the differences observed in soil GHG emissions (Yan et al. 2014). Some studies found that applying N fertilizer could stimulate soil N2O emissions in farmland soil (Jäger et al. 2013) and vineyard soil (Tatti et al. 2012). However, compared with CM, IM did not significantly increased soil N2O emissions (Fig.3), which may be attributed to infrequent fertilization(once a year).
The Q10value reflects the temperature dependence of soil CO2efflux, calculated from a series of soil CO2efflux measurements over a time period while soil temperature changes (Rey et al. 2002; Ma et al. 2014).The Q10value of the soil CO2efflux in plantations under CM is 2.13, which is close to the Q10value of Moso bamboo forests in the Wanmulin Natural Reserve (2.08;Wang et al. 2011) and the average Q10of bamboo forests in China (2.10; Song et al. 2014). Compared with CM,IM significantly increased the Q10of soil CO2efflux(Table S2). Tang et al. (2016) observed the same result and concluded that the main reason might be the increase in Q10of soil microbial respiration. In the present study and the previous study on the same site, IM significantly increased soil MBC (Table S4; Li et al.2016). Moreover, a significant positive correlation between soil CO2flux and soil MBC was found in this study (Table 2), which supports the conclusion of Tang et al. (2016). Li et al. (2020) have found that root respiration does not affect Q10of soil CO2efflux in forest ecosystems.
Compared with CM, IM significantly increased the annual GWP in Moso bamboo plantations (Fig. 4), which can be mainly attributed to the increase in CO2emissions (Table S5). The results suggest that IM induces greater GHG emissions from soils than CM did, although IM may enhance the productivity of Moso bamboo plantations (Zhou et al. 2010). Therefore, the C benefits of IM to Moso bamboo plantations need further comprehensive evaluation, especially in the scenarios of increasing atmospheric N deposition.
Effect of N addition on soil CO2, CH4, and N2O fluxes
In this study, N addition enhanced CO2emissions in both CM and IM plots, which partially supports our second hypothesis, i.e.,N addition promotes CO2emissions.Tu et al. (2009) observed that simulated N deposition promoted soil CO2emission in a bitter bamboo plantation. Some short-term simulated N deposition studies have also shown similar results (Madritch and Hunter 2003; Mo et al. 2005; Song et al. 2007). Soil CO2emissions are related to above-ground biomass, litter mass,underground root biomass, and soil biological factors(e.g., microorganisms and animals) (Zhang et al. 2008a).Our previous studies showed that N input increased the amount of leaf litter (Zhang et al. 2017), decomposition of leaf litter (Song et al. 2015), fine root litter (Song et al. 2017c), and soil microbial biomass (Li et al. 2016)in the current study site, all of which contributed to oxidizing organic C to CO2(Steudler et al. 1991; Emmett 1999), thus increasing CO2emissions. In this study, soil MBC significantly and positively correlated with CO2flux (Table 2), which supports the conclusion that N addition increased soil CO2emissions by increasing MBC. However, Li et al. (2017) found that the CO2emissions of the Moso bamboo forest soil did not change after N addition (40 kg N·ha−1, KNO3) in their incubation experiment, which was different from our experimental results. This difference may be owing to the difference in N source, the external environment of the experiment, and the processing time.
N addition significantly inhibited soil CH4uptake in CM, which supports our second hypothesis, i.e., N addition decreases CH4uptake in CM. Similar results have been observed in a Douglas fir stand (Jassal et al.2011) and a young Japanese larch plantation (Kim et al.2012). Soil CH4uptake rate is usually negatively correlated with soil NH4+concentration (Zhang et al. 2012),as was observed in this study (Table 2). The inhibitory effect of NH4+on soil CH4oxidation can be attributed to the production of the intermediates, hydroxylamine and NO2−, during the nitrification of NH4+, which likely inhibits the activity of methane-oxidizing bacteria,thereby extending the inhibition time (Nyerges and Stein 2009). Further, low pH can reduce the activity of methane-oxidizing bacteria (Semenov et al. 2004), because soil acidification may increase the concentration of Al3+in the forest soil solution, while Al3+ions have an obvious toxic effect on CH4-oxidizing bacteria(Nanba and King 2000;Tamai et al. 2007).
In this study, N addition (N60 and N90) significantly increased N2O emissions, which partially supports our second hypothesis, i.e., N addition increases soil N2O emissions.Similar results were observed in a young Japanese larch forest (Kim et al. 2012) and in incubated Moso bamboo forest soil (Li et al. 2017). N input can increase soil N availability, nitrification, and denitrification and, thus increase N2O emissions (Repo et al. 2009). N addition decreased soil pH significantly (Table S4), while NO2−may have induced aerobic denitrification in acidic soils (Mørkved et al. 2007), thereby increasing N2O emissions. A significant negative correlation between soil pH and soil N2O flux was found in this study (Table 2).N addition increased soil total N content and, thus decreased the soil C/N ratio (Li et al. 2019), which is beneficial for the proportion of external N input converted to N2O (Zhang et al. 2008a). Similarly, a significant negative correlation between soil C/N ratio and soil N2O flux was found in this study (Table 2).
N addition had no effect on Q10of soil CO2efflux relative to that in the control treatment in plots under CM but significantly decreased Q10value in IM plots(Table S2).Similar results have been reported by Mo et al. (2007), who found three-year high-N addition (150 kg N·ha−1·year−1) reduced Q10values in a mature tropical forest. Tu et al. (2013) also observed that N addition decreased Q10of soil CO2efflux in a bamboo ecosystem in southwestern China. Karhu et al.(2014) suggested that microbial community responses increase the temperature sensitivity of soil heterotrophic respiration. Our previous studies have shown that, although N addition significantly increased soil MBC, it also decreased soil pH(Li et al.2016),which might result in microbial activity being inhibited by soil acidity(Kunito et al.2016).This,in turn, may hamper the microbial community responses,whereby the microbial community has no effect on the temperature sensitivity of soil CO2efflux. Furthermore, our previous studies have shown that increases in soil MBC are inhibited when N addition exceeds 60 kg N·ha−1·yr−1(Li et al.2016).We suspect that N input from N addition and N fertilizer in IM treatments inhibited any increase in soil MBC and even reduced soil MBC. This might be why N addition did not affect the temperature sensitivity of soil CO2efflux under CM, yet it reduced the Q10value in the plots under IM.
The GWP of a GHG is a measure of how much energy the emissions of 1 kg of a gas will absorb over a given period of time relative to that absorbed by the emissions of 1 kg of CO2(Tian et al. 2015). The larger the GWP,the more that a given gas warms the Earth compared with CO2over that time period.Moderate N addition significantly increased annual GWP of soil GHG fluxes in both CM and IM plots, which is mainly attributed to the increase in annual soil CO2emissions. Annual soil CH4uptake and N2O emissions did not significantly affect annual GWP. The reason was that the GWP values of CH4and N2O were much larger than that of CO2(25 and 298 times, respectively), but the annual soil CH4uptake and N2O emissions were only 0.12‰-0.18‰ and 0.16‰-0.31‰of the annual CO2emissions,respectively.
In addition, IM enhanced soil CO2and N2O emissions under low N addition (N30), which partly supports our third hypothesis, i.e., IM promotes soil CO2and N2O emissions under N addition. The finding that IM provided more N input may be why IM significantly increased soil CO2and N2O emissions under low N addition. IM did not affect soil CH4uptake under N addition, which was consistent with the effect on plots without N addition, but it did not support our third hypothesis that IM inhibits soil CH4uptake under N addition. This may due to the combination of IM and N addition offsetting the inhibitory effect of N addition on soil CH4uptake. In summary, IM significantly increased GWP under low N addition owing to the main contribution of CO2emissions to GWP.
Conclusion
Compared with CM, IM significantly increased the GWP of soil GHG emissions and sensitivity of soil CO2efflux to soil temperature (Q10), mainly owing to an increase in soil CO2emissions. Nitrogen deposition (≤60 kg N·ha−1·year−1) significantly increased soil CO2and N2O emissions but inhibited CH4uptake, which resulted in a significant increase in GWP. However, N addition(>60 kg N·ha−1·year−1) decreased all soil CO2and N2O emissions and CH4uptake.Concomitantly, the Q10value of soil CO2efflux was significantly reduced after N addition in plots under IM, which indicates that N addition might mitigate the effect of future climate warming on soil CO2efflux in intensively managed Moso bamboo plantations. Soil MBC correlated significantly and positively with soil CO2and N2O fluxes but correlated negatively with soil CH4fluxes,indicating that soil microbes have a strong influence on soil GHG emissions. These results demonstrate that management scheme and N application influenced the GWP of the Moso bamboo plantation ecosystem under study.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1186/s40663-021-00285-0.
Additional file 1:Table S1.Two-way ANOVA of the effects of nitrogen(N)addition and management scheme on soil CO2, CH4,and N2O fluxes in a Moso bamboo forest. Table S2 Sensitivities of soil CO2efflux to soil temperature (Q10)at a depth of 5 cm. Table S3 Annual soil CO2,N2O and CH4emissions calculated in different treatment plots.Table S4 Soil physicochemical properties under different treatments (mean±standard error, n=12). Table S5 CO2emission, N2O emission and CH4uptake contribution to GWP in different treatment plots.
Acknowledgements
Many thanks are due to Honghao Gu and others for participation or helps in the data collection.
Authors’contributions
Xinzhang Song and Changhui Peng designed research, Junbo Zhang and Quan Li performed research, collected and analyzed data; All authors discussed the results and revised the manuscript. The author(s) read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China(Grant Nos.31270517 and 31470529).
Availability of data and materials
The datasets used and/or analyzed in this study are available from the corresponding author on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest.
Author details
1State Key Laboratory of Subtropical Silviculture,Zhejiang A&F University,Hangzhou 311300, China.2Department of Biology Sciences, Institute of Environment Sciences, University of Quebec at Montreal, Case Postale 8888,Succursale Centre-Ville, Montreal H3C 3P8, Canada.3Huzhou Research Institute of Forestry,Huzhou 313000, China.4International Centre for Bamboo and Rattan, Beijing 100102, China.5Zhejiang Academy of Forestry, Hangzhou 310023, China.
Received: 9 June 2020 Accepted: 13 January 2021
- Forest Ecosystems的其它文章
- Using GEDI lidar data and airborne laser scanning to assess height growth dynamics in fast-growing species:a showcase in Spain
- White oak(Quercus fabri Hance)regenerated stump sprouts show few senescence symptoms during 40 years of growth in a natural forest
- A multi-purpose National Forest Inventory in Bangladesh:design,operationalisation and key results
- Decomposition and stabilization of organic matter in an old-growth tropical riparian forest:effects of soil properties and vegetation structure
- Seamless integration of above-and undercanopy unmanned aerial vehicle laser scanning for forest investigation
- Effects of three coniferous plantation species on plant-soil feedbacks and soil physical and chemical properties in semiarid mountain ecosystems

