Nonlinear Phenomena Conveying Body Size Information and Improving Attractiveness of the Courtship Calls in the Males of Odorrana tormota
Yatao WU ,Jiahui BAO ,Pingshin LEE ,Jinmei WANG ,Sheng WANG and Fang ZHANG,2*
1 College of Life Sciences,Anhui Normal University,Wuhu 241000,Anhui,China
2 Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources,Wuhu 241000,Anhui,China
Abstract Nonlinear phenomena are commonly shown in the vocalization of animals and exerts different adaptive functions.Although some studies have pointed out that nonlinear phenomena can enhance the individual identification of male Odorrana tormota,whether the nonlinear phenomena play a specific role in the sexual selection of O.tormota remain unclear.Here we presented evidence that there was a significant negative correlation(Pearson:n=30,r=0.65,P < 0.001) between the nonlinear phenomena content and snout-vent length in the male O.tormota,and two-choice amplexus experiments showed that female O.tormota preferred male with smaller body size containing higher nonlinear phenomena content in its calls.Phonotaxis experiments also revealed that females preferred calls with higher nonlinear phenomena content.Additionally,compared to the calls with lower nonlinear phenomena content and higher fundamental frequency,there was shorter response time in phonotactic behaviour of female induced by the calls with higher nonlinear phenomena content and lower fundamental frequency.We argue that the nonlinear phenomena content in the calls of male O.tormota can convey its body size information and may provide important clues for female frogs in darkened surroundings to identify males’ body size during mate choice,meanwhile,higher nonlinear phenomena content in males’ calls may increase the attractiveness of males to females.The results of this study provide confirmation that,for O.tormota,nonlinear phenomena have specific function in mate choice.
Keywords body size,mate choice,nonlinear phenomena,Odorrana tormota,phonotaxis
1.Introduction
Exchange of information among animals will be conducted by means of molecules,light,electricity or vocalization(Madison,1977;Hagedorn and Heiligenberg,1985;Ordet al.
,2002).Vocal communication has advantages over other forms of communication as it can be rapidly and widely spread,and plays an important role in the recognition (Daleet al.
,2001;Feareyet al.
,2019) and localization (Stabelet al.
,1989;Branstetter and Mercado III,2006) of individuals among animals,as well as in the life activities of animals such as predation (Banner,1972;Fletcher and Index,2005),defense (Gottfriedet al.
,1985;Virant-Doberletet al.
,2019),territorial occupation (Bunnell,1973) and reproduction (Emerson and Boyd,1999).Frogs are species that are highly dependent on acoustic signals for reproduction,and male frogs’ call is an important clue to the female’s sexual selection.Nonlinear phenomena (NLP),as one of the structural features of acoustic signals,is ubiquitous in the acoustic signals of fish,frogs,birds,and mammals,including humans (Fitchet al.,2
002;Fenget al
.,2009;Riceet al
.,2011;Digbyet al
.,2014).In the process of animal communication,calls containing NLP can not only convey the individual relevant information such as the age (Fitchet al
.,2002),health (Fitchet al
.,2002) and mental state (Kaltwasser,1991;Facchiniet al
.,2005) to the receiver,but also play roles in socializing (Schneider and Anderson,2011),alarming (Tokudaet al
.,2002;Blumstein and Recapet,2009;Townsend and Manser,2010;Labraet al
.,2013;Karpet al
.,2014),attracting attention (Fitchet al
.,2002;Tokudaet al
.,2002;Blumstein and Recapet,2009;Townsend and Manser,2010;Labraet al
.,2013;Karpet al
.,2014),preventing the receiver from habituating to certain call types (Karpet al
.,2014).However,in some cases,NLP may be a by-product of animal vocalization which does not involve the adaptive function of the caller (Fitchet al
.,2002),or simply the result of speech impairment (Fitchet
al.
,2002).Some studies on the variables affecting the occurrence of NLP in vertebrates have focused on alarm signals (Riede and Zuberbühler,2003;Townsend and Manser,2010;Matrosovaet al
.,2012;Volodinet al
.,2017),rarely considering the roles of NLP in reproduction,and the adaptive function of NLP in frogs’mate choice has not been explored (Serranoet al
.,2019).In order to adapt to communication in a noisy environment,O.tormota
has undergone long-term adaptive evolution from the body structures (such as tympanic pits and short auditory hair cells) to functions,including a series of highly specific vocal and auditory mechanisms (Feng and Narins,2008;Shenet al.
,2011).The calls of maleO.tormota
contain ultrasonic and NLP components,and the abundance (93%) and diversity of the NLP combination in its calls are relatively rare in the communication system of anuran.Regarding to the mate choice,high-frequency calls of animals includingO.tormota
have advantages over lower-frequency calls in overcoming the masking of ambient noise (Schwartz and Bee,2013).The function of NLP inO.tormota
,however,has not been reported except in its contribution to individual signatures (Fenget al.
,2009).At the individual level,the frequency of calls of animals are affected by their body sizes and weight (Wermke and Robb,2010).Although the calls of maleO.tormota
contain a large number of NLP components,whether these components can reflect the individual characteristics of the caller remain unknown.For instance,does NLP have a role in reflecting the body size of maleO.tormota
? In addition,although Fenget al.
(Fenget al.
,2009;Shenet al.
,2011;Zhanget al.
,2017) pointed out that NLP can increase the vocalization specificity of maleO.tormota
,the effect of NLP content on females’ mate choice remains unclear.Therefore,the main purpose of this study is to explore the relationship between the body size and NLP content in maleO.tormota
,and the preference of femaleO.tormota
towards different NLP content,which is critical for further understanding the evolution of adaptive function of NLP.2.Materials and Methods
2.1.Study site
Field study was performed in the mountain range of Huangshan (Anhui Province,China) in the village Fuxi (118°08'44.89'' E,30°05'01.61'' N,Elevation:600 m a.s.l.),along Fu Creek,in the month of April,2019.The nightly ambient temperature at the study site during the study periods fluctuated widely but the temperature and humidity at which males were spotted in the field within limited ranges,from 15.0℃ to 18.0 ℃ and from 80% to 95%,respectively.2.2.Sound recordings
A digital audio recorder (Sound Devices 702,WI,USA;frequency range:10 Hz-96 kHz) and a miniature omni-directional condenser microphone with a flat frequency response over 20-20 000 Hz and a drop of a mere 10 dB at 30 000 Hz (AKG model C417,Vienna,Austria) were employed to record the sounds of the maleO.tormota
with a sampling rate of 96 kHz and 16-bit accuracy.The AKG microphone was placed above malesat a height of 10 cm.Recordings were carried out during their peak period (mainly between 19:00 and 23:00) under similar ambient conditions (temperature:15-18 °C;humidity:80%-95%;ambient noise:68-72 dB SPL peak).The long calls (described in the part of result) of each male frog is not less than 7.After recording the calls,each maleO.tormota
was captured and housed in a separate plastic terraria.Using a caliper (Wiha Spi2000,Germany) and a gravimeter (Liangping JY10001,Shanghai,China),we immediately measure their snout-vent length (SVL) to the nearest 0.1 mm and their body weight to the nearest 0.1 g.To avoid repeating the recording,all captured frogs (n
=30) were not released to their individual calling sites until the whole experiment was completed.2.3.Amplexus experiment
We captured 15 amplexed pairs ofO.tormota
in the wild (the female frogs did not oviposit).Then,the females and amplectant males were housed in separate plastic terrarium (L × W × H:22 ×14 × 12 cm).Liu and colleagues previously found that only females who have ovulated,but have not yet oviposited,would form amplexus in captivity (Liuet al.
,2019).Therefore,two-choice amplexus experiment (2C experiments) were carried out exclusively on newly captured amplectant females,within 30 hours of capture before they oviposited.In the 2C experiment,we placed the amplectant female in the center of the aquarium (L × W × H:32 × 22 × 16 cm),at equidistance to the two randomly captured males with varying body sizes (the males that were captured after recording).The presence of female typically caused males to compete for access to the females and the competition among males generally yielded a victor.If the pairing did not result in amplexus within 15 minutes,we substituted the two males with two new males and repeated the experiment.Upon formation of amplexus,we separated the pair and measured the SVLs of the amplectant male and non-amplectant males (see Zhanget al.
,2020 for details).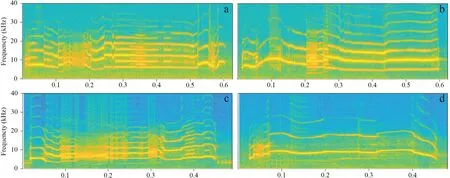
Figure 1 Spectrogram of stimuli pairs.a (NLP:55 %;AFF:6500 Hz) and b (NLP:11 %;AFF:6600 Hz) are For Review Only a stimuli pairs for NLP-2SP.c (NLP:58 %;AFF:5800 Hz) and d (NLP:7 %;AFF:8800 Hz) are a stimuli pair for NLP-Freq-2SP.This plot is made by MATLAB R2018a,with FFT frame set to 512.
2.4.Phonotaxis experiment
Two-loudspeaker phonotaxis experiments were performed using the calls with different NLP content (NLP-2SP) as the acoustic stimuli to females ofO.tormota.
Two long calls with different NLP content were selected as stimuli pairs,and the differences in average fundamental frequency (AFF) and call duration in each stimuli pair was no more than 200 Hz and 30 ms,respectively(Figure 1 a,b).We used acoustic foams (thickness 180 mm) to build the experimental arena (L × W × H:200 × 120 × 150 cm)in a quiet and darkened indoor room under dim infrared illumination (Shenet al.
,2008),~ 1.2 km from the frog’s natural habitat to conduct phonotaxis tests at an ambient temperature and humidity level of~ 17 ℃ and~ 85 % respectively.The phonotaxis behaviours of female frogs were recorded by a camcorder (Sony modle HF M40,Tokyo,Japan).In the indoors,male calls at the rate of 1 call per 15 s at~ 85 dB SPL(measured at 50 cm from the playback loudspeaker) were broadcast through two loudspeakers (Altec Lansing modle Orbit-M iM227,Pennsylvania,USA),which were placed in opposite to each other.Each isolated female frog was placed at the midpoint of the two loudspeakers (the distance from loudspeaker to the midpoint was 1 m) and covered with an acoustically-transparent container (L × W × H:27 × 15 ×17 cm),which allowed the female to rest for at least 5 min.After the resting period,we broadcast stimulis about 1 min before moving the acoustically-transparent container,then the container was immediately lifted up and any phonotaxis behavior (orientation or approaching) of female frogs was recorded.Each phonotaxis test lasted for 10 minutes,which was counted from the time when the transparent container was lifted up.To eliminate the habituation to one side acoustic stimuli,we reversed the position of loudspeaker each time.In order to test the effect of the frequency and the NLP content on the females’ phonotaxis behavior,we performed the second two-loudspeaker phonotaxis experiment (NLP-Freq-2SP).We selected two types of long call for NLP-Freq-2SP,that was,one call contained higher NLP and lower fundamental frequency(HN-LF),and another call contained lower NLP content and higher fundamental frequency (LN-HF) (Figure 1.c and d).The experimental procedures of NLP-Freq-2SP were the same as above.To determine whether a particular call characteristc was similarly chosen by the same female,we would perform the second time phonotaxis experiments for the same female with the different position of designated target loudspeaker(a rest period of at least 2 h elapsed prior to the next test).We scored a response if a female approached to within 10 cm of one of the two loudspeakers or showed phonotactic orientation movements (Gerhardt 1995;Bee 2007).If focal female failed to show phonotactic behavior for 10 min after the chamber was raised (e.g.move randomly between the two loudspeakers),we interpreted this as a lack of mating motivation and discarded the trial from the data set (Talyoret al.
,2011).In total,15 of the 20 (75%) amplexus females were documented.Afterwards,the frogs were released into the wild,to their individual calling sites.2.5.Statistics of calls and data analysis
Adobe Audition CC 2017 (Adobe Inc.,USA) was employed to edit the calls.PRAAT(6.1.14) was applied to analyze calls (Boersma and Weenink;Herzel,1993).By referring to Feng’s classification method of maleO.tormota
calls (Fenget al.
,2009),the long-calls (> 135 ms)were selected for analysis,and the numbers of long calls of each male frog is not less than 7.Based on the visual inspection of narrowband spectrogram (Herzel,1993),calls can be segmented into NLP and linear phenomena (see Zhanget al.
,2017 for details).NLP refers to four acoustic characteristics not found in vocalizations produced by linear sound production systems,i.e.,frequency jump,subharmonic,deterministic chaos,or biphonation (Wildenet al
.,1998).Frequency jump refers to an abrupt or rapid change within a few milli seconds,in the call fundamental frequency (f
);subharmonics refers to harmonic segments of a call where there is energy at 0.5,0.33,0.25,or 0.2f
;deterministic chaos refers to segments of a call with noiselike spectra;biphonation refers to a bifurcation of thef
or of the overtones.Segment borders were placed at bifurcations,i.e.,at boundaries between different dynamic regimes.The categories of different dynamic regimes were:harmonic phonation,deterministic chaos,biphonation,subharmonics,and signal break (Wildenet al
.,1998;Fenget al
.,2009).Following call segmentation (Figure 2),we measured the durations of the various segments (harmonic phonation,deterministic chaos,biphonation,subharmonics).Subsequently,the percentages of these durations to the total call duration were calculated.Pearson correlation analysis (PCA) was used to explore the correlation between SVL and NLP content,SVL and body weight.The Binomial test was used to evaluate the females’preference in 2C experiments,NLP-2SP,and NLP-Freq-2SP.All data analysis is completed by R 4.0.0.3.Results
Due to the geographical and other factors (e.g.temperature,body size etc.),there may be some variability in spectrotemporal features of the vocal signals in the same species (i.e.,dialect phenomenon) (Bowman,1979;Mitaniet al.
,1992;DeWolfe and Baptista,1995).Therefore,by referring to Feng’s classification method of maleO.tormota
(Fenget al.
,2009),we set new standards for long-and short-calls in this study.According to the frequency distribution of note durations (Figure 3),this study defines a call of maleO.tormota
with a note duration of not less than 135 ms as a long-call;on the contrary,a short-call is defined as a call with less than 135 ms per note durations.The results showed that there was a significant positive correlation(Pearson:n
=30,r
=0.73,P
< 0.001) between SVL and body weight,and a significant negative correlation (Pearson:n
=30,r
=-0.76,P
< 0.001) between SVL and NLP content (Figure 4)in maleO.tormota
.The results of the 2C experiment (Figure 5)indicated that females prefer to mate with males of smaller body sizes (Binomial test:P
< 0.05).Phonotaxis test (Figure 5)demonstrated that female frogs showed higher preference to the calls with higher NLP content (Binomial test,P
< 0.05) as they showed phonotaxis behavior to those calls in relatively less time than that of low NLP content (143 s versus 306 s).In the NLP-Freq-2SP,12 female frogs and 3 female frogs exhibited phonotaxis to HN-LF (the calls with higher NLP content and lower frequency) and LN-HF (the calls with higher frequency and lower NLP),respectively (Figure 5).Besides,the results of NLP-Freq-2SP indicated that the time of phonotaxis for HN-LF(118 ± 143 s) is significantly less than that of LN-HF (513 ± 34 s)(Binomial test,P
< 0.05).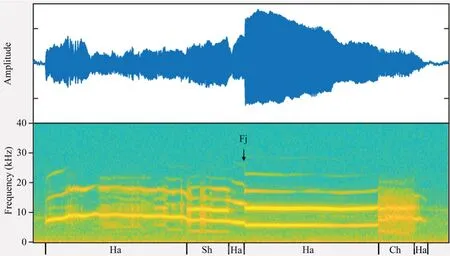
Figure 2 Amplitude-modulated waveform (top trace) and spectrogram(bottom trace) of a long call of O.tormota.The call was segmented into segments containing harmonic (Ha),subharmonics (Sh),deterministic chaos (Ch),frequency jumps (Fj,in the direction of the black arrow).This particular call does not show biphonation segments.This plot is made by MATLAB R2018a,with FFT frame set to 512.
4.Discussion
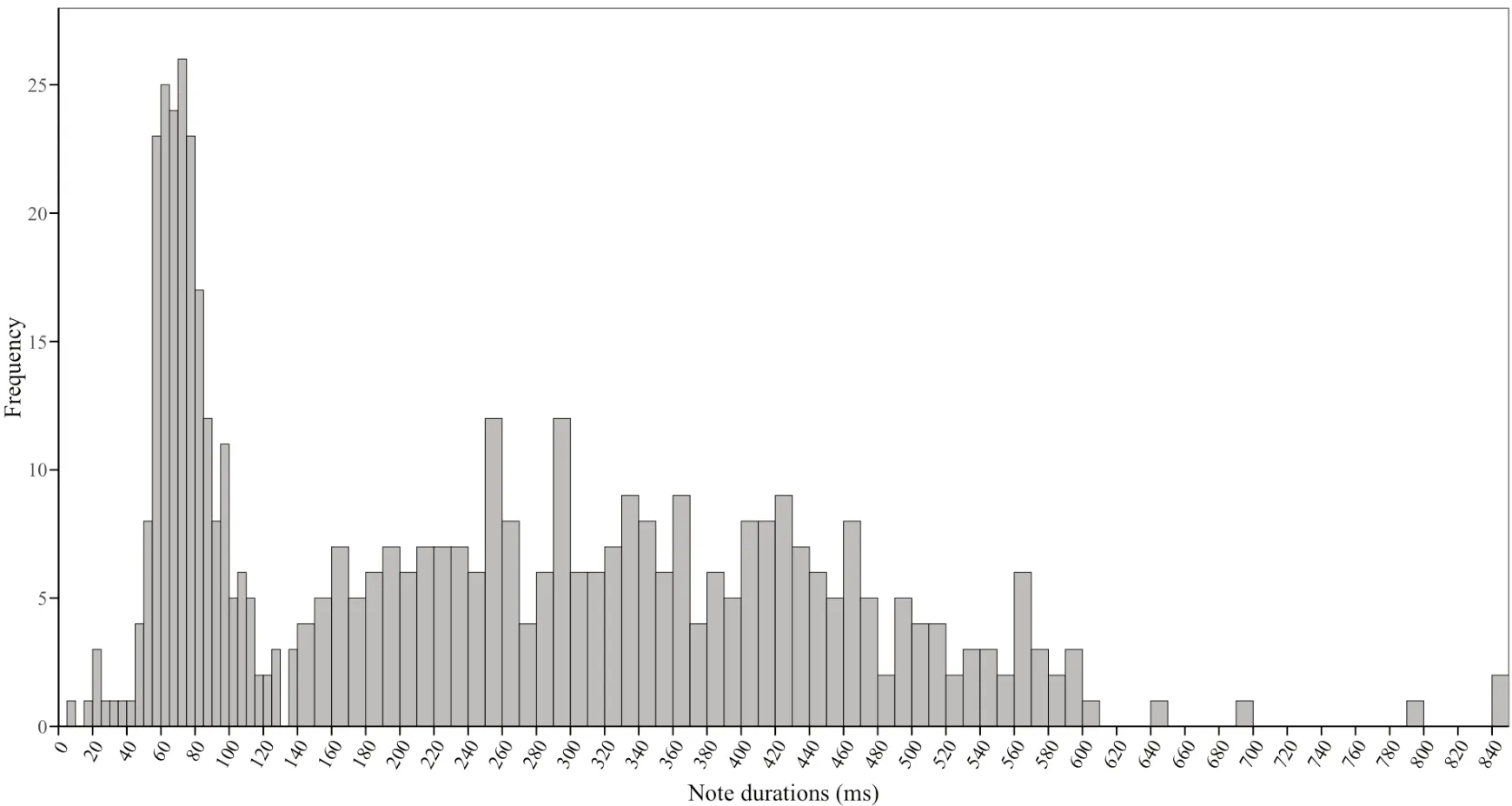
Figure 3 Frequency distribution of notes duration of male O.tormota.
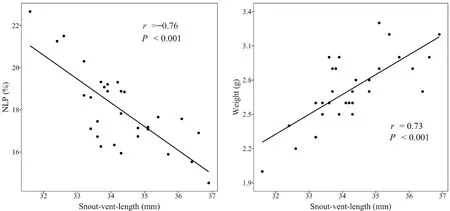
Figure 4 Graphs illustrating (a) the relationship between SVL and NLP content,and (b) the relationship between SVL and weight for male O.tormota.
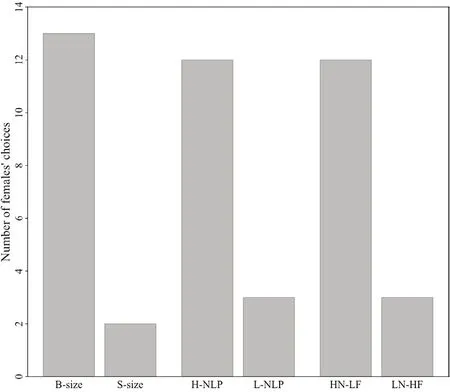
Figure 5 The results of 2C experiments (binomial test:P < 0.05)and phonotaxis tests (binomial test:P < 0.05) for female O.tormota.B-size:big-size;S-size:small-size;H-NLP:high-NLP;L-NLP:low-NLP;HN-LF:long call with high NLP content and low fundamental frequency;LN-HF:long call with low NLP content and high fundamental frequency
The results showed that there was a significant positive correlation (Pearson:n
=30,r
=0.73,P
< 0.001) between SVL and body weight,and a significant negative correlation(Pearson:n
=30,r
=-0.76,P
< 0.001) between SVL and NLP content in maleO.tormota
.In other words,the larger body size the males have,the fewer NLP content are found in their calls.Larger frogs,in general,tend to have larger larynges and their related morphological structures,which are responsible in producing calls that are negatively related to the calling frequency (Bowman,1979).This phenomenon is common among animal taxa,including anurans (Castellanoet al.
,2000;Fenget al.
,2009),lizard (Labraet al.
,2013),birds (Digbyet al.
,2014) and mammals (Charlton and Reby,2016).The influence of body size variation on NLP components has been only inferred from studies employing vocal apparatus models(Cazauet al.
,2016),but this has never been reported from studies on living animals varying in body size (Serranoet al.
,2019).With regards to the relationship between the NLP components and SVL,Fitchet al.
(2002) suggested that the nonlinear phenomena might subserve the estimation of size,e.g.mimicry of larger individuals,as producing subharmonics may lower the perceived frequency and thus causing signalers appear larger than they are.It is expected that NLP characteristics in calls are correlated to the variation in body size (Serranoet al.
,2019).Serranoet al.
(2019) had revealed that body size has an influence on NLP component at intra-and inter-population levels,e.g.in Darwin´s frogs (Rhinoderma darwinii
),the smaller individuals had higher proportions of the relative duration of chaos.To our knowledge,it was the first data showing that the NLP contents are correlated to body size of frogs.Field and indoor experiments have proven that femaleO.tormota
prefered smaller males in mate choice (Zhanget al.
,2020).The results of 2C experiment and NLP-2SP showed that females ofO.tormota
tend to choose smaller males with higher NLP content to mate.Previous studies in mammalian vocalizations revealed that NLP could prevent habituation(Serranoet al.
,2019),convey mental state (Kaltwasser,1991;Facchiniet al.
,2005),and draw attention (Townsend and Manser,2010;Blesdoe and Blumstein,2014).It also has been suggested that the presence of NLP in the vocalizations of some frogs could not only contribute to individual signatures (Wildenet al.
,1998;Fitchet al.
,2002;Volodinaet al.
,2006) and improve the individual specificity (Fenget al.
,2009),but also be more appropriate for transmission in noisy environments (Riedeet al.
,2000;Tokudaet al.
,2002;Zhanget al.
,2015).Evidently,calls with high NLP content could mean multiple types or/and duration of NLP components,which can combine in various ways,and which could significantly improve the specificity of calls and prevent the habituation to the calls (Riceet al.
,2011).Therefore,the calls of maleO.tormota
in smaller individuals containing higher NLP content can make their calls more complicated and attractive,which are more likely to be noticed and favored and were more prone to be selected by female.Calls of maleO.tormota
containing higher NLP or higher frequency are more likely to be favored by females (Zhanget al
.,2020).The result of NLP-Freq-2SP further revealed that the male’s calls with higher NLP content and lower frequency were more attractive to female,compared to male's calls of higher frequency and lower NLP content.This suggests that higher NLP content rather than higher frequency in calls had stronger effects over phonotaxis behavior of females.The fact that calls of maleO.tormota
have higher percentages of NLP content compared to the vocalizations of most other anurans may be the result of long-term sexual selection (Stabelet al.
,1989).The males with the calls containing higher NLP content could mean more opportunities for mating,and thus higher success of reproducing more offsprings.Therefore,we speculate that sexual selection may be responsible for the higher fitness of males with high NLP content,that is,females prefer males whose calls contain more NLP content.For maleO.tormota
,having smaller body sizes means higher NLP content in their calls,as evolution might dictate females to perceive how much NLP content a male has in its calls based on body size.It has been suggested that NLP contributes to boosting the auditory salience and providing cues to the fitness of vocalizers(Wildenet al.
,1998;Riedeet al.
,2000;Fitchet al.
,2002).The contribution of NLP beyond individual signatures inO.tormota
remains poorly understood (Feng and Narins,2008;Fenget al.
,2009).Results of our study inO.tormota
revealed that NLP content,as well as fundamental frequency,can reflect body sizes of males,and that higher NLP content contribute to higher attractiveness of the male’s call to the female.The exact primary and secondary function of NLP deserves attentions for further investigations.Acknowledgements
We thank Albert S.FENG for his constructive suggestions on the field and laboratory study.We also thank anonymous referees for their valuable comments and suggestion for revision on an earlier version of this manuscript.This research is supported by a grant from the Chinese Natural Science Foundation to Fang ZHANG(NSFC grants 31 872230;31640073) and Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources (No.591601). Asian Herpetological Research2021年1期
Asian Herpetological Research2021年1期
- Asian Herpetological Research的其它文章
- An Integrative Taxonomy of Amphibians of Nepal:An Updated Status and Distribution
- A New Species of the Gekko japonicus Group (Squamata:Gekkonidae) from Southwest China
- Genetic Diversity and Population Structure of the Oriental Garden Lizard,Calotes versicolor Daudin,1802 (Squamata:Agamidae) along the Mekong River in Thailand and Lao PDR
- Species Diversity,Distribution,and Microhabitats of Anurans on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park,Bukidnon,Philippines
- Species Account of Anurans from the Western Slope of Mt.Kitanglad,Mindanao Island,Philippines
- Behavioral and Neurogenomic Responses to Acoustic and Visual Sexual Cues are Correlated in Female Torrent Frogs
