Thromboembolic events in metastatic testicular cancer treated with cisplatin-based chemotherapy
Lisa B E Shields, Michael W Daniels, Nataliya Mar, Arash Rezazadeh Kalebasty
Lisa B E Shields, Norton Neuroscience Institute, Norton Healthcare, Louisville, KY 40202,United States
Michael W Daniels, Department of Bioinformatics and Biostatistics, University of Louisville,Louisville, KY 40292, United States
Nataliya Mar, Arash Rezazadeh Kalebasty, Division of Hematology/Oncology, Department of Medicine, UCI Medical Center, Orange, CA 92868, United States
Abstract BACKGROUND Testicular germ cell tumor (TGCT) is the most curable solid tumor and most common cancer among men 18-39 years. While cisplatin-based chemotherapy has significantly lengthened the survival of patients with TGCT, it is associated with a high rate of thromboembolic events (TEE).AIM To summarize our single-center experience highlighting patients who were diagnosed with TGCT and received platinum-based chemotherapy, with special attention to those patients who suffered a TEE.METHODS A retrospective analysis of the medical records and imaging studies of 68 consecutive individuals who were diagnosed with TGCT and received platinumbased chemotherapy at our Institution in a metropolitan community between January 1, 2014 and December 31, 2019.RESULTS A total of 19 (28%) patients experienced a TEE following orchiectomy which occurred during chemotherapy in 13 (68%) of these patients. Patients with a higher pathologic stage (stage III) were significantly (P = 0.023) more likely to experience a TEE compared to patients who had a lower stage. Additionally,patients who were treated with 3 cycles of bleomycine, etoposide, and cisplatin and 1 cycle of etoposide and cisplatin or 4 cycles of etoposide and cisplatin were significantly 5 (P = 0.02) times more likely to experience a TEE compared to patients who were treated with only 3 cycles of bleomycine, etoposide, and cisplatin.CONCLUSION Due to numerous factors that predispose to a TEE such as large retroperitoneal disease, higher clinical stage, greater number of chemotherapy cycle, central venous catheter, cigarette smoking, and possible cannabis use, high-risk ambulatory patients with TGCT treated with cisplatin-based chemotherapy may benefit from prophylactic anticoagulation. Randomized studies to evaluate the safety and efficacy of prophylactic anticoagulants are warranted in this young patient population generally devoid of medical co-morbidities.
Key Words: Oncology; Testicular cancer; Thromboembolic; Cisplatin; Pulmonary embolism; Thromboprophylaxis
INTRODUCTION
According to the American Cancer Society, approximately 9610 new cases of testicular cancer will be diagnosed in 2020 with 440 deaths[1]. One in every 250 males will develop testicular cancer during their lifetime[1,2]. With the emergence of cisplatinbased chemotherapy in the 1970’s[3], patients with testicular cancer are able to attain a 5-year survival rate of more than 95%[1,4,5]. However, the risk of a thromboembolic event (TEE) poses a significant concern in this population who are usually young- and middle-aged adults without medical co-morbidities. Several factors predispose to experiencing a TEE in the testicular oncologic setting including bulky retroperitoneal disease leading to vascular compression and stasis, endothelial damage resulting from vascular invasion, platinum-based chemotherapy with cisplatin and bleomycin that causes alterations of the clotting system, cigarette smoking, stress, central venous catheter (CVCs), presence of the germ cell tumor marker bhCG that predisposes to thrombosis, and recent surgery that enhances inflammation and disrupts thrombotic and hemostatic pathways[6-11].
The standard chemotherapy regimen is 3 cycles of bleomycine, etoposide, cisplatin(BEP) or 4 cycles etoposide, cisplatin (EP) for good-risk testicular germ cell tumor(TGCT), or 4 cycles of BEP or 4 cycles of vasoactive intestinal peptide (etoposide,ifosphamide, cisplatin) for intermediate- or poor-risk TGCT[5,12,13]. Given the cumulative toxicity of bleomycine, a regimen of 3 cycles of BEP followed by 1 cycle of EP has been utilized in certain patients with intermediate risk. This schedule may be curtailed if a patient experiences a TEE due to safety concerns. Treating a TEE in cancer patients is challenging due to the risk of recurrent TEE with continuation of systemic chemotherapy and a higher bleeding risk than in patients without cancer[14].
Herein, we present 68 patients with TGCT all of whom underwent cisplatin-based chemotherapy. The factors associated with an increased risk of TEE in this patient population are discussed. An analysis of thromboprophylaxis in the ambulatory setting for patients with high-risk cancer is also presented.
MATERIALS AND METHODS
Study population and data collection
Under an Institutional Review Board-approved protocol, we reviewed the medical records and imaging studies of 68 consecutive individuals who were diagnosed with TGCT and received platinum-based chemotherapy at our Institution between January 1, 2014 and December 31, 2019. All patient in our study underwent orchiectomy(without any exceptions), and the pathology was determined based on the orchiectomy. Standard dosing and cycles of BEP and EP were planned to be completed unless patients experienced complications from chemotherapy. A TEE was defined as a venous or arterial complication that occurred following the patient’s orchiectomy for TGCT. A TEE was confirmed by venous Doppler ultrasonography or contrasted computed tomography scans. Patients with a deep venous thrombosis (DVT) had a contrasted chest computed tomography scan at baseline to rule out pulmonary embolism (PE) and to determine the length of anticoagulation treatment. None of the patients was treated with primary thromboprophylaxis.
Statistical analysis
The statistical analysis was performed by stratisfying each metric (TEE, retroperitoneal lymph nodes (RPLN) ≥ 3.0 cm before chemotherapy, age, body mass index (BMI),cigarette smoker, marijuana use, side of orchiectomy, pathologic stage, CVC, type of chemotherapy, number of chemotherapy cycles, RPLN dissection (RPLND), and recurrence after chemotherapy by those patients who experienced a TEE and by those who did not. When comparing the two groups, the Fisher’s exact test and the Mann-WhitneyU-test compared binary/categorical and continuous data, respectively. All analyses were performed using R software (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Demographics and clinicopathologic characteristics
The mean age of the 68 patients with TCGT at orchiectomy was 31 years (16-53 years)(Table 1). The mean BMI at the first medical oncology visit following orchiectomy and before the initiation of chemotherapy was 29 (18.6-58.4). A total of 35 (51%) patients were current or previous cigarette smokers, and 16 (23.5%) patients were current cannabis users.
The majority [49 (72%)] of patients were diagnosed with a non-seminoma and were pathologic stage II (Table 1). A total of 18 (26%) patients had retroperitoneal lymph nodes RPLN ≥ 3.0 cm before chemotherapy, and a slightly higher number of TCGT were on the left [35 (52%)]. Most patients were treated with 3 cycles of BEP. A total of 18 (26.5%) patients underwent a RPLND, consisting of 6 before chemotherapy and 12 afterwards. RPLND is an option prior to chemotherapy in cases of mixed germ cell tumors with negative tumor markers and evidence of retroperitoneal adenopathy.RPLND is a viable possibility especially if a teratoma is suspected, the tumor is not bulky, or if the patient prefers to avoid chemotherapy. Only 1 patient had a family history of testicular cancer, specifically, in a paternal uncle. One patient was treated with 17 fractions of 2250 cGy radiation after the orchiectomy and prior to the chemotherapy.
Five patients experienced recurrent disease necessitating a second course of chemotherapy after their initial cisplatin-based chemotherapy regimen. One of these patients underwent several additional courses of chemotherapy including high dose chemotherapy with autotransplant, however, succumbed to progressive disease 5 years after his orchiectomy. His death was the only one in our study.

Table 1 Characteristics of patients with testicular germ cell tumors at our Institution
Thromboembolic events
A total of 19 (28%) patients experienced a TEE, consisting of 8 PEs, 8 DVTs, and both a PE and DVT in 3 patients (Table 2). Six patients with a TEE had a CVC for delivering chemotherapy. Four of the DVT were peripheral inserted central catheter-line related.Two patients had infusaports, one of whom had no evidence of a fibrin sheath orpericatheter thrombus detected by fluoroscopic evaluation. The other patient had a family history of thromboembolic conditions.
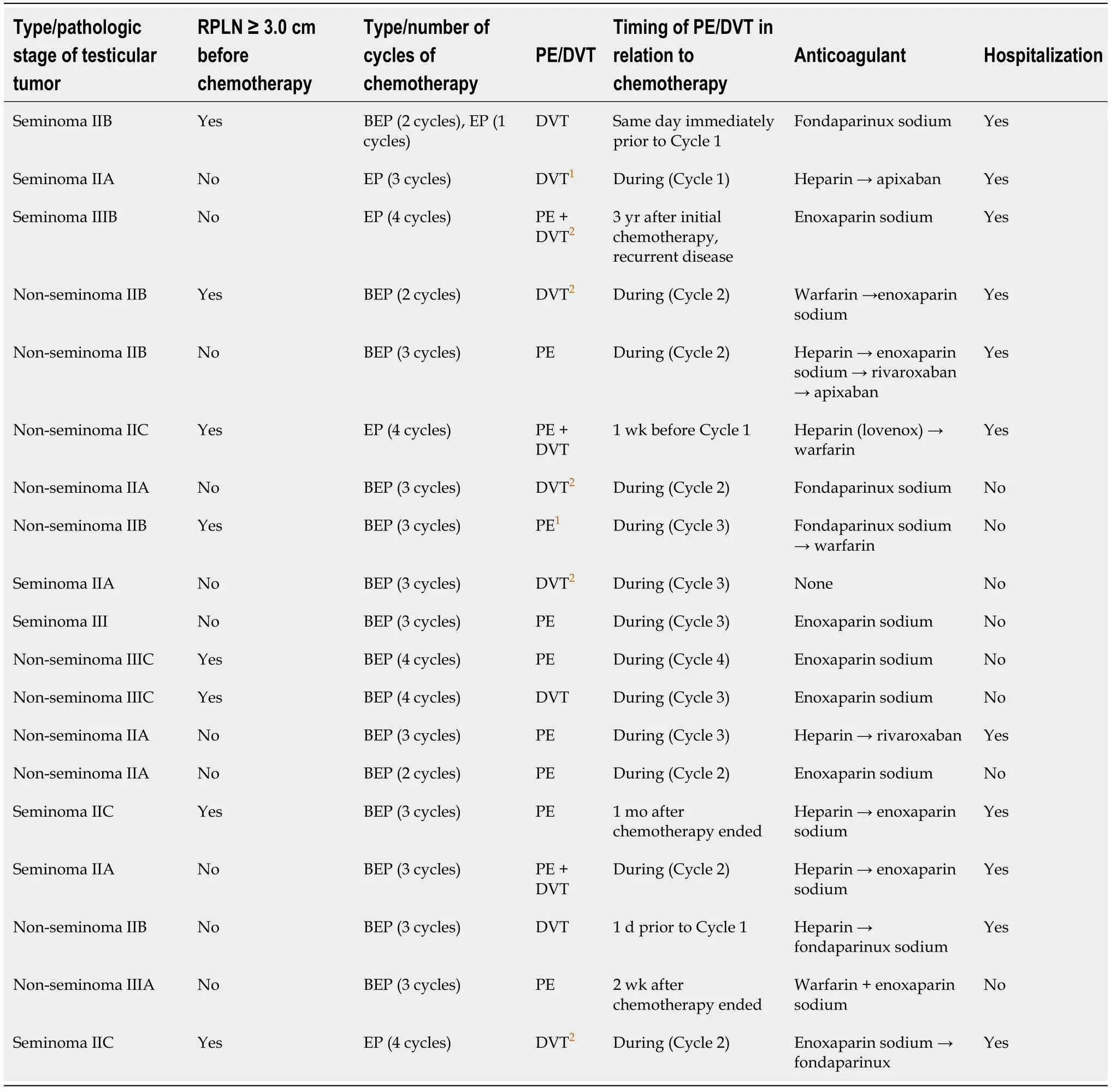
Table 2 Thromboembolic events in patients with testicular germ cell tumors who underwent cisplatin-based chemotherapy at our Institution (n = 19)
Eight (42%) patients were diagnosed with a seminoma, and patients were either pathologic stage II [14 (74%)] or stage III [5 (26%)] (Table 2). The RPLN were ≥ 3.0 cm before chemotherapy in 8 patients (42%). The relative proportions of TEEs by at least one type stage was significantly different from the other stages (P= 0.05) (Table 3).Patients with stage IS testicular cancer were significantly (P= 0.03) less likely to experience a TEE compared to patients who had stages II or III. Note that no patients with stage IS testicular cancer had a TEE.
Most patients were scheduled to undergo 3 cycles of BEP. The relative proportions of TEEs by at least one type of chemotherapy treatment was significantly different from the other chemotherapy types (P= 0.004) (Table 3). Patients who were treated with BEP/EP and EP were significantly 5-times (1.02-28.3,P= 0.02) more likely to experience a TEE compared to patients who were only treated with BEP.
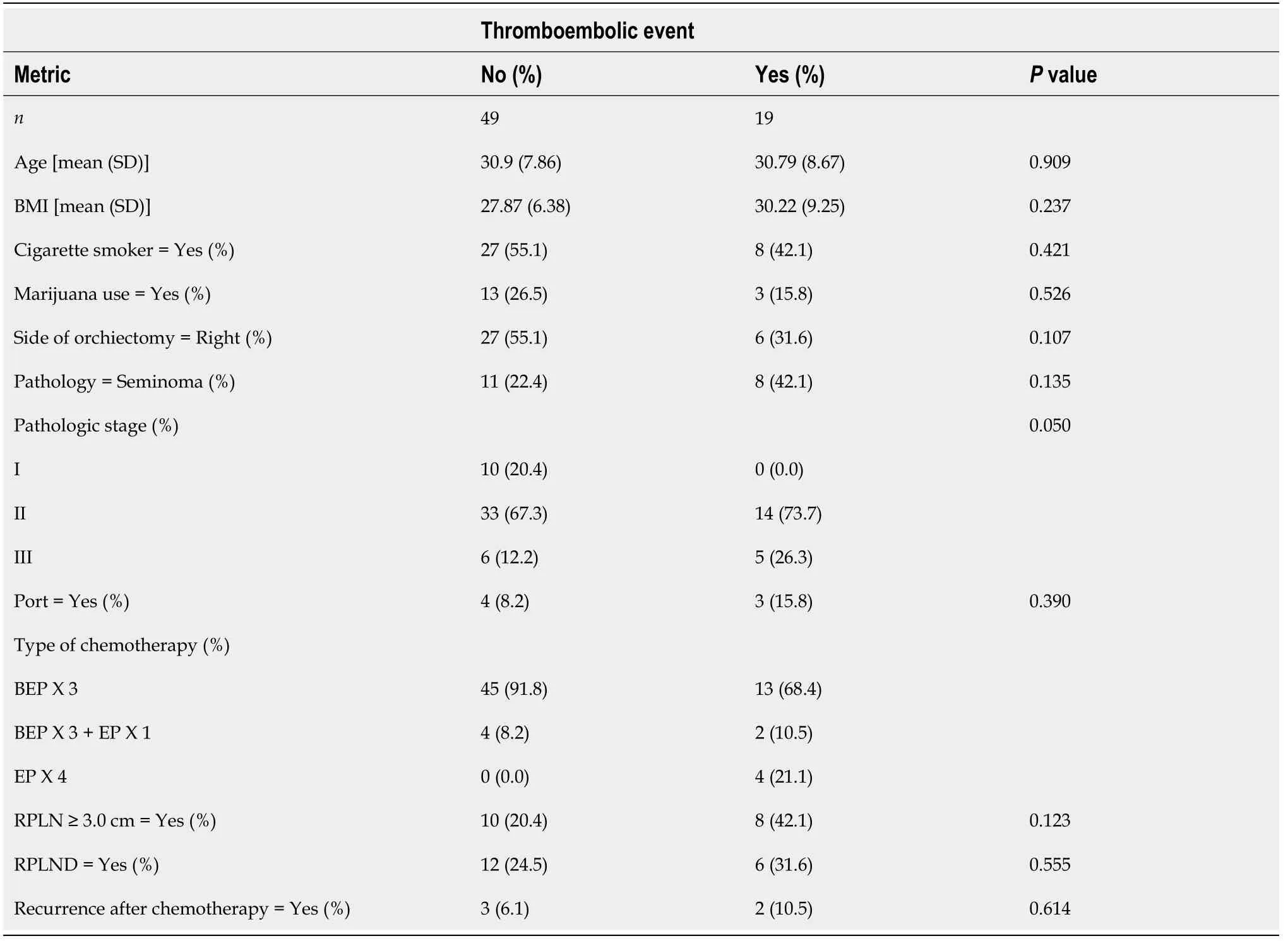
Table 3 Comparison of metrics between patients with testicular germ cell tumors at our Institution stratified by those who experienced a thromboembolic event and those who did not
The TEE occurred during the cisplatin-based chemotherapy in 13 (68%) patients. A total of 18 (95%) patients underwent anticoagulant therapy, and 10 (56%) patients were hospitalized for management of their TEE. Eight (42%) patients were current or excigarette smokers, and 3 (16%) were current cannabis users.
One patient sustained bilateral PE and a DVT 3 years after the completion of 4 cycles of EP when he experienced recurrent testicular disease. He was hospitalized and treated with enoxaparin. This patient also experienced recurrent disease and was the sole death in our study.
DISCUSSION
Cisplatin chemotherapy has been reported to cause PE, DVT, myocardial infarction,and peripheral arterial thrombosis[11,15,16]. Several pathogenic mechanisms have been identified between cisplatin and thrombosis, including endothelial cell damage marked by increased plasma levels of Von Willebrand factor during chemotherapy,platelet activation, and up-regulation of prothrombotic factors. Lauritsenet al[17]investigated cardiovascular risk factors and disease following BEP treatment for male germ cell cancer[17]. These authors reported that patients had a more than 20-fold increased hazard ratio of venous TEE compared with the normal population which decreased to a hazard ratio of 1.4 after 10 years.
In our literature review the percentage of patients who experienced a TEE with testicular cancer ranged between 5.2% and 19%[6,9,16,18,19]. Most of the TEE occurred during chemotherapy[9,16,18,19]. Various risk factors for a TEE were confirmed, including retroperitoneal and supraclavicular lymph node metastases, elevated serum lactic acid dehydrogenase, higher clinical stage, CVC, high body surface area, and febrile neutropenia. Paffenholzet al[16]reported a significantly reduced overall survival in patients with a TEE during a median follow-up of 8 mo[16]. The present study concurs with this finding, as the TEE occurred during the cisplatin-based chemotherapy in 13(68%) patients.
The Khorana score is a predictive model for chemotherapy-associated TEE and is based on the following: (1) Site of cancer; (2) Prechemotherapy platelet count 350 × 109/L or more; (3) Hemoglobin level less than 100 g/L or use of red cell growth factors;(4) Prechemotherapy leukocyte count more than 11 × 109/L; and (5) BMI 35 kg/m2or more[2,20,21]. The risk of TEE is 4-6-fold higher in patients with cancer than in those without, and the TEE incidence is 3%-15% annually in cancer patients[14,18]. The use of thromboprophylaxis to reduce the recurrence of cancer-associated TEE have been examined primarily in cancers such as breast, colorectal, lung, pancreatic, and multiple myeloma that usually affect older individuals[14,21-24].
Carrieret al[22]determined that apixaban resulted in a significantly lower rate of venous thromboembolism (VTE) than placebo among intermediate-to-high-risk ambulatory patients with cancer who started chemotherapy[22]. In the intention-to-treat analysis, major bleeding occurred in 10 (3.5%) patients in the apixaban group and in 5(1.8%) patients in the placebo group (P= 0.046). Khoranaet al[25]concluded that highrisk ambulatory patients with metastatic cancer who were receiving chemotherapy and were treated with rivaroxaban did not have a significantly lower incidence of VTE or death due to VTE in a 180-d trial period[25]. Despite these two large randomized phase III trials, routine prophylaxis for TEE has not been embraced by clinicians in the outpatient setting as the absolute risk reduction is small and thromboprophylaxis is associated with a heightened risk of major bleeding and high cost[14,18,22].
Of the 574 patients in Carrier and colleagues’ placebo-controlled study, only 3 patients were diagnosed with testicular cancer. Khoranaet al[25]’s study of 1080 patients did not specify the number of patients with testicular cancer, however, mentioned that there were 32 cases of “genitourinary” cancers that included renal, bladder, ureteral,and testicular cancers but not prostate cancer. Young men with TGCT are generally without co-morbidities. This patient population was not represented in large trials investigating thromboprophylaxis in metastatic cancer during chemotherapy. This is largely due to inclusion and exclusion criteria based on the Khorana scoring system used in these trials.
The cisplatin-based chemotherapy used to treat TGCT often does not cause severe thrombocytopenia. A relatively short course of chemotherapy in metastatic testicular cancer often leads to a cure despite the need for ongoing systemic therapy in most other metastatic solid tumors. Additionally, the cisplatin dose in the BEP regimen is higher compared to most regimens used for non-testicular cancer. This important difference may lead to better outcomes with the use of thromboprophylaxis. TGCT possesses numerous risk factors that increase the risk for clotting including bulky retroperitoneal disease, cisplatin-based chemotherapy, cigarette smoking, and possibly marijuana use. Furthermore, the risk of bleeding in TGCT is likely to be lower compared to other types of cancers due to the shorter duration of chemotherapy in TGCT and younger patient population with better bone marrow function. These factors support further investigation into the use of thromboprophylaxis as patients receive chemotherapy for TGCT.
We propose conducting a multicenter randomized double blind placebo control trial examining the efficacy and safety of thromboprophylaxis in patients undergoing systemic treatment for metastatic TGCT. Such a trial will use a novel inclusion scoring system specific for TGCT that incorporates risk factors often encountered with this cancer. Carrieret al[22]’s as well as Khoranaet al[25]’s studies only included patients who had 2 risk factors on the Khorana scale[22,25]. Most of the patients who experienced a TEE in the present study would not have fulfilled the criteria for inclusion for thromboprophylaxis in these 2 trials. The new TGCT scoring system should incorporate the following risk factors: Higher pathologic stage and greater number of chemotherapy cycles as confirmed by the current study as well as elevated serum lactate dehydrogenase, CVC, large RPLN, and seminoma[6,9,16,18,19].
Several studies have reported the limited use of thromboprophylaxis in TGCT with cisplatin-based chemotherapy (Table 4). Paffenholzet al[16]reported that 93% of all of their TCGT received prophylactic anticoagulation which is customary in Germany[16].Thromboprophylaxis during chemotherapy for TGCT is not customary in the United States.
Kentucky has both the highest incidence and death rates for lung cancer in the United States[26,27]. In 2017, a total of 24.6% of adults in Kentucky smoked while the national average was 14.0%[26,28]. Cigarette smoking is a well-recognized risk factor for both atherosclerotic disease and TEE[29]. Half of the patients in our study were eithercurrent or previous cigarette smokers, and 42% of these patients experienced a TEE while undergoing cisplatin-based chemotherapy. Cannabis is the most commonly used recreation drug in the United States and has been suggested to cause myocardial infarction and ischemic stroke[30]. Only a single case of PE in a young male following heavy cannabis use has been reported[30]. It has been suggested that cannabinoids, the active component of cannabis, cause endothelial cell disruption leading to vascular thrombosis. A total of 23.5% of the patients in our study were current cannabis users,and 16% of the cannabis users suffered a TEE while undergoing chemotherapy.

Table 4 Patients with testicular germ cell tumors who underwent cisplatin-based chemotherapy and experienced a thromboembolic event in the literature
Our study of TCGT offers several unique aspects that shed light on the risk factors for a TEE and educational opportunities for preventing a TEE. Our study, consisting of a single Institution in a metropolitan community in Kentucky, has a large number of patients with TCGT over a 6-year period. The high percentage (28%) of patients who experienced a TEE compared to the literature may be attributed to numerous cumulative factors leading to a hypercoagulable state that may provoke a TEE,including bulky retroperitoneal disease, higher pathologic stage, greater number of chemotherapy cycles, metastatic disease, the remarkably large number of patients who smoke cigarettes and use cannabis, high number of patients with CVC, and lack of patients who were treated with thromboprophylaxis. The limitation of the present study is its retrospective nature and limited number of patients.
Several risk factors have been identified as increasing the risk of developing VTE,including the patient’s age (> 60 years), obesity, and history of anterior VTE[31]. The tumor’s site, histological type, and stage also elevate the risk of VTE. Pancreatic cancer is the solid tumor with the highest likelihood of VTE, while lymphoma, acute leukemia, and multiple myeloma represent hematologic malignancies that pose a strong risk of VTE. Adenocarcinomas have a higher risk of VTE compared to squamous cell carcinomas, and an advanced tumor stage increases the prospect of developing a VTE[31]. Advanced tumor stage and use of subclavian catheters are the main risk factors for CVC-associated thrombosis[32]. Our Institution generally does not use CVC for delivering chemotherapy in patients with TCGT. However, CVC placement may be unavoidable for patients with poor venous access or patient preference. Educating patients and providers about the thromboembolic risk of CVC may discourage them from selecting CVC as the desired route of chemotherapy infusion. Further educational opportunities with patients that may decrease the likelihood of TEE and enhance overall medical health include an emphasis on discontinuing cigarette smoking and cannabis use as well as promoting weight loss.
CONCLUSION
Although patients with TGCT are a relatively healthy population due to their young age, myriad risk factors such as bulky retroperitoneal disease, higher clinical stage,CVC, cigarette smoking, and cannabis use may heighten their risk of developing a TEE during cisplatin-based chemotherapy. Patients with TGCT who experience a TEE may be unable to complete the course of prescribed chemotherapy despite initiating treatment with an anticoagulant. There may be a significant increase in cost of care following a TEE, including hospital stay, potential requirement for an aggressive procedure, and need for a longer duration of anticoagulation. Patients who could not complete the course of prescribed chemotherapy due to TEE will likely need more frequent medical oncology appointments with monitoring tumor markers as well as closer follow-up imaging studies which can substantially escalate the cost of patient care. High-risk ambulatory patients with TGCT treated with cisplatin-based chemotherapy may benefit from treatment with primary prophylactic anticoagulation to mitigate the potential morbidity and mortality associated with a TEE. Multiinstitutional randomized studies are warranted to evaluate the safety and efficacy of thromboprophylaxis during chemotherapy for TGCT in this young patient population.
ARTICLE HIGHLIGHTS
Research background
Cisplatin-based chemotherapy has significantly increased the survival of patients with testicular germ cell tumor (TGCT), although it is associated with a high rate of thromboembolic events (TEE).
Research motivation
As TGCT is the most curable solid tumor and most common cancer among men 18-39 years, we sought to evaluate our single-center experience of patients who were diagnosed with TGCT and received platinum-based chemotherapy. Patients who suffered a TEE were the primary focus of this study.
Research objectives
Our objective was to identify patients who were diagnosed with TGCT and received platinum-based chemotherapy, with particular attention to those patients who experienced a TEE.
Research methods
The medical records and imaging studies of 68 consecutive individuals who were diagnosed with TGCT and received platinum-based chemotherapy at our Institution in a metropolitan community between January 1, 2014 and December 31, 2019 were reviewed. Statistical analysis was performed by stratisfying each metric (TEE,retroperitoneal lymph nodes ≥ 3.0 cm before chemotherapy, age, body mass index,cigarette smoker, marijuana use, side of orchiectomy, pathologic stage, central venous catheter, type of chemotherapy, number of chemotherapy cycles, retroperitoneal lymph nodes dissection, and recurrence after chemotherapy by those patients who experienced a TEE and by those who did not.
Research results
A total of 19 (28%) patients experienced a TEE following orchiectomy which occurred during chemotherapy in 13 (68%) of these patients. Patients with a higher pathologic stage (stage III) were significantly (P= 0.023) more likely to experience a TEE compared to patients who had a lower stage. Patients who were treated with 3 cycles of bleomycine, etoposide, and cisplatin and 1 cycle of etoposide and cisplatin or 4 cycles of etoposide and cisplatin were significantly 5 (P= 0.02) times more likely to experience a TEE compared to patients who were treated with only 3 cycles of bleomycine, etoposide, and cisplatin.
Research conclusions
Since myriad factors predispose to a TEE such as large retroperitoneal disease, higher clinical stage, greater number of chemotherapy cycle, central venous catheter, cigarette smoking, and possible cannabis use, high-risk ambulatory patients with TGCT treated with cisplatin-based chemotherapy may benefit from prophylactic anticoagulation.
Research perspectives
Randomized studies to evaluate the safety and efficacy of prophylactic anticoagulants are essential in this young patient population generally lacking medical comorbidities.
ACKNOWLEDGEMENTS
We acknowledge Norton Healthcare for their continued support.
 World Journal of Clinical Oncology2021年3期
World Journal of Clinical Oncology2021年3期
- World Journal of Clinical Oncology的其它文章
- GOECP/SEOR radiotherapy guidelines for small-cell lung cancer
- Cardiovascular risk management in cancer survivors: Are we doing it right?
- Systemic adverse effects and toxicities associated with immunotherapy: A review
- Overview of recent advances in metastatic triple negative breast cancer
