Effect of Ultrasound-Assisted Thawing on Protein Oxidation and Quality of Frozen Jumbo Squid (Dosidicus gigas)
ZHU Wenhui, HUAN Haizhen, LI Yue, BU Ying, LI Xuepeng, LI Jianrong
(National & Local Joint Engineering Research Center of Storage, Processing and Safety Control Technology for Fresh Agricultural and Products,College of Food Science and Engineering, Bohai University, Jinzhou 121013, China)
Abstract: The objective of the present study was to explore the relationship of protein oxidation, muscle structural changes and jumbo squid mantle quality during ultrasound-assisted thawing. Frozen jumbo squid mantles were thawed by ultrasonic treatment using different power gradients (100, 300, and 500 W) at different temperatures (5 and 10 ℃). The U5-5 treatment (5 ℃, 500 W)caused the smallest thawing and cooking loss. Low-field nuclear magnetic resonance (LF-NMR) suggested that U5-5 could reduce juice loss. The seven different thawing methods showed significant differences in carbonyl content, total sulfhydryl content,dityrosine content and surface hydrophobicity (P < 0.05). U5-3 (5 ℃, 300 W) and U5-5 had little effect on the carbonyl content of myofibrillar protein. U5-3 caused the highest total sulfhydryl content and the smallest dityrosine content, while refrigerator thawing and U5-1 (5 ℃, 100 W) caused the least exposure of hydrophobic groups on the protein surface. Raman spectroscopy showed that ultrasonic-assisted thawing could retard the conversion of α-helices to β-sheets, β-turns and random coils. These results demonstrate that ultrasonic-assisted thawing at 5 ℃ (U5-3 and U5-5) can improve product quality and defer protein oxidation.
Keywords: ultrasonic-assisted thawing; jumbo squid; protein oxidation; quality; structural transformation
Freezing technology plays a very important role in seafood storage, transportation and processing[1]. Frozen foods must be thawed before processing or consumption[2].Thawing is an important factor affecting the final quality of frozen products. The formation of large ice crystals during thawing causes cell damage leading to irreversible drip loss,protein denaturation and the oxidation of food stuffs[2].
Recently, scientists are constantly looking for new thawing methods with fast thawing rate and good quality of thawed products[3]. Rapid thawing at low temperatures is helpful to prevent loss of food quality during production. There are many frozen food thawing methods available, including high-pressure thawing, ohmic thawing, microwave thawing and high-voltage electrostatic field thawing[4-8]. Some drawbacks of these thawing methods limit their application in thawing direction, such as high cost of high pressure thawing, different structure characteristics of meat caused by ohmic thawing, and local overheating phenomenon of microwave thawing[3,9].
Ultrasonic-assisted thawing has been explored as a new and efficient thawing technology[10]. Ultrasonic processing has been introduced as a novel approach to alter the quality and biophysical attributes of meat[11]. Gambuteanu et al[12]reported that low-intensity ultrasonic thawing not only accelerated the thawing process but also had a positive effect on the quality of frozen pork. Saleem[11]and Stadnik[13]et al reported that ultrasonic treatment improved water holding capacity of beef and poultry. Miles et al[14]reported that a combination of 500 kHz and 0.5 W/cm2produced an effective ultrasonic thawing method which could minimize surface heating for frozen meat and fish samples. Meanwhile, the function and structure of proteins can be changed by applying ultrasonic processing[15].However, the use of ultrasonic processing may result in quality impairment, such as protein oxidation[16]. Ultrasonic treatment is carried out in fluid, usually in water, because the characteristics of ultrasound require an energy-propagating medium[17]. Highly reactive free radicals are generated from cavitation zone initiated by ultrasound, and these degradation products can induce free radical chain reactions in foods[18-19].Kang Dacheng et al[15]found that ultrasound leads to protein oxidation and structural changes of beef proteins. However,the effect of ultrasonic thawing on squid oxidation has rarely been reported so far.
In the present study, the effects of ultrasonic-assisted thawing on protein oxidation and structure of jumbo squid mantle were evaluated. The aim is to provide insights into the relationship among protein oxidation, structural modifications and jumbo squid mantle quality.
1 Materials and Methods
1.1 Materials and reagents
Jumbo squid mantle was provided by the Dalian Donglin Food Co. Ltd., and had been stored at -20 ℃ for less than 3 months.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel kit Beijing Solarbio Science & Technology Co. Ltd.; All other chemicals and reagents (analytical grade) were purchased from Nanjing St.Bio Biotechnology Company.
1.2 Instruments and equipments
JHG-Q60-P100 laboratory homogenizer Shanghai Fusion Machinery Equipment Co. Ltd.; Sorvall Stratos centrifuge Germany Thermo Fisher Scientific Inc.;UV-2550 UV-Vis spectrophotometer Japan Shimadzu Company; 970 CRT fluorescence spectrophotometer Shanghai Precision Scientific Instrument Co. Ltd.; 106 high precision food thermometer Germany Testo Group; TA.XT Plus texture analyser UK Stable Micro Systems Company;Benchtop low-filed nuclear magnetic resonance (LF-NMR)analyser Shanghai Niumag Electric Corporation;XO-120L-II ultrasonic assisted refrigerating machine Nanjing Xianou Instrument Manufacturing Co. Ltd.; CR-400 color difference meter Hangzhou Xiangsheng Technology Co. Ltd.; Labram HR800 Evolation high resolution Raman spectrometer France Horiba Jobin Yvon S.A.S.; NanoBrook 90 Plus laser particle size analyzer USA Brookhaven Co. Ltd..
1.3 Methods
1.3.1 Preparation of samples
Each jumbo squid mantle was cut into the same size(10 cm × 8 cm × 3 cm) and weighted about 0.2 kg, then stored at -40 ℃ refrigerator for less than 1 week. The ultrasonic power was chosen according to the unpublished results of a pilot study on jumbo squid mantles conducted in our laboratory, and commercially, the thawing of aquatic products is normally done at about 10 ℃. Jumbo squid mantles were packed with polyethylene film and thawed by three types of methods: 1) refrigerator thawing (4 ℃) considered as control;2) ultrasound-assisted thawing at power of 100, 300 and 500 W in water bath at 5 ℃ (U5-1, U5-3, U5-5, respectively);3) ultrasound-assisted thawing at power of 100, 300 and 500 W in water bath at 10 ℃ (U10-1, U10-3, U10-5,respectively). Ultrasonic frequency was 25 kHz. Samples were considered thawed when the center temperature reached 0 ℃ as measured with a probe, and record the thawing time.
1.3.2 Thawing loss rate determination
The samples were weighed before thawing (m0/g) and then packed in polyethylene film. After thawing, the samples were taken from the bags, and weighed again (m1/g). The thawing loss rate was calculated using the formula (1).

1.3.3 Cooking loss rate determination
Detailed information about the method of cooking loss rate is available from Zhu Wenhui et al[20]. The thawed jumbo squid mantle was sliced into 2 cm × 2 cm × 2 cm pieces and placed in polyethylene bags, and then cooked in water bath at 85 ℃ for 20 min. The cooking loss rate was calculated using the formula (2).

1.3.4 Water distribution determination
Spin-spin relaxation time (T2) were measured by LFNMR. The Carr-Purcell-Meiboom-Gill (CPMG) sequence was used to measure theT2and then fitted the distributed exponential of CPMG decay curves using MultiExp Inv Analysis software. Detailed information about the method is available from Zhu Wenhui et al[20].
1.3.5 Texture determination
The texture properties of thawed jumbo squid mantles were measured using a TA.XT Plus texture analyser.Mantles were cut into the size of 2.5 cm × 2.5 cm × 2.5 cm.Parameters for texture analysis were as follows: cylindrical probe (P/50, 50 mm in diameter) strain was 30%, pre-test speed was 1 mm/s, test speed was 1 mm/s, post-test speed was 1 mm/s, pause time between cycles was 5 s. All samples were measured 6 times.
1.3.6 Colour determination
The surface colour of jumbo squid mantles were measured by a color difference meter. The values were expressed asL*(lightness),a*(“+” measuring the redness;“-” measuring the greenness) andb*(“+” measuring the yellowness; “-” measuring the blueness). The samples were measured 6 times.
1.3.7 Protein oxidation analysis
The jumbo squid of myofibrillar protein (MP) was extracted according to our previous methods[20]. Protein oxidation index of carbonyl, total sulfhydryl (SH), surface hydrophobicity and dityrosine content were also determined by our previous methods[20].
1.3.7.1 Determination of carbonyl content
Carbonyl groups were reacted with 2,4-dinitrophenylhydrazine (DNPH) to form protein hydrazones. MP samples were treated with 10 mmol/L DNPH in 2 mol/L HCl for 1 h at room temperature. After incubation,MP solution was precipitated with 20% trichloroacetic acid. The precipitate was washed 3 times with ethanol:ethyl acetate mixture (1:1,V/V), then dissolved in 6 mol/L guanidine hydrochloride for 30 min at 37 ℃. Absorbance was measured at 370 nm for the DNPH-treated sample.The amount of carbonyl was calculated using an absorption coefficient 22 000 L/(mol·cm).
1.3.7.2 Determination of total sulfhydryl content
Total SH contents were determined using 5,5’-dithiobis(2-nitrobenzoic acid). The absorbance at 412 nm was recorded on a UV-Vis spectrophotometer, and the SH content was calculated using the extinction coefficient of 13 600 L/(mol·cm).
1.3.7.3 Determination of surface hydrophobicity
The surface hydrophobicity of MP was measured using 8-anilino-1-naphthalene sulfonate (ANS) fluorescence probe by a fluorescence spectrophotometer. MP samples were diluted in 20 mmol/L phosphate buffer (containing 0.6 mol/L KCl, pH 7.0), protein concentration gradient was 0.8-0.2 mg/mL. Surface hydrophobicity of sample was calculated from the slope of relative fluorescence to protein concentration/ (mg/mL) by linear regression analysis.
1.3.7.4 Determination of dityrosine content
Dityrosine content was measured by fluorescence spectrophotometer. MP sample was diluted in 20 mmol/L phosphate buffer (containing 0.6 mol/L KCl, pH 6.0) to 1 mg/mL and then was filtered. Results were expressed in arbitrary units (A.U.).
1.3.8 MP structure determination
1.3.8.1 SDS-PAGE
Detailed information about the method is available from Zhu Wenhui et al[20]. The gel was scanned and analyzed by a gel image scanning system with a Quantity One software.
1.3.8.2 Particle size determination
The protein particle size was determined by a NanoBrook 90 Plus laser particle size analyzer according to the methods of Liu Chengmei et al[21]. The concentrated MP solution was diluted to approximately 0.5 mg/mL with extraction buffer (0.01 mol/L Tris-HCl, pH 7.2, containing 0.6 mol/L NaCl). A scattering angle of 90° was used for the dynamic light scattering measurements of particle size, and all measurements were repeated 5 times.
1.3.8.3 Secondary structure determination
The proportions of protein secondary structure were measured with a high resolution Raman spectrometer[22-23].About 1 g sample was placed on glass slide under the Raman microscope. Amide I was analyzed using Labspec 6.0 software.
1.4 Statistical analysis
All data were presented as mean values ± standard deviations, which were analyzed using the one-way analysis of variance (ANOVA) with Duncan’s multiple range test of the SPSS software. Statistical significance was defined asP<0.05.All figures were drawn using Origin Pro 8.5 software.
2 Results and Analyses
2.1 Effect of ultrasonic-assisted thawing on thawing time,thawing loss rate and cooking loss rate of jumbo squid mantle
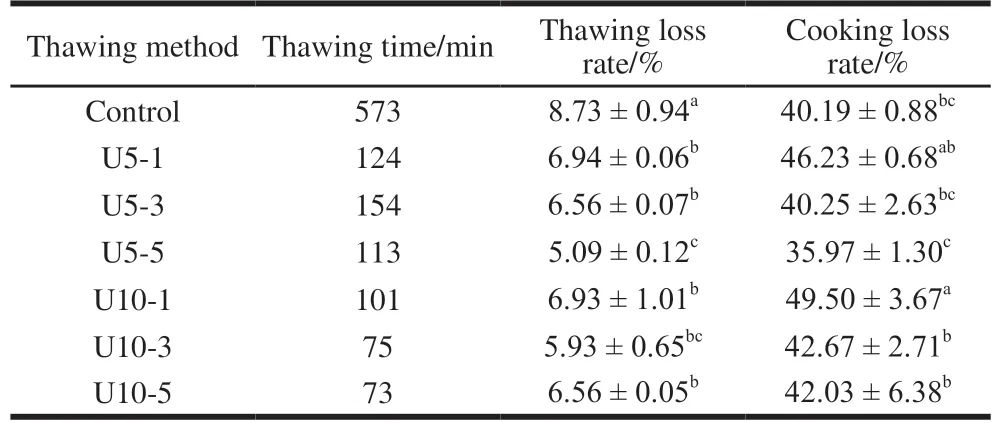
Table 1 Effect of ultrasonic-assisted thawing on the thawing time,thawing loss rate, cooking loss rate of jumbo squid mantle
The results of ultrasonic-assisted thawing on the thawing time, thawing loss rate and cooking loss rate of jumbo squid mantle were presented in Table 1. The thawing time varied greatly according to the different thawing methods.Among the ultrasonic-assisted thawing methods, U10-5 had the shortest thawing time. In general, the thawing time of refrigerated thawing was between 3.72-7.85 times longer than ultrasonic-assisted thawing. Therefore, ultrasonic-assisted thawing significantly increased the thawing rate.
The U5-5 had the least thawing loss rate (5.09%) and cooking loss rate (35.97%), while refrigerated thawing had the highest thawing loss rate (8.73%) and U10-1 had the highest cooking loss rate (49.50%). The drip loss of muscle leads to poor consumer satisfaction, due to the loss of tasteful constituents, such as some amino acids and nucleotides[2].The cooking yield is related to the amount of water loss or the decrease in the weight of muscle during cooking as a result of protein denaturation[24]. With the ultrasonic power increasing,ultrasonic-assisted thawing can significantly reduce thawing loss rate at the same temperature, and selecting proper ultrasonic temperature and power can also reduce cooking loss rate compared to refrigerator thawing.
2.2 Effect of ultrasonic-assisted thawing on water distribution and composition of jumbo squid mantle

Fig. 1 Transverse relaxation time (T2) spectra of water in jumbo squid
TheT2relaxation time distribution was shown in Fig. 1.Four kinds of water distributions were evident in the jumbo squid mantle. The two small peaks between 0-35 ms(T21) represent combined water. The second peak, which occurred between 40-100 ms (T22), probably corresponds to immobilized water. The third peak, which appeared between 100-1 000 ms (T23), represents free water.
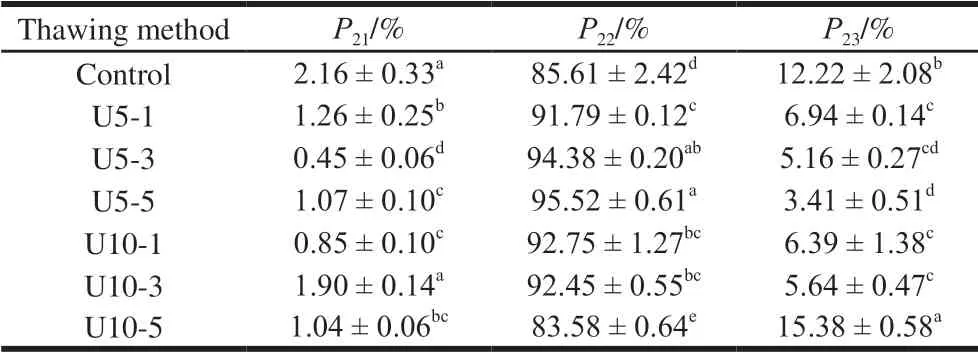
Table 2 Water distribution and composition of jumbo squid mantle
P21,P22, andP23, respectively were calculated by the percentage of each peak respectively. As shown in Table 2,there were significant differences in the three states of water content among jumbo squid mantle upon different thawing treatments (P<0.05). When the temperature is 5 ℃, with the ultrasonic power increasing, theP22increased andP23decreased drastically. This indicated that high power ultrasonic-assisted thawing may reduce water loss. Overall,the results of LF-NMR were basically consistent with the results of thawing loss rate and cooking loss rate.
2.3 Effect of ultrasonic-assisted thawing on texture properties of jumbo squid mantle
The texture profile of food is an important characteristic because it contributes to the satisfaction of consumers and determines the marketability of the final products[25]. Poor texture will reduce the satisfaction of consumers and can cause economic losses[26]. The changes in texture indicators(hardness, springiness, cohesiveness, chewiness and resilience) of the jumbo squid mantle upon ultrasonic-assisted thawing were presented in Table 3. There were significant differences in hardness, cohesiveness, chewiness, and resilience of jumbo squid among different ultrasonic-assisted thawing treatments (P< 0.05), while there was no significant difference in springiness (P> 0.05).Lee et al[27]reported that frozen meat had a different texture when thawed at varying temperatures. Ultrasonic thawing(except for U10-1) caused a different degree of decrease of hardness and chewiness compared with refrigerated thawing.Jayasooriya et al[28]also reported that ultrasound treatment(24 kHz, 12 W/cm2) significantly reduced the hardness of bovinesemitendinosusandlongissimusmuscles, while Chang Haijun et al[29]found that the overall meat tenderness was increased during ultrasound treatment (40 kHz, 1 500 W),resulting in a more tender meat. However, in another study,a frozen porklongissimus dorsimuscle was subjected to ultrasonic thawing, which resulted in no significant difference in hardness compared to control[12]. This may be related to the sample characteristics, sizes and the ultrasonic parameters. The tendency of changes in chewiness was consistent with elasticity,and similar results were reported by LiXiuxia et al[30].

Table 3 Effect of ultrasonic-assisted thawing on texture properties of jumbo squid mantle
Texture is an important quality parameter in the acceptability of cephalopods, and may vary depending on species, muscle area, storage, and processing[31-32]. During thawing, differences in the texture parameters of muscles are related to changes in the physicochemical properties of proteins and freshness of muscle. Improper thawing increases the degree of protein oxidation, which affects the texture characteristics. Xia Xiufang et al[2]reported that MP oxidation during the thawing process may have a significant effect on meat.
2.4 Effect of ultrasonic-assisted thawing on colour of jumbo squid mantle
Colour determines the appearance and attractiveness of frozen jumbo squid mantle. Colour of frozen jumbo squid mantle changed due to different thawing procedures,as shown in Table 4. Theb*values of U5-5 and U10-5 were significantly different compared with other groups(P< 0.05). TheL*value decreased anda*value increased with the increasing of ultrasonic power both at 5 ℃ and at 10 ℃.Colour is one of the most obvious quality changes caused by thawing[33]. Improper thawing methods can lead to undesirable colour which would reduce the likelihood of consumers purchasing the product, and thus result in industry losses[34].

Table 4 Effect of ultrasonic-assisted thawing on the color of jumbo squid mantle
2.5 Effect of ultrasonic-assisted thawing on protein oxidation index of jumbo squid mantle
2.5.1 Carbonyl content

Fig. 2 Effect of ultrasonic-assisted thawing on the carbonyl content of jumbo squid myofibrillar protein
Fig. 2 showed that there were significant differences in carbonyl content of MP due to different thawing methods,and the carbonyl content of U10-1, U5-3 and U5-5 were significantly lower than that of the control group (refrigerator thawing). The U5-5 group exhibited the least carbonyl content, and there was no significant difference in carbonyl content of MP between U5-5 and U5-3. This suggested that U5-3 and U5-5 ultrasonic-assisted thawing were both appropriate methods to reduce the carbonyl content of MP.
2.5.2 Total SH content
SH content is an important indicator of protein oxidation degree[35]. Fig. 3 indicated that different thawing methods had different effects on the total SH group content of MP.U5-3 had the highest total SH content, while the U10-5 had the lowest total SH content. The total SH content decreased with an increase of ultrasonic power at 10 ℃. On the whole,the total SH content upon ultrasonic-assisted thawing was lower than upon refrigerator thawing. Jin Jian et al[36]reported that sequential dual frequency ultrasound pretreatment of corn protein decreased the SH content significantly, while the sweeping frequency and pulsed ultrasound pretreatment did not change the SH content compared with traditional enzymolysis. Zhou Cunshen et al[37]likewise reported that the SH content of wheat germ protein changed with ultrasound frequency as well as power and duration.

Fig. 3 Effect of ultrasonic-assisted thawing on total SH content ofjumbo squid myofibrillar protein
2.5.3 Surface hydrophobicity

Fig. 4 Effect of ultrasonic-assisted thawing on the surface hydrophobicity of jumbo squid myofibrillar protein
Surface hydrophobicity is a structural characteristic,which reflects the amount of hydrophobic groups exposed on the surface of protein molecules[36]and which is useful for evaluating the conformational change in proteins. Fig. 4 showed the changes of surface hydrophobicity of MP in different thawing procedures. Different thawing methods had significantly different effects on the surface hydrophobicity(P< 0.05). The surface hydrophobicity of jumbo squid upon ultrasonic-assisted thawing gradually increased with increasing power and temperature, and were significantly higher than that of refrigerator thawing, except in the case of U5-1. The increase of surface hydrophobicity indicated that hydrophobic groups were exposed to the surface of protein molecule upon ultrasonic-assisted thawing. JinJian et al[36]reported that the increase of surface hydrophobicity was due to the cavitation phenomenon induced by ultrasound.
2.5.4 Dityrosine contents

Fig. 5 Effect of ultrasonic-assisted thawing on the dityrosine content of jumbo squid myofibrillar protein
Fig. 5 showed the effect of different thawing methods on the content of dityrosine. Refrigerated thawing caused the highest dityrosine content, while U5-3 caused the least dityrosine content. The content of dimeric tyrosine due to ultrasonic thawing was significantly lower than that of refrigerator thawing (P< 0.05). The experimental results showed that ultrasonic-assisted thawing led to a reduction in the degree of protein oxidation compared with refrigerator thawing. The degree of oxidation reduction by ultrasonic thawing has a nonlinear relationship with power, and a positive correlation with temperature.
2.6 Effect of ultrasonic-assisted thawing on MP structure aggregation and degradation of jumbo squid mantle
2.6.1 SDS-PAGE analysis
In order to observe the effect of ultrasonic-assisted thawing on squid proteins, the muscle protein of jumbo squid was analyzed by SDS-PAGE, and the results were shown in Fig. 6. It can be seen from Fig. 6A that the myosin heavy chain (MHC) of the samples thawed by U5-1, U5-3 and U5-5 had different degrees of protein reduction compared with refrigerator thawing. U10-1, U10-3 and U10-5, and the electrophoretic bands were found at the top of the separation gel. This may due to the cross-linking of proteins, leading to the formation of aggregates of high molecular mass,which accumulate at the interface of the separating gel and concentrating gel, and cannot enter the separating gels[38].Notably, there was no detectable change of actin in the electrophoresis spectrums. We also found that the MHC band increased but not fully recovered and several faint bands of aggregates of high molecular weight (above 200 kDa)were still present at the top of the gel, even in the presence ofβ-mercaptoethanol (Fig. 6B). It thus appears that the high molecular mass aggregates were not only cross-linked by disulfide linkages but also by non-disulfide linkages. In addition, new bands appeared between 29-44.3 kDa and several bands disappeared near 66.4 kDa, which indicated that there was not only cross-linking but also protein degradation during the thawing process. In agreement with our results, Zhang Mingcheng et al[39]observed the simultaneous aggregation and degradation of proteins during freeze-thaw cycles.

Fig. 6 SDS-PAGE analysis of myofibrillar proteins from jumbo squid
2.6.2 Particle size
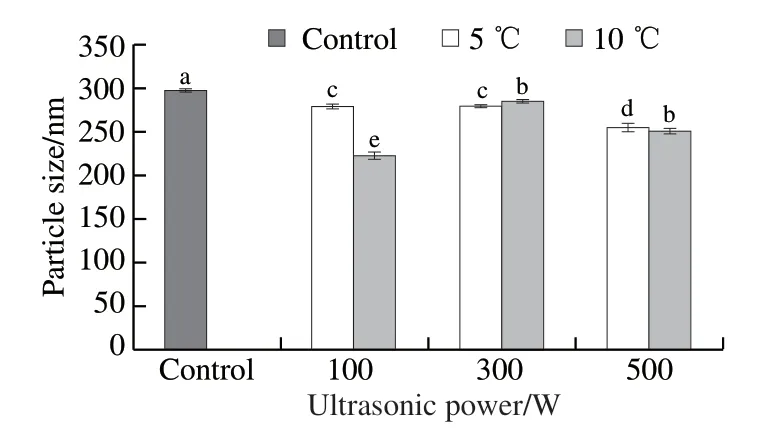
Fig. 7 Effect of ultrasonic-assisted thawing on the mean particle size of jumbo squid myofibrillar protein
The mean particle size under different thawing methods was shown in Fig. 7. There were significant differences in mean particle size of MP with different thawing treatments,and the mean particle size in ultrasonic thawing was significantly lower than in the control group. This indicated that ultrasonic thawing reduced the particle size of MP.Zhang Qiuting et al[40]reported that the mean particle size of peanut protein isolates decreased significantly with ultrasound treatment. Additionally, the increase in foaming properties of soy protein isolate was found to be related to the reduction of particle size with high ultrasound treatment[41].
2.6.3 Protein secondary structure

Fig. 8 Raman spectra of jumbo squid myofibrillar protein
Raman scattering spectroscopy which is mainly indicative of changes in the secondary structure and variations in local environments is a valuable tool to get insight into the conformational change of proteins[42]. Raman spectra of MP from jumbo squid upon ultrasonic-assisted thawing in the 800-1 800 cm-1region were shown in Fig. 8. The amide I bands in the 1 650-1 658, 1 660-1 665, 1 665-1 680 and 1 680-1 700 cm-1representα-helices, random coils and disorder structure forβ-sheets andβ-turns, respectively[43].
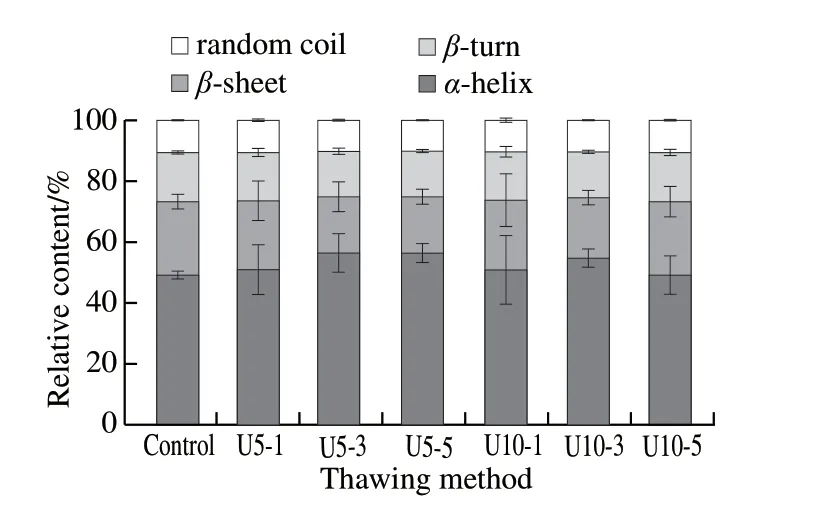
Fig. 9 Changes in secondary structures of jumbo squid myofibrillar protein
The percentage of secondary structure can be calculated according to Alix’s formula[32]. As shown in Fig. 9, different thawing methods had different effects on the overall relative content of secondary structure. Treatments U5-3 and U5-5 resulted in the highestα-helical relative content, while refrigerated thawing and U10-5 resulted in the leastα-helical relative content. Protein denaturation results in the disorder and increased conformational change of myofibrillar protein during thawing. The decrease inα-helices relative content was associated with an increase in the relative content ofβ-sheets, and the relative content inβ-turns and random coil or in disordered structure also increased to varying degrees.This may explained by a gradual transformation ofα-helices intoβ-sheets,β-turns and random coil structure. Compared with refrigerated thawing, ultrasonic assisted thawing slowed the conversion ofα-helices toβ-sheets,β-turns and random coils, with the effect being most obvious for U5-3 and U5-5.This indicates that ultrasound can inhibit protein denaturation.
3 Conclusion
The present study examined the effects of different ultrasonic-assisted thawing conditions on the protein oxidation and structure of jumbo squid mantle. Ultrasonic-assisted thawing significantly increased the thawing rate compared with refrigerated thawing. U5-5 had the least detrimental impact on the squid mantle quality compared with the other thawing methods. Compared to refrigerator thawing, ultrasonicassisted thawing reduced the denaturation and oxidation of proteins. The results demonstrate that ultrasonic-assisted thawing at 5 ℃ could improve thawing speed and maintain the quality of frozen jumbo squid.

