Application of Ag/ZnO composite materials in nitrogen photofixation: Constructing Schottky barrier to realized effective charge carrier separation
XIAO Yu, OUYANG Yu-xin, XIN Yue, WANG Liang-bing
Application of Ag/ZnO composite materials in nitrogen photofixation: Constructing Schottky barrier to realized effective charge carrier separation
XIAO Yu, OUYANG Yu-xin, XIN Yue, WANG Liang-bing*
(State Key Laboratory for Powder Metallurgy, School of Materials Science and Engineering, Central South University, Changsha 410083, China)
The photocatalytic reduction of nitrogen (N2) to ammonia (NH3) is a sustainable energy product method. Plasmonic photocatalysts can achieve effective conversion of solar energy via surface plasmon resonance (SPR), and therefore have attracted more and more attention. However, the photo-induced hot charge-carriers tend to recombine during N2reduction process. Ag nanoparticles with plasmon resonance effect with ZnO semiconductor (Ag/ZnO) and apply them to N2photofixation were studied. Compared with pure ZnO, Ag/ZnO exhibited enhanced catalytic activity in N2photofixation, and the NH3production rate at room temperature can reach 120 µmol·gcat.-1·h-1. Mechanism studies revealed that a Schottky barrier was formed at the interface between Ag nanoparticles and ZnO, which boosted the separation of photo-induced electron-hole pairs. Ag nanoparticles generated hot charge carriers via SPR effect, and the formed Schottky barrier facilitated the transfer of electrons from Ag to ZnO. The electron-rich Zn+species in ZnO was speculated to serve as active sites to adsorb and activate N2molecules, thereby promoting N2photofixation.
silver; surface plasmon resonance; Schottky barrier; N2photofixation; plasmonic catalysis
Nitrogen (N2) photofixation with water to ammonia (NH3) is a sustainable approach for producing valuable energy sources under mild conditions[1-2]. A wide range of catalysts have been designed and fabricated for N2photofixation[3-10]. With the capability to efficiently harvest and convert solar energy via SPR, plasmonic photocatalysts have attracted more and more attention recently[11-14]. SPR in plasmonic metals not only extends the adsorption range of light, but also generates hot electrons with enough energy for N2reduction during decaying[14-16]. For instance, light-harvesting plasmonic Au was coupled with catalytic Ru for N2photoreduction by Xiong and co-workers, and a NH3production rate of 101.4 µmol·gcat.-1·h-1was obtained[9]. The plasmonic hot electrons along with the enhanced electric field induced by SPR effect of Au were considered to promote the dissociation of N2molecular and further the conversion of N2to NH3. Recently, we constructed porous CuFe bimetals for plasmonic N2photofixation, where Cu frameworks generated hot electrons by SPR, while surface Fe atoms functioned as the active sites to adsorb and activate N2molecules[10]. Though some progress has been made in plasmon-assisted N2photofixation, the photo-generated charge carriersSPR often suffer from severe recombination during photocatalysis, resulting in low hot electrons concentration and low N2reduction rate. Fortunately, to integrate plasmonic metals with appropriate semiconductors is a promising approach for improving the efficiency of charge carriers separation through forming Schottky barrier at the interface[17-21]. Generally, Schottky barrier is formed at the interface through the downward bending of the conduction band of the semiconductor,so that the Fermi levels in the metal and the semiconductor are aligned[18]. The Schottky barrier filters energetic electrons to pass through the interface and inhibits the recombination of plasmonic charge carriers, leading to the effective separation of electron-hole pairs for N2photo-fixation[19-20].
Herein, we successfully synthesized Ag/ZnO photocatalyst by depositing plasmonic Ag nano-particles onto ZnO. Without adding any sacrificial agent, Ag/ZnO photocatalyst achieved a NH3production rate of 120 µmol·gcat.-1·h-1, which is about 2.1-fold higher than ZnO. NH3production rate retained more than 90% of initial activity after five successive reaction cycles, indicating the outstanding stability of Ag/ZnO. Mechanism studies demonstrated that the Schottky barrier was formed in Ag/ZnO photocatalyst, promoting the efficiency of charge separation. In situ XPS and XAS measurement revealed that Ag NPs generated hot charge carriers via SPR effect, which then moved to ZnO under the photocatalytic conditon. We further detected the electron-rich Zn+species in Ag/ZnO photocatalyst by ESR measurement, which was speculated to serve as active sites to adsorb and activate N2molecule. As a result, the Schottky barrier facilitated the transfer of electrons from Ag NPs to ZnO and the Zn+species enhanced the N2photofixation.
1 Experiments
1.1 Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O), 2-methylimidazole, silver nitrate (AgNO3), methane sulfonic acid (CH3SO3H), methanol (CH3OH), and ethanol (C2H5OH) were purchased from Sinopharm Chemical Reagent Co., Ltd. High purity nitrogen (N2, ≥99.999%) used in this work was gained from Saizongtezhong gas Co. LTD in Changsha. All solvents and chemicals were in analytical grade. All aqueous solutions were prepared using deionized water with a resistivity of 18.2 MΩ·cm–1.
1.2 Synthesis of ZnO and Ag/ZnO
Zeolitic imidazolate framework-8 (ZIF-8) was firstly prepared for the synthesis of ZnO. 3.7 g of Zn(NO3)2·6H2O and 4.1 g of 2-methylimidazole were added into 500 mL of methanol at room temperature. After 24 h, ZIF-8 was obtained by collecting the precipitation in the suspension solution. 100 mg of ZIF-8 was then heated to 500℃ in a muffle furnace and kept it at 500℃ for 3 h, he resulted powder was ZnO. The synthesized ZnO powder was immersed in a solution containing 1.07 mg of AgNO3, 35 mL of methanol, and 35 mL of ethanol, followed by stirring them at room temperature for 6 h. The resulted mixture was then transferred to a Teflon-lined stainless-steel autoclave and kept it at 120℃ for 8 h. The Ag/ZnO composite was finally acquired via suction filtration with copious deionized water and dried at 40℃ under vacuum for 2 hours.
1.3 Photocatalytic experiments
Catalytic tests for N2photofixation were carried out in a 100-mL glass reactor. For a typical reaction, 10 mg of photocatalysts and 20 mL of deionized water were added into a 100-mL photo-reactor, followed by bubbling N2at a flow rate of ca. 30 mL·min−1for 30 min. Subsequently, the suspension was irradiated under vigorous stirring by a Xenon lamp (Perfectlight PLS-SXE300) under full-spectrum with an intensity of 250 mW·cm-2. The generated NH3was analyzed via an ion chromatograph method. To test the recycling of Ag/ZnO, the first measurement was conducted as described above, and the used catalysts were then washed and collected by centrifugation with deionized water for the next catalytic cycle.
1.4 Detection of NH3
The concentration of NH3was determined by an ion chromatograph method. 4.5 mmol·L-1of methane sulfonic acid was used as the eluent solution with a flow rate of 1 mL·min-1. The column temperature and self-regenerating suppressor current were kept as 35℃ and 75 mA, respectively. 25 μL of quantitative injection loop was injected into the column. The retention time of NH4+cations was at ~4.0 min. A standard curve of peak areasthe concentrations of standard solution was firstly plotted. To determine the generated NH3, 25 μL of reaction solution was analyzed via this method. Based on the peak areas and standard curve, the concentration of NH3was obtained. It was worth mentioning that the acid in eluent solution guaranteed the generated NH3completely transformed to NH4+which can be determined by ion chromatograph.
1.5 In situ XPS measurements
XPS measurements were performed at the photoemission end-station and beamline BL10B in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. The beamline is connected to a bending magnet and covers photon energies from 100 to 1,000 eV with a resolving power () better than 1,000,and the photon flux was 1 ×1010photons per s. The end-station is composed of four chambers: an analysis chamber, a preparation chamber, a load-lock chamber, and a high-pressure reactor. The analysis chamber, with a base pressure of <2×10−10torr, is connected to the beamline with a VG Scienta R3000 electron energy analyser and a twin anode X-ray source. In addition, the analysis chamber is equipped with a window to allow the irradiation of light during the measurement of XPS spectra. In this work, theXPS measurements of Ag/ZnO were conducted with/without light irradiation, which were named as “Ag/ZnO” and “Ag/ZnO+light”, respectively.
1.6 In situ Zn L-edge XAS measurements.
Zn L-edge measurements were performed at the photoemission end-station and beamline BL10B in the National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. The beamline and end-station are the same as those described in Section 2.5. The high-pressure reactor contains a reaction cell where the samples can be treated with different gases up to 20 bar and simultaneously heated up to 650℃. After the sample treatment, the reactor can be pumped down to < 10−8torr for sample transfer. In this work, theZn L-edge measurements of Ag/ZnO were conducted with/without light irradiation, which were named as “Ag/ZnO” and “Ag/ZnO+light”, respectively.
1.7 Instrumentations
TEM images were collected on a JEOL ARM-200F field-emission transmission electron microscope operating at 200 kV accelerating voltage.X-ray diffraction (XRD) patterns were recorded by using a Philips X’Pert Pro Super diffractometer with Cu-Kradiation (= 1.54178 Å), and the 2range is from 10 to80°. X-ray photoemission spectroscopy and X-ray absorption spectroscopy experiments were conducted at the Catalysis and Surface Science End-station connected to the BL10B beamline in NSRL in Hefei, China. UV-Vis tests were conducted on a TU-1901 at room temperature. ESR spectra were taken on JEOL JES-FA200 ESR spectrometer at 298 K.
2 Results and discussions
2.1 Characterization of materials
The morphology and structure of the samples were characterized by TEM as shown in Fig.1.

Fig.1 TEM images of ZnO (a) and Ag/ZnO (b)
Fig.1(a) shows the microstructure of ZnO prepared via heating ZIF-8 to 500℃ in a muffle furnace. It can be seen from Fig.1(a) that the average particle size of ZnO was ~500 nm. Fig.1(b) illustrated the TEM image of Ag/ZnO formed through a hydrothermal process by dispersing ZnO powders into alcoholic solution of Ag ions. It is clear that Ag NPs besides ZnO.
XRD analysis was further adopted to characterize the composition of the as-prepared powders as shown in Fig.2.

Fig.2 XRD patterns of ZnO and Ag/ZnO
2.2 Photocatalytic performance
The photocatalytic performance of obtained catalysts was evaluated through N2photofixation reaction. Each reaction cycle was conducted in 20 mL of deionized water purged with pure N2after adding 10 mg of catalysts at room temperature. The generated NH3was measured through an ion chromatography method. No NH3was detected for blank experiments.
As shown in Fig.3(a), the NH3production rates over ZnO were 57 and 6 μmol·gcat.-1·h-1under full-spectrum light irradiation (320~780 nm, 250 mW/cm2) and visible light (400~780 nm, 200 mW/cm2), respectively. After depositing Ag NPs with SPR on ZnO, the NH3production rate was up to 120 μmol·gcat.-1·h-1under the same full-spectrum range. Meanwhile, Ag/ZnO showed higher photocatalytic activity under visible light with 81 μmol·gcat.-1·h-1of NH3production rate as shown in Fig.3(a). To further corroborate the superior property of Ag/ZnO in photocatalytic reduction of N2, Ag/ZnO was recycled to test its stability as shown in Fig.3(b). More than 90% of initial activity was retained in N2photofixation after five cycles, indicating the remarkable catalytic stability of Ag/ZnO. As a result, Ag/ZnO exhibited remarkable activity and endurance towards the photocatalytic reduction of N2.

Fig.3 (a). The NH3 production rates catalyzed by ZnO and Ag/ZnO under full-spectrum and filtered light irradiation of Xenon lamp; (b). NH3 production rate of Ag/ZnO catalystsat five successive reaction cycles
2.3 Photocatalytic mechanisms
To explore the origin of the excellent catalytic performance of Ag/ZnO in N2photofixation, the catalytic mechanism was investigated in details as shown in Fig.4.

Fig.4 (a). Diffuse reflectance UV-Vis spectra of the obtained samples of ZnO and Ag/ZnO;(b). Transformed K-M function for ZnO; (c). Valence band spectrum; (d). Secondary electron cutoff of ZnO
Fig.4(a) displayed the diffuse reflectance ultraviolet-visible (UV-Vis) spectra of ZnO and Ag/ZnO. The absorption spectrum of Ag/ZnO was similar to that of ZnO with a steep absorption increase at 350~400 nm. However, the adsorption of Ag/ZnO was obviously enhanced in visible region owing to the SPR of metallic Ag. As shown in Fig.4(b), the calculated band gap of ZnO was 3.2 eV via a trans-formed Kubelka-Munk (K-M) plot. In addition, synchrotron radiation photoemission spectroscopy (SRPES) was conducted with a photon energy of 169.4 eV. Fig.4(c) displayed the valence band (VB) spectra of ZnO. It can be seen from Fig.4(c) that the maximum VB of ZnO was 2.4 eV which is below the Fermi level (E) secondary electron cutoff was performed to confirm the position ofEversus vacuum level (E), where the value of work function (Φ) for ZnO was 5.1 eV (Fig.4(d)). Thus, the calculated value of maximum VB for ZnO was 7.5 eVE. As normal hydrogen electrode (NHE) was 4.5 eVE[22], the values of maximum VB andEfor ZnO were determined to be 3.0 and 0.6 eVNHE, respectively. Furthermore, considering the 3.2 eV of band gap for ZnO, the calculated conduction band (CB) of ZnO was -0.2 eVNHE. TheE versusNHE was generally 1.0 eV for metal Ag.
Based on above analysis, the corresponding electronic band structureNHE of Ag/ZnO was depicted in Fig.5.

Fig.5 Schematic illustration of the band structure of Ag/ZnO
The contact between metallic Ag and ZnO semiconductor equalized theEvalues of the two materials, creating Schottky barrier at the interface between Ag nanoparticle and ZnO. Ag NPs generated hot electrons by SPR effect under illumination, while the formed Schottky barrier facilitated the transfer of electrons from Ag NPs to the CB of ZnO, directly boosting the separation of electron-hole pairs for further N2reduction process (Fig.5).
A series ofexperiments were further conducted to reveal the electronic dynamics of Ag/ZnO during N2photofixation, as shown in Fig.6.

Fig.6 In situ XPS spectra of Ag 3d (a) and Zn 2p (b) for Ag/ZnO measured with/without light irradiation
In order to confirm the electronic properties,Zn L-edge X-ray absorption spectroscopy (XAS) was also analyzed, as shown in Fig.7. The lower peak intensity of the spectrum of Zn L-edge XAS under illumination was attributed to the decrease of the valence state of Zn species, which further demonstrated electrons accumulation in Zn species under light irradiation(Fig.7)[29]. As a result, the formation of Schottky barrier promoted the separation of photo-excited electron-hole pairs, lending to the electron transfer from Ag NPs to ZnO.

Fig.7 In situ Zn L-edge XAS spectra for Ag/ZnO under dark and light
Electron spin resonance (ESR) spectroscopy was conducted (Fig.8) for further characterization. As depicted in Fig.8, an ESR signal with=1.999 assigned to the oxygen vacancy was observed for ZnO[6]. More importantly, an obvious ESR signal with=1.960ascribedto Zn+cations (3d104s1) appeared for both ZnO and Ag/ZnO, suggesting that Zn+species was presented on the ZnO and Ag/ZnO[30]. Thus, the electrons were speculated to prefer to be trapped by Zn+rather than delocalize over the lattice, which favored the efficient electron separation and transfer. The electron-rich Zn+was considered as active sites to chemisorb and activate N2molecules for the further transformation to NH3.
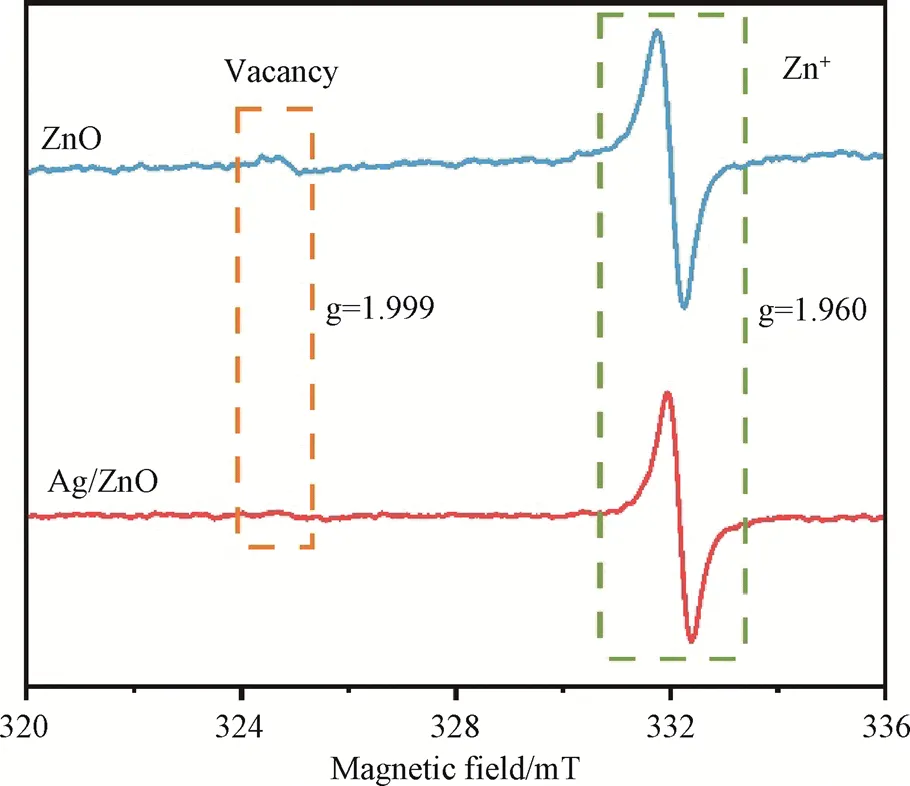
Fig.8 ESR signal of ZnO and Ag/ZnO
3 Conclusions
In conclusion, we successfully deposited Ag NPs on ZnO containing Zn+and formed Schottly barrier. Ag/ZnO was witnessed to exhibit excellent activity and stability towards N2photofixation. The investigation of band structure for Ag/ZnO revealed the Schottky barrier generated at the interface between Ag and ZnO, which facilitated electrons transfer from Ag NPs to ZnO. Furthermore, the electron-rich Zn+species was considered as the active sites to chemisorb and activate N2molecules. This work not only promotes the separation efficiency of plasmonic charge carriers by constructing Schottky barrier, but also highlights a promising approach to prepare Zn+containing Ag/ZnO catalysts for long-term N2photoreduction.
[1] WANG S, ICHIHARA F, PANG H, et al. Nitrogen fixation reaction derived from nanostructured catalytic materials[J]. Advanced Functional Materials, 2018, 28(50): 1803309.
[2] YANG J, GUO Y, LU W, et al. Emerging applications of plasmons in driving CO2reduction and N2fixation [J]. Advanced Materials, 2018, 30(48): 1802227.
[3] HOU T, PENG H, XIN Y, et al. Fe single-atom catalyst for visible-light-driven photofixation of nitrogen sensitized by triphenylphosphine and sodium iodide [J]. ACS catalysis, 2020, 10(10): 5502-5510.
[4] ZHAO Y, ZHAO Y, SUI R, et al. Tuning oxygen vacancies in ultrathin TiO2nanosheets to boost photocatalytic nitrogen fixation up to 700 nm [J]. Advanced Materials, 2019, 31(16): 1806482.
[5] ZHANG N, JALIL A, WU D, et al. Refining defect states in W18O49by Mo doping: A strategy for tuning N2activation towards solar-driven nitrogen fixation [J]. Journal of the American Chemical Society, 2018, 140(30): 9434-9443.
[6] HOU T, LI Q, ZHANG Y, et al. Near-infrared light-driven photofixation of nitrogen over Ti3C2Tx/TiO2hybrid structures with superior activity and stability [J]. Applied Catalysis B: Environmental, 2020, 273: 119072.
[7] WANG W, ZHANG H, ZHANG S, et al. Potassium ion assisted regeneration of active cyano groups in carbon nitride nanoribbons: Visible light driven photocatalytic nitrogen reduction [J]. Angewandte Chemie-International Edition, 2019, 58(46): 16644-16650.
[8] HOU T, XIAO Y, CUI P, et al. Operando oxygen vacancies for enhanced activity and stability toward nitrogen photofixation [J]. Advanced Energy Materials, 2019, 9(43): 1902319.
[9] HU C, CHEN X, JIN J, et al. Surface plasmon enabling nitrogen fixation in pure water through a dissociative mechanism under mild conditions [J]. Journal of the American Chemical Society, 2019, 141(19): 7807-7814.
[10] HOU T, CHEN L, XIN Y, et al. Porous CuFe for plasmon-assisted N2photofixation [J]. ACS Energy Letters, 2020, 5(7): 2444-2451.
[11] ASLAM U, RAO V, CHAVEZ S, et al. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures [J]. Nature Catalysis, 2018, 1(9): 656-665.
[12] ZHAN C, MOSKOVITS M, TIAN Z. Recent progress and prospects in plasmon-mediated chemical reaction [J]. Matter, 2020, 3(1): 42-56.
[13] LI S, MIAO P, ZHANG Y, et al. Recent advances in plasmonic nanostructures for enhanced photocatalysis and electrocatalysis[J]. Advanced Materials, 2020, 33(6): 200086.
[14] GELLÉ A, JIN T, GARZA L, et al. Applications of plasmon-enhanced nanocatalysis to organic transform-ations[J]. Chemical Reviews, 2020, 120(2): 986-1041.
[15] ZHANG Y, HE S, GAO W, et al. Surface-plasmon-driven hot electron photochemistry [J]. Chemical Reviews, 2017, 118(6): 2927-2954.
[16] LINIC S, ASLAM U, BOERIGTER C, et al.Photo-chemical transformations on plasmonic metal nano-particles [J]. Nature Materials, 2015, 14(6): 567-576.
[17] LINIC S, CHRISTOPHER P, INGRAM D. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy [J]. Nature Materials, 2011, 10(12): 911-921.
[18] YU K, JIANG P, YUAN H, et al. Cu-based nanocrystals on ZnO for uranium photoreduction: Plasmon-assisted activity and entropy-driven stability [J]. Applied Catalysis B: Environmental, 2021, 288: 119978.
[19] YANG J, GUO Y, JIANG R, et al. High-efficiency “working-in-tandem” nitrogen photofixation achieved by assembling plasmonic gold nanocrystals on ultrathin titania nanosheets [J]. Journal of the American Chemical Society, 2018, 140(27): 8497-8508.
[20] XU X, LUO F, TANG W, et al. Enriching hot electrons via NIR-photon-excited plasmon in WS2@Cu hybrids for full-spectrum solar hydrogen evolution [J]. Advanced Functional Materials, 2018, 28(43): 1804055.
[21] YANG Y, LUO M, ZHANG W, et al. Metal surface and interface energy electrocatalysis: Fundamentals, performance engineering, and opportunities [J]. Chem, 2018, 4(9): 2054-2083.
[22] WANG L, HOU T, XIN Y, et al. Large-scale synthesis of porous Bi2O3with oxygen vacancies for efficient photo-degradation of methylene blue [J]. Chinese Journal of Chemical Physics, 2020, 33(4): 500-506.
[23] MUTHUCHAMY N, GOPALAN A, LEE K. A new facile strategy for higher loading of silver nanoparticles onto silica for efficient catalytic reduction of 4-nitrophenol [J]. RSC Advances. 2015, 5(93): 76170-76181.
[24] GAO J, WEI C, DONG M, et al. Evolution of Zn species on Zn/HZSM-5 catalyst under H2pretreated and its effect on ethylene aromatization [J]. ChemCatChem, 2019, 11(16): 3892-3902.
[25] ZHANG C, KWAKA G, LEE Y, et al. Light hydrocarbons to BTEX aromatics over Zn-modified hierarchical ZSM-5 combined with enhanced catalytic activity and stability [J]. Microporous and Mesoporous Materials, 2019, 284: 316-326.
[26] DING Y, DU X, ZHANG X. Controlled synthesis and high performance of Zn-Ni-Co-M (M = O, S, P and Se) nanoneedle arrays as an advanced electrode for overall water splitting [J]. Applied Surface Science, 2021, 543: 148818.
[27] CHEN G, ZHAO Y, SHANG L, et al. Recent advances in the synthesis, characterization and application of Zn+-containing heterogeneous catalysts [J]. Advanced Science, 2016, 3(7): 1500424.
[28] GUO W, SHIM K, KIM Y. Ag layer deposited on Zn by physical vapor deposition with enhanced CO selectivity for electrochemical CO2reduction [J]. Applied Surface Science, 2020, 526: 146651.
[29] WANG M, REN F, ZHOU J, et al. N doping to ZnO nanorods for photoelectrochemical water splitting under visible light: Engineered impurity distribution and terraced band structure [J]. Scientific Reports, 2015, 5: 12925.
[30] JIANG W, LOW J, MAO K, et al. Pd-modified ZnO-Au enabling alkoxy intermediates formation and dehydro-genation for photocatalytic conversion of methane to ethylene [J]. Journal of the American Chemical Society, 2021, 143(1): 269-278.
Ag/ZnO复合材料在氮气光固定中的应用:构造肖特基势垒以实现有效的电荷载流子分离
肖 宇,欧阳宇欣,辛 月,王梁炳*
(中南大学 材料科学与工程学院 粉末冶金国家重点实验室,长沙 410083)
将氮气(N2)光催化还原为氨(NH3)是一种可持续的能源生产方法。等离激元共振光催化剂能够通过表面等离激元共振效应实现太阳能的有效转化,也因此受到越来越广泛的关注。然而,热载流子往往会在催化固氮的过程中发生重新结合。本研究将具有等离激元共振效应的Ag纳米粒子与ZnO半导体复合(Ag/ZnO)并应用于氮气光固定。与ZnO相比,Ag/ZnO在氮气光固定的催化活性得到了提高,室温下氨生成速率达到120 µmol·gcat.-1·h-1。进一步的机理研究表明在Ag纳米颗粒与ZnO的界面处形成了肖特基势垒,这大幅度促进了光生电子-空穴对的分离。Ag纳米粒子通过表面等离激元共振效应生成热载流子,所形成的肖特基势垒则促进了电子从Ag向ZnO转移。此外,ZnO中的富电子Zn+可能作为活性位点以吸附和活化氮气分子,从而促进氮气光固定的进行。
银;表面等离激元共振;肖特基势垒;氮气光固定;等离激元共振催化
2021-03-04
国家自然科学基金(51801235, 51674303);中南大学创新驱动项目(2018CX004);中南大学启动资金项目(502045005)
肖 宇,男,硕士研究生,研究方向:金属氧化物的光催化性能。E-mail: Xiaoyu2018@csu.edu.cn
通讯作者:王梁炳,男,教授,研究方向:有色金属催化、小分子的高效活化转化。E-mail: wanglb@csu.edu.cn
O643.36; O644
A
1004-0676(2021)04-0047-08

