Species Diversity,Distribution,and Microhabitats of Anurans on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park,Bukidnon,Philippines
Von Carlo P.DELA TORRE and Olga M.NUNEZA
1 Department of Biological Sciences and Environmental Studies,University of the Philippines Mindanao,Mintal,Tugbok District,Davao City 8022,Philippines
2 Wildlife-Human Interaction Studies,Ecological Research,and Biodiversity Conservation Laboratory,University of the Philippines Mindanao,Mintal,Tugbok District,Davao City 8022,Philippines
3 Department of Biological Sciences and Biodiversity Laboratory,Premier Research Institute of Science and Mathematics,Mindanao State University-Iligan Institute of Technology,A.Bonifacio Ave,Iligan City 9200,Philippines
Abstract Anurans are considered good indicators of habitat quality due to their bi-phasic life mode,limited dispersal abilities,and sensitivity to environmental changes brought about by habitat fragmentation,pollution,climate change,and emerging infectious diseases.This study aimed to determine species diversity and local distribution of anurans in lower (1000-1400 masl) and upper (1400-1600 masl)montane forests on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park,Central Mindanao,Southern Philippines by employing standard sampling techniques for amphibians.We recorded a total of 251 individual anurans,representing 12 species during a brief recent study period.Mt. Kalo-Kalo’s upper montane forest has a higher diversity index (H’=1.276)than the lower montane forest (H’=0.851).Despite the very low diversity indices,the level of endemism of anurans was 100% where ten of the recorded species are Mindanao Faunal Region endemics and an additional two species are Philippine endemics.Ansonia muelleri exhibited the highest local abundance and individuals of this species which constituted 73% of individual anurans observed.Most of the species encountered prefer terrestrial and aquatic microhabitats,specifically on the rocks and bank substrates along the rivers and streams.Three species of anurans (Philautus acutirostris,P.poecilius,and Rhacophorus bimaculatus) were strictly found in arboreal microhabitats.Limnonectes magnus and A.muelleri have overlapping microhabitats.Results indicate that for such a short survey and modest sampling effort,the lower and upper montane forests of Mt.Kalo-Kalo support high endemism of anuran species suggesting that conservation efforts continue to be a priority in this unique protected area.
Keywords conservation,endemic,indicator species,montane forest,protected area
1.Introduction
The herpetofauna of the Philippine Archipelago is taxonomically diverse and species-rich (Inger,1954;Alcala,1986;Brownet al
.,2001,2009;Brown and Siler,2013) with a total of 112 Philippine amphibians (84% endemic) and 368 (66%endemic) Philippine reptile species (Diesmoset al
.,2015;Uetzet al
.,2020).These high levels of diversity and endemism have been continuously reaffirmed by biogeographical hypothesis testing,based on the Pleistocene Aggregate Island Complex(PAIC) model (Heaney,1985;Brown and Diesmos,2002,2009;Esselstyn and Brown,2009;Lomolinoet al
.,2010;Sileret al
.,2010).Employing this perspective,nine herpetological subregion Pleistocene Aggregate Island Complexes (PAICs) have been recognized as centers of biological diversity and endemism(Batanes Island Group,Babuyan Island group,Luzon PAIC,Mindoro Island,Romblon Island Group,Palawan PAIC,Visayan PAIC,Mindanao PAIC,and Jolo-Tawi-Tawi PAIC),each supporting unique herpetofaunal communities (Brownet al
.,2001;Diesmos and Brown,2011;Brown and Siler,2013;Diesmoset al
.,2014).The faunal regions of the Philippines have been described by Heaney and colleagues based on more than 40 years of research (Heaney 1986,1998,1999,2000,2004;Heaneyet al
.,1990,1998,2005).Past efforts to document the natural history and biology of amphibians and reptiles by herpetologists and biogeographers have revealed the herpetological and conservation importance of the Philippines (Brownet al
.,2008,2012,2013;Diesmos and Brown,2011;Diesmoset al
.,2015;Sanguilaet al
.,2016).However,complete knowledge of the ecology and distribution of many species is still lacking,which is a challenge to effective conservation planning and species-specific intervention(Margules and Pressey,2000).Several studies have been conducted to describe the species diversity and distribution of the herpetofauna in the Philippines,including forested mountains of the Luzon PAIC (Mcleodet al.
,2011;Devan-Song and Brown,2012;Brownet al.
,2012;Brown and Siler,2013;Gojo Cruzet al
.,2018),the Babuyan Island Group (Oliveroset al
.,2011),Romblon Island Group (Sileret al.
,2012),Panay Island (Ferneret al
.,2000;Gaulke,2011),Cebu Island (Supsupet al
.,2016),and Leyte Island (Aureo and Bande,2017).On Mindanao Island,herpetological surveys are increasing in frequency (Davidet al.
,2006;Delimaet al.
,2007;Nuñezaet al.
,2010,2014,2015,2017;Beukema,2011;Almeria and Nuñeza,2013;Warguezet al.
,2013;Sularteet al.
,2015;Calo and Nuñeza,2015;Plaza and Sanguila,2015;Sanguilaet al
.,2016;Toledo-Brunoet al
.,2017;Vidalet al
.,2018;Delima-Baronet al
.,2019).However,large areas of Mindanao are still unexplored (Heaneyet al
.,2006;Petersonet al.
,2008;Sileret al
.,2009;Beukema,2011;Sanguilaet al
.,2016),and many new species are still being described (Brownet al.
,2009;Sileret al.
,2009).Mindanao is located in the southern part of the Philippine archipelago.It is considered a unique island for biogeographers and conservationists to study impacts of evolutionary diversification in archipelagos,since it is home to faunal elements that are both of Sundaic and Sulawesian origins(Esselstynet al
.,2009;Setiadiet al
.,2011).In the late twentieth century,the southern portions of the archipelago have been viewed to be more diverse than the northern portions of the archipelago in terms of faunal diversity (Inger,1954;Leviton,1963;Brown and Alcala,1970),in the sense that the northern islands are perceived as the ends of colonization routes and the extreme endpoints of dispersal for Sundaic faunal elements(Huxley,1868;Dickerson,1928;Inger,1954,1999;Myers,1962;Diamond and Gilpin,1983;Brown and Guttman,2002;Jones and Kennedy,2008).However,recent faunal surveys in the northern portions of the archipelago showed high species diversity and endemism (Linkemet al
.,2010;Heaneyet al
.,2011;Brownet al
.,2013).Hence,northern portions of the archipelago may be more diverse than generally appreciated (Heaneyet al
.,2010,2011;Baleteet al
.,2011;Duyaet al
.,2011;Sileret al
.,2011;Brownet al
.,2013;Diesmoset al
.,2015).Mt.Kalatungan Range Natural Park (MKaRNP) is located in the central section of Mindanao Island,Bukidnon Province.It was designated as a protected area on 5 May 2000,under Proclamation 305. Mt.Kalatungan Range Natural Park is under the administrative jurisdiction of the Department of Environment and Natural Resources (DENR),through the Protected Area Management Board (PAMB) and administered in accordance with the provisions of the National Integrated Protected Areas System (NIPAS) Act of 1992 (Official Gazette of the Republic of the Philippines,2000).Mt.Kalatungan Range Natural Park covers an area of approximately 35,139.230 hectares of which 21,247.730 hectares consist of the strict protected area whereas 13,891.500 hectares make up the buffer zone.Its current status is considered threatened by habitat fragmentation.Efforts to assess the anuran species diversity and distribution in MKaRNP have been done previously by Warguezet al
.(2013) and Toledo-Brunoet al
.(2017).However,they were at different sampling sites,elevations,sampling efforts,and seasons.A large part of the mountain range on MKaRNP is still in need of biodiversity assessment of anurans(Warguezet al
.,2013;Toledo-Brunoet al
.,2017).One of these mountains in MKaRNP is Mt.Kalo-Kalo which is located at the southeastern part of the MKaRNP (Figure 1),with a peak of 1730 masl.The climate falls within Type III,characterized as having a short dry season lasting only from 1 to 3 months.The highest amount of rainfall occurs in June while March is the driest month (Toledo-Brunoet al
.,2017).The Philippines is considered one of the most imperiled of the global biodiversity conservation hotspots because of high levels of endemism and alarming rates of destruction of fauna and flora brought about by overexploitation,deforestation,habitat degradation,climate change,and pollution (including biological pollution),among other factors (Heaney and Mittermeier,1997;Mittermeieret al
.,1999;Catibog-Sinha and Heaney,2006;Mittermeieret al
.,2011).From originally >85%forested,the archipelago now retains only 4%-8% of original forest cover (Catibog-Sinha and Heaney,2006).The Philippines needs at least 52% of total land area covered by forests to maintain a healthy and stable ecosystem (DENR,1989).However,the country has already reached the point below this threshold from about 68% forest cover in 1876 to 24% in 2003(Fernando,2005).Anurans are considered good indicators of habitat quality due to their bi-phasic life mode and sensitivity to environmental changes.They are notoriously affected by ha bitat fragmentation and logging.These environmental changes elicit changes in microclimatic variables,which manifest as a disturbance gradient in the habitat structure which is impactful to amphibians due to their limited dispersal capabilities (Alcala and Custodio,1995;Hampson,2001;Davic and Welsh,2004;Bickfordet al
.,2010;Heatwole and Wilkinson,2012;Jianget al.
,2016).Anurans are one of the severely threatened faunal groups within the Philippines,with one“Critically Endangered”(Platymantis insulatus
),one“Endangered”(Platymantis spelaeus
),and 21“Vulnerable”species (Philippine Red List Status,2019).The Philippine Archipelago has a significant and an increasing trend of forest cover loss exceeding that anywhere else in the planet (Sodhiet al
.,2004) with forest loss of at least 2.61 km/yr (Fallarcuna and Perez,2016).This may threaten anuran diversity,so new survey efforts on anurans are desirable and justified.Here we provide species accounts and determine the species diversity,distribution,and microhabitats of anurans in the upper and lower montane forests on Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park.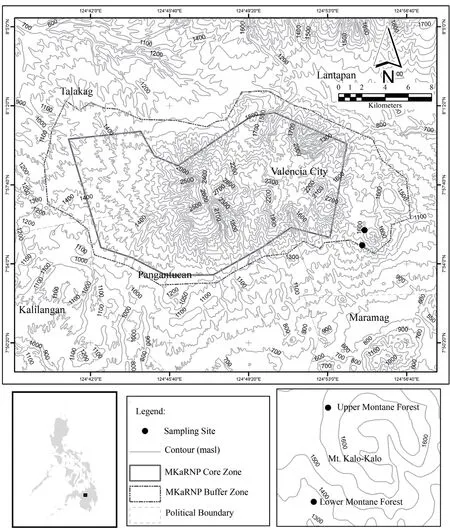
Figure 1 Center:Map of the Mt.Kalatungan Range Natural Park (MKaRNP).Lower Bottom Left:Map of the Philippines.Lower Bottom Right:Map of the Mt.Kalo-Kalo in MKaRNP showing the two sampling sites in the lower montane (1000-1400 masl) and upper montane (1400-1600 masl) forests.
2.Materials and Methods
2.1. Study Sites
Sample collection was done in the lower montane forest at 1000-1400 meters above sea level (masl)(Figure 2) and upper montane forest (1400-1600 masl) (Figure 3),that is,from submontane to montane forest of Mt.Kalo-Kalo,Mt.Kalatungan Range Natural Park,Sitio Bauhon,Barangay Dagumbaan,Municipality of Maramag,Bukidnon Province,Philippines which is geographically located at 7°54’27”N,124°54’32”E (WGS84) (Figure 1).The distance between the upper and lower montane forests was ca 2-3 kilometers.Delineation of upper and lower montane forests was based on vegetation types of sampling areas (Toledo-Brunoet al
.,2017). Descriptions of the habitats were based on the Habitat Description Form(HDF) (National Research Council,1981).Site 1
Lower Montane Forest (7°54’6”N,124°54’42”E):has an altitude of 1000-1400 masl (Figure 2) characterized by tall and big“Ulayan”(Lithocarpus
spp.)emergent trees up to 45 m tall and 90 cm diameter at breast height (dbh).“Repetek”(Kokoona ochracea)
is the canopy tree with a dbh of 84 cm and height of 40 m.Wild orchids,Bird’s nest fern,and vines are observed.Thespesia populnea,Syzgium
spp.,Agathis philippinensis
,Cof fea arabica
,Sphaeropteris
spp.,Alsophila
spp.,Pandanaceae,
and Araceaeare observed in moderate numbers.Some of the trees observed are covered with mosses and ferns.The ground is covered with moderately dense leaf litter of 2-5 layers.Site 2
Upper Montane Forest (7°55’34”N,124°54’43”E):has an altitude of 1400-1600 masl (Figure 3) characterized by“Ulayan”(Lithocarpus
spp)as emergent trees with a dbh of 70 cm and height of 40 m.and“Tampoy”(Syzygium
spp.) canopy trees with a dbh of 61 cm and height of 37 m.Thespesia populnea
,Agathis philippinensis
,Phyllocladus hypophyllus
,Sphaeropteris
spp.
,Alsophila
spp.,Colocasia esculenta,
andCallamus deeratus
are observed in moderate densities in the montane forest.Trees are mostly covered with mosses and epiphytic plants like ferns.The ground is covered with 3-7 layers of leaf litter.Generally,the montane forests in the Philippines are characterized by the presence of theLithocarpus
spp.,Syzygium
spp.,and gymnosperms such asAgathis philippinensis
andPhyllocladus hypophyllus
,including tree ferns of the family Cyatheaceae and Dicksoniaceae (Whitford,1991;Gruezo,1997;Buot and Okitsu,1998;Fernandoet al
.,2004;Amorosoet al
.,2011).Microhabitats in this study were divided into four categories according to the microhabitat types of Gonzales and Dans(1994),namely:Type I:Arboreal microhabitats as characterized by clumps of moss and other bryophytes and epiphytic plants such as orchids,ferns,and vines/climbers present on trees or above the ground (Figure 2);Type II:Leaf-axils as characterized by water-filled axils ofAraceae,Pandanaceae,Musaceae,and Cyatheaceae(tree ferns) (Figure 4);Type III:Vertical stratum as characterized by tree holes and similar crevices in the main branches approximately along the middle stratum of the forest,including tree bark and buttresses (1-10 m high) (Figure 5);and lastly,Type IV:Substrate level or ground litter as characterized by rocks along the streams,tree stumps,burrows,fallen logs or tree fall,and leaf litter (Figure 6).2.2. Sampling Technique
Field surveys were conducted during the rainy season from 29 April-30 May 2019,for a total of 32 field-days and 896 person-hours.Anurans were collected using random sampling,visual encounter techniques(Plaza and Sanguila,2015),and cruising techniques (Nuñezaet
al
.,2017).Cruising techniques were conducted during diurnal(700-1000) and nocturnal (1800 -2200) times,employing an opportunistic approach,such as capture by hand.Standard collection and specimen preservation techniques were utilized(Heyer,1994;Simmons,2002) and the collected specimens were photographed alive.Notes on microhabitat preferences and coloration were taken.After capture,one to two voucher specimens under Wildlife Gratuitous Permit No.R10 2019-48 were processed immediately in the field station and euthanized with denatured alcohol by injecting a small amount in the vent of each specimen (Heyer,1994;Simmons,2002).Digital vernier calipers and Pesola® spring balances were used to obtain the measurements and body weight of each specimen,respectively.Measurements include snout-vent length (SVL),head width(HW),head length (HL),tympanum width (TW),eye diameter(ED),forelimb length (FL),and hind limb length (HiL) (Watterset al
.,2016).These measurements and body weight were taken for accurate identification of anurans.Initial identification of specimens followed Inger (1954),Alcala and Brown (1998),Nuñeza (2012),Diesmoset al
.(2015),and Sanguilaet al
.(2016).Once identified,most specimens were returned to the wild.Representative samples were initially fixed in 10% formalin in the field and were later transferred to 70% ethanol at the Mindanao State University -Iligan Institute of Technology,Premier Research Institute of Science and Mathematics,Biodiversity Laboratory.Accounts for each species are provided and identifications validated by Dr.Rafe M.Brown of the University of Kansas,Biodiversity Institute,Lawrence,Kansas,United States of America.Determination of socioeconomic importance of anurans and potential threats to the anurans and their habitats was based on key informant interviews in a local community and on direct observations.Three respondents in the community of Sitio Bauhon,Barangay Bagumbaan,Maramag,Bukidnon,who are knowledgeable of anurans and have at least ten years of residency in the area,provided information such as abundance and threats to anurans at the sampled sites.2.3. Data analysis
Paleontological Statistics Software (PAST)version 3.06 (Hammeret al
.,2001) was used to determine biodiversity indices.Biodiversity indices were used to analyze the data gathered.These indices were the relative frequency,richness,diversity,evenness,and dominance.Relative species frequency refers to how common or rare a species is relative to others in a given location or community (Magurran,1988;Iskandar and Kotanegara,1993).3.Results

Figure 2 Characteristic appearance of the lower montane forest and Type I microhabitat.

Figure 3 Characteristic appearance of the upper montane forest.
3.1. Species Composition and Abundance
Twelve species of anurans were documented in the upper montane forest of Mt.Kalo-Kalo on MKaRNP.Of these species,10 are Mindanao Faunal Region endemics and two are Philippine endemics.There are three species in the family Rhacophoridae,two species in Megophryidae,Ranidae,and Dicroglossidae,and one species in Bufonidae,Cetatobatrachidae,and Microhylidae (Table 1).Ansonia muelleri
(Bufonidae) was the most abundant species with 70.13% relative frequency followed byRhacophorus bimaculatus
(Rhacophoridae)(6.25%),Megophrys stejnegeri
(Megophryidae)(4.86%),Leptobrachium lumadorum
(Megophryidae)(4.86%),Staurois natator
(Ranidae) (4.16%),Limnonectes magnus
(Dicroglossidae) (2.08%),Pulchrana grandocula
(Ranidae)(2.08%),Philautus acutirostris
(Rhacophoridae) (2.08%),Kalophrynus sinensis
(Microhylidae) (1.37%),Philautus poecilius
(Rhacophoridae) (1.37%),Limnonectes parvus
(Dicroglossidae) (0.69%),andPlatymantis
cf.dorsalis
(Cetatobatrachidae)(0.69%).
Figure 4 Characteristic appearance of Type II microhabitat.

Figure 5 Characteristic appearance of Type III microhabitat.
In the lower montane forest (1000-1400 masl),eight species belonging to five families were documented,namely:Bufonidae,Megophyridae,Microhylidae,Ranidae,and Rhacophoridae.Still,the Mindanao Faunal Region endemic speciesAnsonia muelleri
was the most abundant species recorded with 78.30%relative abundance followed byLimnonectes magnus
(9.43%),Staurois natator
(3.77%),Megophrys stejnegeri
(3.77%),Rhacophorus.bimaculatus
(2.83%),Pulchrana grandocula
(0.94%),andPhilautus poecilius
(0.94%).3.2. Species Accounts
Bufonidae
(Boulenger,1887)
Ansonia muelleri
(Figure 7) was documented from 1000-1600 masl and was observed in aquatic and terrestrial microhabitats.Specimens were collected and observed those exposed on forest ground,on top or among leaf litter on forest trails,and also on or beneath rocky crevices in mountain river systems or on stream banks.A.muelleri
has been recorded on mountains in Mindanao such as Mt.Sinaka and Mt.Hamiguitan (Ates and Delima,2008),Mt.Malindang (Nuñezaet al
.,2010),Mt.Pasian(Sileret al
.,2009) and across Mindanao island (Sanguilaet al
.,2016).This species is widely distributed in the Mindanao Faunal Region.Ansonia muelleri
had SVL ranging from 22-30 mm;HL=9-12 mm;HW=8-4 mm;tympanum width (TW)=7-8 mm;eye diameter (ED)=2-4 mm;forelimb length (FL)=17-23 mm;hind limb length (HiL)=23-40 mm;body weight (BW)=1-4 g.A.muelleri
has a variable color pattern.However,most of the observedA.muelleri
had alternate dark brown,green,and black straight lines running from the snout to vent;prominent dark bands on its forelimbs and hind limbs;and toes are webbed except for the 4th toe,which has the distal portion free of webbing.Ceratobatrachidae
cf.(Duméril,1853)

Figure 6 Characteristic appearance of type IV microhabitat.

Figure 7 Ansonia muelleri (Least Concern;NSM4001),a Mindanao Faunal Region endemic,abundant in terrestrial and aquatic microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.
Platymantis
cf.dorsalis
(Figure 8)was found inhabiting terrestrial microhabitat from 1400-1600 masl,specifically in the leaf litter along the riparian system.This species is a Philippine endemic.The capturedPlatymantis
cf.dorsalis
had the following measurements:SVL=1.6 mm;HL=2.4 mm;HW=0.7 mm;TW=0.8 mm;FL=1.1 mm;HiL=2.4 mm;ED=0.2 mm;BW=3 g.This recorded species had a prominent golden-yellow color from snout to the middle of the orbit.It also had numerous dark crossbars on dorsal surfaces of its hindlimbs and forelimbs.Dicroglossidae
(Stejneger,1910)
Limnonectes magnus
(Figure 9) was recorded in both aquatic andterrestrial microhabitats at 1000 -1600 masl on Mt.Kalo-Kalo.Specimens were observed sitting on rocks,boulders of mountain streams,or hidden among leaf litter on the forest ground near stream banks.This species has a wide distribution and is endemicto the Mindanao Faunal Region.
Figure 8 Platymantis cf.dorsalis (Least Concern;NSM4002),a Philippine endemic,abundant in terrestrial microhabitats(1400-1600 masl).Photo by V.C.P.DELA TORRE.

Figure 9 Limnonectes magnus (Near-Threatened;NSM4003),a Mindanao Faunal Region endemic,abundant in aquatic and terrestrial microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.
Limnonectes magnus
had the following measurements:SVL=57.5-73 mm;HL= 27.5-33 mm;HW=22--3 mm;TW=23.5-29;FL=31-52 mm;HiL=94-105;ED=7.5-10 mm;BW=20-60 g.Specimens encountered had a visible tympanum;and dark bands on forelimbs and hind limbs.Laterally,they had light yellow spot near the groin;tympanic fold was present from snout to base of forelimbs;dorsal body granular to tuberculate skin;and the ventral body had smooth skin.(Taylor,1920)
Limnonectes parvus
(Figure 10) was recorded inhabiting terrestrial microhabitats at 1400-1600 masl.This species can be commonly encountered in central,southern,western,and northeastern Mindanao (Sanguilaet al
.,2016).This species is endemicto the Mindanao Faunal Region.The capturedLimnonectes parvus
had the following measurements:SVL=3.5 mm;HL=1.5 mm;HW=6 mm;TW=1.2 mm;FL=2.8 mm;HiL=1.5 mm;ED=3 mm;BW=3 g.This species had a prominent golden-yellow color from its snout to vent.Also numerous dark crossbars in its hindlimbs and forelimbs can be observed.Megophryidae
(Brown,Siler,Diesmos and Alcala,2009)
Leptobrachium lumadorum
(Figure 11) is widely distributed throughout the upper montane forest of Mt.Kalo-Kalo inhabiting riparian habitats.Moreover,this species was recorded as widespread on Mindanao and Basilan islands,but not present on Dinagat,Siargao,Leyte,Samar,and Bohol(Brownet al
.,2009). This species is endemic to the Mindanao Faunal Region.Leptobrachium lumadorum
had the following measurements:SVL=4.2-4.5 mm;HL=2.1-2.3 mm;HW=1.9-2.1 mm;TW=2.0-2.2 mm;ED=0.7-0.9 mm;FL=2.8-3.2 mm;HiL=5.3-5.6 mm;BW=5-6 g.TheL.lumadorum
encountered had alternating brown bands on the forelimbs and hindlimbs,a visible tympanum,and a spotted-brown pattern on its dorsum.(Taylor,1920)
Megophrys stejnegeri
(Figure 12) was observed in the lower montane forest from 1000-1400 masl on Mt.Kalo-Kalo.It uses terrestrial and aquatic microhabitats as well as on standing water pools.This species is considered widespread and endemic to the montane regions of the Mindanao Faunal Region like Mt.Sinaka,Mt.Hamiguitan (Ates and Delima,2008),Mt.Pasian(Sileret al
.,2009),Mt.Malindang (Nuñezaet al
.,2010),Samar,Leyte,Bohol,and Siargao Island (Diesmoset al
.,2014;Diesmoset al
.,2015).
Figure 10 Limnonectes parvus (Least Concern;NSM4004),a Mindanao Faunal Region endemic,abundant in terrestrial microhabitats (1400-1600 masl).Photo by V.C.P.DELA TORRE.

Figure 11 Leptobrachium lumadorum (Least Concern;NSM4005),a Mindanao Faunal Region endemic,utilizes aquatic and terrestrial microhabitats (1400-1600 masl).Photo by V.C.P.DELA TORRE.

Figure 12 Megophrys stejnegeri (Least Concern;NSM4006),a Mindanao Faunal Region endemic,utilizes aquatic and terrestrial microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.
Megophrys stejnegeri
had the following measurements:SVL=41 mm;HL=13 mm;HW=13.5 mm;TW=12.5 mm;FL=21 mm;HiL=67 mm;ED=5.5 mm;BW=10 g. This specimen had an observable brownish-orange color;a pair of longitudinal folds on the back;dark bands on forelimbs and hind limbs present;visible tympanum,and a horn-like projection over each eyelid.(Peters,1867)
A juvenile ofKalophrynus sinensis
(Figure 13) was captured in the upper montane forest at 1400-1600 masl.It occupies terrestrial and aquatic microhabitats.It is considered a widespread species,having been recorded from Basilan,Bohol,Camiguin Sur,Culion,Dinagat,Leyte,Mindanao,and Samar islands (Diesmoset al
.,2015).Kalophrynus sinensis
is frequently encountered in the rainy season,calling while floating on temporary pools or water-filled cavities in a variety of habitats of varying levels of disturbance.This species is endemic to the Mindanao Faunal Region.The juvenileKalophr ynus sinensis
had the following measurements:SVL=15 mm;HL= 7 mm;HW=6 mm;TW=6 mm;FL=10 mm;HiL=8 mm;ED=3 mm;BW=0.5 g.This species had a brown-orange color,usually with dark spots anteriorly and a dark triangle in the upper part of the head.The lower surfaces were dirty white with numerous black spots and the hindlimbs had dark crossbars.Ranidae
(Taylor,1920)

Figure 13 Kalophrynus sinensis (Least Concern;NSM4007),a Mindanao Faunal Region endemic,utilizes aquatic and terrestrial microhabitats (1400-1600 masl).Photo by V.C.P.DELA TORRE.
Pulcharana grandocula
(Figure 14) is commonly encountered at high abundances and distributed throughout the Mindanao PAIC islands (Brown and Siler,2013).This species can be found in a variety of disturbed habitats and is distributed across much of the elevational relief of Mindanao (Sanguilaet al
.,2016).In this study it was recorded in the lower and upper montane forests at 1000-1600 masl,inhabiting the aquatic and terrestrial microhabitats.The discovery of a new,morphologically similar,and exceedingly rare stream frog species that had previously been confused withHylarana grandocula
(Brown and Siler,2013;Brown,2015) led Brown and Siler (2013) and Brown(2015) to speculate that mountains of northeast Mindanao may also harbor undocumented populations of this second MindanaoHylarana
taxon.Oliveret al
.(2015) recently published a phylogeny for many members of the African,Papuan,and Southeast Asian members of the genusHylarana
and recognized“Pulchrana
”as the available name corresponding to theHylarana signata
complex (Brown and Siler,2013).Although this action is arbitrary and unnecessary and no justification for a maximally reduced classification was provided (Wienset al
.,2009;Poe,2013;Brown,2015),the most recently published name was adopted.Pulchrana grandocula
had the following measurements: SVL=54 mm;HL=29 mm;HW=22 mm;TW=18 mm;FL=41mm;HiL=121 mm;ED=8 mm;BW=15 g.This species had a brown-orange color,usually with dark spots anteriorly and a brown margin on the side of its dorsal body.Hindlimbs and forelimbs had prominent dark bands.(Günther,1858)
Staurois natator
(Figure 15) was observed to be widely distributed in the lower and upper montane forests at 1000-1600 masl.Mainly inhabiting the terrestrial and arboreal microhabitats,this species was commonly found on leaf axils of ferns and Pandaceae.Common throughout the Mindanao Faunal Region,Staurois natator
is a frequently observed component of most amphibian communities of the southern Philippines (Alcala and Brown,1998).Arifinet al
.(2011) demonstrated the distinction between Palawan faunal region populations (S.nubilis
) versus those of the Mindanao PAIC (S.natator
).
Figure 14 Pulchrana grandocula (Least Concern;NSM4008),a Mindanao Faunal Region endemic,utilizes aquatic and terrestrial microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.

Table 1 Species composition,relative frequency (),distribution,and conservation status of anurans on Mt.Kalo-Kalo,Mt.Kalatungan Range Natural Park,Philippines.
Staurois natator
had the following measurements:SVL of 25-28.5 mm;HL=11-13 mm;HW=8-8.5 mm;TW=12-13.5 mm;FL=14-16 mm;HiL=59-62 mm;ED=4-4.5 mm;BW=1.5-2 g.The species observed had a uniformly brownish dorsal skin color,usually with green spots anteriorly and a green venter.Hind limbs and forelimbs had brown bands.Rhacophoridae
(Peters,1867)
Philautus acutirostris
(Figure 16) was recorded in the upper montane forest at 1400 -1600 masl on Mt.Kalo-Kalo inhabiting arboreal microhabitats.This small shrub frog is known to be widely distributed on Jolo and Basilan islands of the Sulu Archipelago,Zamboanga del Sur Province of western Mindanao,and on the mountains of the northeast Mindanao(Brown and Alcala,1994;Sanguilaet al
.,2016).This species is endemic to the Mindanao Faunal Region.Philautus acutirostris
had the following measurements: SVL=0.5-0.6 mm;HL=0.7-0.9 mm;HW=0.5-0.6 mm;TW=0.6-0.8 mm;FL=1.0-1.1 mm;HiL=2.2-2.4 mm;ED=0.1-0.2 mm;BW=3 g.The species observed had a uniformly brownish dorsal skin color,usually with brown spots anteriorly and brown venter,and black lateral line from snout to vent.It had prominent lateral folds from its eyes down to its forelimbs.Hind limbs and forelimbs had black bands.(Brown and Alcala,1994)

Figure 15 Staurois natator (Least Concern;NSM4009),a Mindanao Faunal Region endemic,abundant in arboreal and terrestrial microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.

Figure 16 Philautus acutirostris (Least Concern;NSM4010),a Mindanao Faunal Region endemic,abundant in arboreal microhabitats (1400-1600 masl).Photo by V.C.P.DELA TORRE.
This Mindanao Faunal Region endemic shrub frog was recorded in the lower and upper montane (1000-1600 masl)forests of Mt.Kalo-Kalo inhabiting arboreal microhabitats.Philautus poecilius
(Figure 17) was also widely observed from the forests of eastern Mindanao (Brown and Alcala,1994;Plaza and Sanguila,2015) up to the high elevation forests of Mt.Lumot(Sanguilaet al
.,2016) and Mt.Malindang of western Mindanao(Nuñezaet al
.,2010).Philautus poecilius
had the following measurements:SVL=1.5-1.8 mm;HL=0.7-0.8 mm;HW=0.5-0.6 mm;TW=0.6-0.7 mm;FL=1.2-1.4 mm;HiL=1.1-1.2 mm;ED=0.2-0.3 mm;BW=0.5 g.The species observed had a uniformly spotted brown and black dorsal skin color,usually with brown spots anteriorly and brown venter.Hind limbs and forelimbs had black bands.(Peters,1867)

Figure 17 Philautus poecilius (Least Concern;NSM4011),a Mindanao Faunal Region endemic,abundant in arboreal microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.

Figure 18 Rhacophorus bimaculatus (Least Concern;NSM4012),widespread in Luzon and Mindanao Faunal Region utilizes arboreal microhabitats (1000-1600 masl).Photo by V.C.P.DELA TORRE.
Rhacophorus bimaculatus
(Figure 18) utilizes arboreal microhabitats.It was observed in the lower and upper montane forests of Mt.Kalo-Kalo at 1000-1600 masl on the leaf axil of ferns.This species is known to be widespread in the Luzon and Mindanao Faunal Regions.This common tree frog inhabits overhanging understory vegetation surrounding rapidly cascading streams in lower to mid-montane forests.Its distinctive advertisement call is a single brief,high frequency,shrill chirp and can be heard over the sound of waterfalls(Sanguilaet al
.,2016).Previously considered uncommon,this species is now appreciated for its very specific microhabitat preference,where it can be predictably encountered by experienced field workers.Originally classified as“Near-Threatened”in 2010 (NT;IUCN 2010),and“Vulnerable”in 2016 (VU;IUCN 2016);this species now qualifies only for“Least Concern”status (LC;IUCN,2018) as a result of the numerous new localities at which it has been recorded (Gonzaleset al
.,2014),and the predictability where it can be found now that its habitat is known and can be purposefully surveyed (Diesmoset al
.,2014,2015).Rhacophorus bimaculatus
had the following measurements:SVL=30-32 mm;HL=13 -14 mm;HW=13.5-14 mm;TW=12.5-13 mm;FL=22.5-23 mm;HiL=59-60 mm;ED=4.5-5.5 mm;BW=3-4 g.This observed species had a highly variable color pattern,usually with grey spots anteriorly and yellow green venter.Hind limbs and forelimbs had brown bands.3.3. Biodiversity Indices
Table 2 shows the biodiversity indices of the two sampling sites on Mt.Kalatungan.Our results indicate very low species diversity following the modified biodiversity scale of Fernandoet al
.(2004) in the upper montane (H’=1.276) and in the lower montane (H’=0.851)forests with uneven distribution.3.4. Microhabitats of Anurans
Gonzales and Dans (1994)defined four microhabitat types,although we have further classified these into five specific microhabitats such as bodies of water,rocks along streams/rivers,shrubs growing near the stream,shrubs growing in the forest,and fallen logs.The majority of the species recorded can be both aquatic and terrestrial that are found within or near bodies of water(Table 3).Most anurans were observed on rocks along streams and rivers,on shrubs growing in the forest,and on fallen logs.Staurois natator
was also found inhabiting the leaf axils of ferns and different shrubs near the stream.Three species,Philautus acutirostris,Philautus poecilius,
andRhacophorus bimaculatus
were strictly found in arboreal microhabitats (forest shrubs),whereasLimnonectes magnus
andAnsonia muelleri
were observed to have overlapping microhabitats.4.Discussion
The presence of endemic anuran species,including ten Mindanao Faunal Region endemics and two Philippine endemic anurans,in this study suggests that Mt.Kalo-Kalo of the Mt.Kalatungan Range Natural Park (MKaRNP) is an area of local conservation value in which suitable habitats still exist to support endemic anuran communities.OnlyLimnonectes magnus
is“Near-Threatened”whereas the rest of the taxa are categorized as“Least Concern”according to IUCN (2018).Despite being listed as“Near-Threatened”,
Limnonectes magnus
was the second most abundant species in the study area.Its ability to inhabit undisturbed and disturbed streams and rivers in lower montane and lowland forests explains its abundance(IUCN SSC Amphibian Specialist Group,2019).Recently conducted herpetofaunal surveys by local herpetologists in the Philippines listedAnsonia muelleri
as“Vulnerable”(Philippine Red List Status,2019). In this study,Ansonia muelleri
was the most abundant species.The depressed body,strong tail,reduced tailfins,and sucker-like mouth of the tadpole ofAnsonia muelleri
suits this species for rapidly-running streams (Boulenger,1912;Taylor,1922;Inger,2007) found in the study area.More anuran species were recorded in the upper montane forest (1400-1600 masl) than in the lower montane forest(1000-1400 masl).Moreover,results also showed higher anuran diversity in the upper montane compared to the lower montane forest.Similar findings were reported by Warguezet al
.(2013) and Toledo-Brunoet al
.(2017) on MKaRNP,in which herpetofaunal species diversity was slightly higher at 1200-1600 masl.Several faunal surveys throughout the Philippines showed decreasing diversity with increasing elevations (Heaney and Rickart,1990;Diesmoset al
.,2002;Nunezaet al
.,2010;Sileret al
.,2006).However,no trend was established in this study because there were only two sites sampled.Although the highest peak of MKaRNP is around 2880 masl,the peak at the sites we sampled within the mountain range on Mt.Kalo-Kalo was lower at 1730 masl.However,due to difficult accessibility and safety issues we did not conduct sampling at higher elevation or at the peak.Previous ecological studies of anuran species richness at different elevations in certain Long-Term Ecological Research(LTER) sites on Mindanao mountains also showed higher anuran species richness at high elevation.For instance,Mt.Malindang at 1600-1700 masl has the highest anuran species richness (n
=11) of the four LTER sites (Mohaganet al
.,2018).The species richness in this study is higher compared to anuran species richness on Mt.Hamiguitan with 10 species at 1400-1700 masl.However,Mt.Malindang has higher total species richness (n
=20) considering various vegetation types from elevation of 900 to 1700 masl (Nunezaet al
.,2010).Mt.Matutum also has higher species richness (n
=13) at 500-1719 masl(Nunezaet al
.,2017),but the montane forest has lower species richness (n
=10) compared to the present study.Moreover,Mt.Kalo-Kalo’s 12 species is lower compared to Mt.Hamiguitan(n
=15) at 545-1435 masl (Delimaet al
.,2007) and Mt.Hilong-Hilong (n
=27) at 700-1300 masl (Plaza and Sanguila,2015).Compared to the four LTER sites of Mohaganet al
.(2018) on Mt.Apo (n
=5) at 1900-2000 masl,Mt.Hamiguitan (n
=10) at 1000-1100 masl,Mt.Malindang (n
=11) at 1600-1700 masl,and Mt.Kitanglad (n
=6) at 2100-2200 masl,Mt.Kalo-Kalo of the MKaRNP has the highest species richness (n
=12).In addition,all of these Mindanao mountains have high anuran endemism.For instance,the four LTER sites of Mohaganet al
.(2018) on Mt.Apo and Mt.Kitanglad has 100% endemism,90% endemism on Mt.Hamiguitan,and 90% endemism on Mt.Malindang.Moreover,Mt.Hilong-Hilong has 100% endemism (Plaza and Sanguila,2015) and 90% endemism on Mt.Matutum (Nunezaet al
.,2017).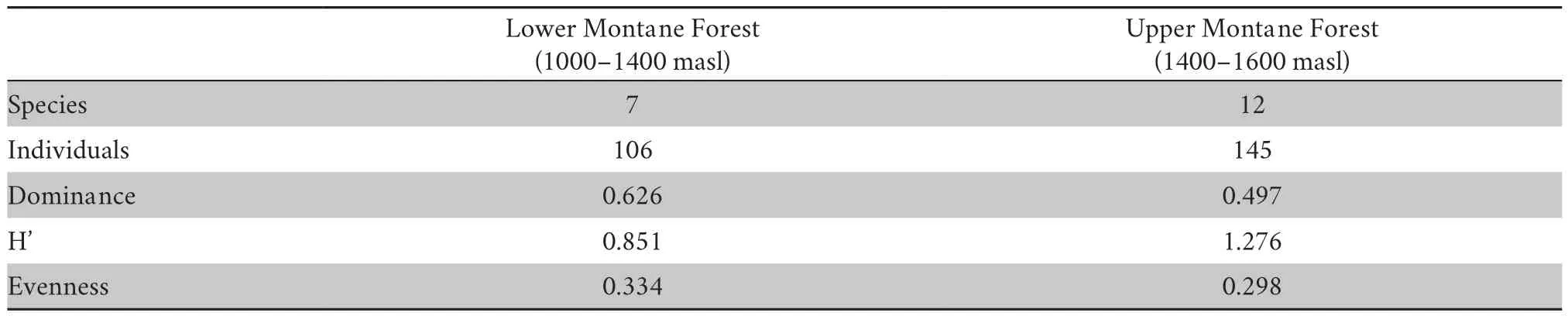
Table 2 Anuran biodiversity indices in the lower and upper montane forests of Mt.Kalo-Kalo,Mt.Kalatungan Range Natural Park,Bukidnon.

Table 3 Microhabitats and relative frequency () of anurans on Mt.Kalo-Kalo,Mt.Kalatungan Range Natural Park,Bukidnon,Philippines.Legend:[]Plant species;*Mindanao faunal region endemic;**Philippine endemic.
Generally,species richness of anurans is highest at lower elevations especially those anurans requiring water bodies for reproduction such as streams and rivers.However,in the case of anurans that are direct developers,diversity increases with increasing elevation particularly in the montane forest(Diesmoset al
.,2005;Fuet al.
,2006;Mohaganet al
.,2018).Some frogs in the families Ceratobtrachidae and Rhacophoridae are known to exhibit a terrestrial development type of reproductive mode,and are thus capable of undergoing direct development(Alcala,1962;Alcala and Brown,1982).In this study,33% of the total number of endemic species belong to these families.This may be related to amphibian ecological and physiological tolerances and microhabitat preferences.The upper montane forest itself has a patchy,range-restricted distribution to higher elevation in the topographically complex Mindanao Faunal Region (Diesmos and Brown,2011;Mohaganet al
.,2018).The study on Mt.Kalatungan Range Natural Park(MKaRNP) conducted by Warguezet al.
(2013) in Sitio San Guinto,Barangay Bacusanon,Municipality of Pangantucan,Bukidnon Province (7.90658°N;124.72382°E;7.92474°N;124.73241°E) at 1208-1600 masl.,recorded 14 species of anurans but Toledo-Brunoet al
.(2017) in Barangay Portulin,Municipality of Pangantucan,Bukidnon Province (4°22’33.36”N,128°21’58.70”E) at 1200-1600 masl,recorded only half this number from May-June 2016,five years later (Table 4). Species earlier reported but not observed in our study includeAnsonia mcgregori
,Pelophryne brevipes,Limnonectes
cf.ferneri,Fejervarya moodiei,Occidozyga laevis,Philautus surrufus,
Philautus worcesteri
,andPolypedates leucomystax
.Seasonal variation and atmospheric variation (precipitation) may also explain some differences in species recorded previously and in the present study on Mt.Kalatungan.Despite not having observed some previously recorded species from another area on Mt.Kalatungan Range Natural Park,we encountered three species that were not documented in earlier studies.These species areLimnoectes parvus,
Leptobrachium lumadorum,
andPhilautus
cf.dorsalis
which inhabit both the lower and upper montane forests at 1000-1600 masl.These species have very low abundance in the study area,which probably explains why Warguez et al
.(2013) and Toledo-Brunoet al
.(2017) failed to document them in their studies.Other species likeMegophrys stejnegeri
,Kalophrynus sinensis
,Pulchrana grandocula,Staurois natator
,andPhilautus poecilius
have low abundance and were not documented by Toledo-Brunoet al
.(2017).Overall,seven families were observed on MKaRNP,namely:Bufonidae (Ansonia mcgregori,A.muelleri,
Polyphryne brevipes
),Ceratobatrachidae (Philautus
cf.dorsalis
) Dicroglossidae(Limnonectes
cf.ferneri,Limnonectes parvus,
Limnonectes magnus
),Megophryidae (Megophrys stejnegeri,Leptobrachium lumadorum
),Microhylidae(Kalophrynus sinensis
);Ranidae (Fejervarya moodiei,Occidozyga laevis,Pulchrana grandocula,Staurois natator
),and Rhacophoridae (Philautus acutirostris,Philautus poecilius,Philautus surrufus,Philautus worcesteri,Polypedates leucomystax,Rhacophorus bimaculatus
).With the previous studies and the present results,MKaRNP has now a total of 20 species of anurans.Despite the protected status of Mt.Kalatungan Range Natural Park (MKaRNP),anthropogenic activities such as unregulated harvesting of frogs (Limnonectes magnus
),slash and burn farming,conversion of some areas in the lower and upper montane forests to farmland,and improper disposal of garbage pose considerable threats to the anuran population of the mountain range.Since most of the endemic anurans on Mt.Kalo-Kalo of MKaRNP were found on rocks along streams,rivers,fallen logs,and leaf litter microhabitat,threats like deforestation and habitat destruction may affect anuran assemblages (Lehtinenet al
.,1999).Results suggest that despite the anthropogenic disturbances,the lower and upper montane forests of Mt.Kalatungan Range Natural Park remain suitable habitats for endemic anurans.Acknowledgments
We would like to acknowledge the OVCRE-MSU-IIT and DOST-ASTHRDP-NSC for the funding support and Department of Environment and Natural Resources—Northern Mindanao (Region X) for the issuanceof the Gratuitous Permit.We would like also to extend our gratitude to Dr.Rafe M.BROWN for validating the anuran species identification and Dr. Christine F.GODINEZ-ORTEGA for editing the manuscript.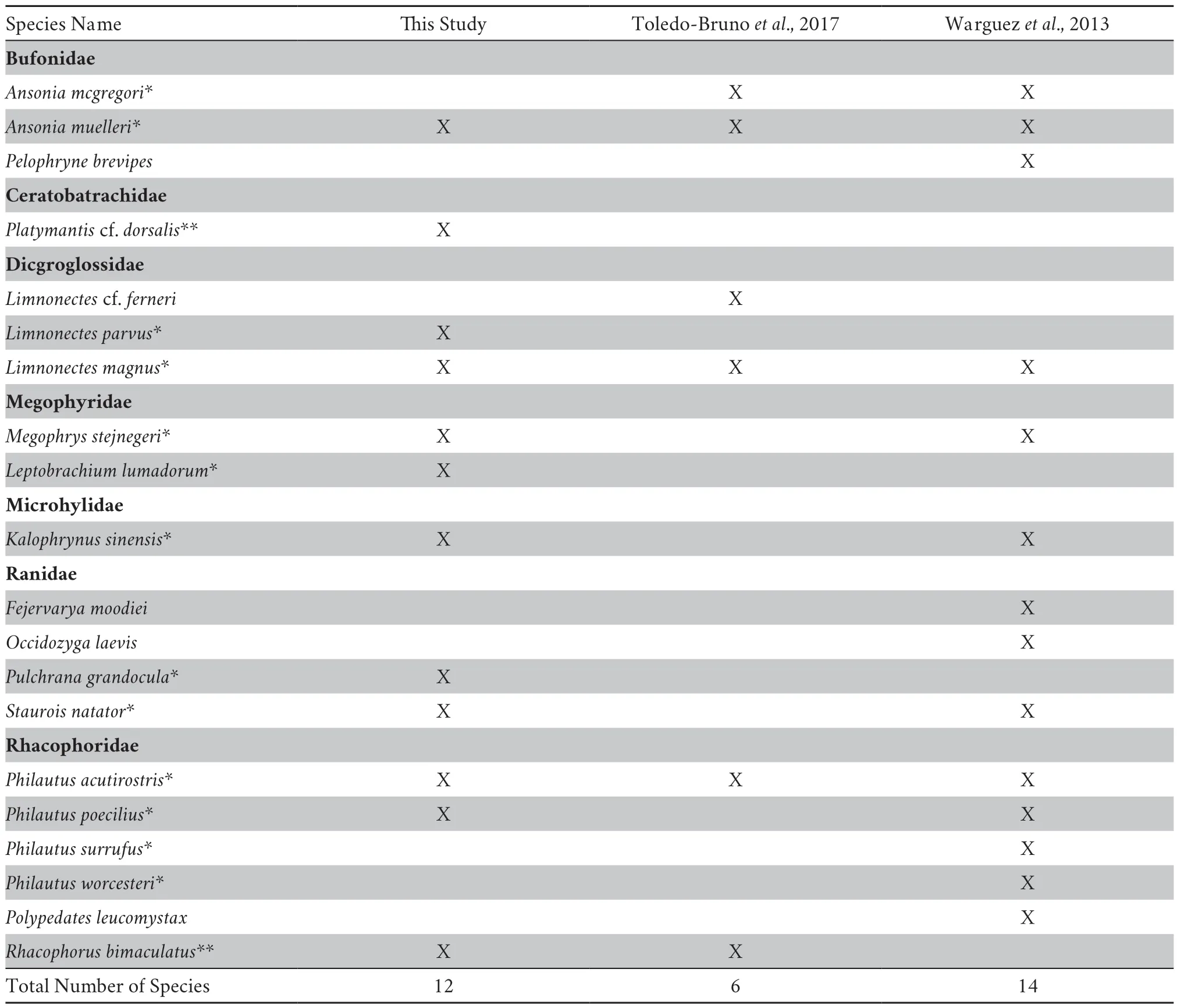
Table 4 Anuran species of Mt.Kalatungan Range Natural Park.Legend:*Mindanao Faunal Region endemic;** Philippine endemic.
 Asian Herpetological Research2021年1期
Asian Herpetological Research2021年1期
- Asian Herpetological Research的其它文章
- An Integrative Taxonomy of Amphibians of Nepal:An Updated Status and Distribution
- A New Species of the Gekko japonicus Group (Squamata:Gekkonidae) from Southwest China
- Genetic Diversity and Population Structure of the Oriental Garden Lizard,Calotes versicolor Daudin,1802 (Squamata:Agamidae) along the Mekong River in Thailand and Lao PDR
- Species Account of Anurans from the Western Slope of Mt.Kitanglad,Mindanao Island,Philippines
- Behavioral and Neurogenomic Responses to Acoustic and Visual Sexual Cues are Correlated in Female Torrent Frogs
- The Influence of Environmental Factors on the Behavior and Mortality Risk of Polypedates megacephalus Tadpoles
