川鄂金丝桃的化学成分研究
邓憬童,郝 吉,马远任,周童曦,黄慧琪,王 强,杨新洲**,庞克坚
(1. 中南民族大学 药学院,湖北 武汉 430074;2. 和田维吾尔药业股份有限公司,新疆 和田 848200)
金丝桃属(Hypericum)归属于藤黄科植物,世界约有400余种,中国有55种8亚种,分布较广泛,各地均有,特别是贵州、陕西、甘肃等地区. 金丝桃属植物由于其化学成分结构新颖和药理活性丰富,近年来备受关注[1]. 目前,关于金丝桃属植物的研究发现其含有多种化学成分,已经发现的有黄酮类、甾体类、萜类,尤其是近年来从金丝桃属植物中发现的代表性间苯三酚衍生物类化合物,如李祖强等[2]在尖萼金丝桃中发现了间苯三酚及黄酮类化合物. 间苯三酚结构新颖且具有有较好的生物活性,如Tian等[3]在连柱金丝桃(H.cohaerens)中分离得到一个具有二环[5.3.1]十一烷新颖骨架的间苯三酚hypercohin A和特殊三元碳环结构的新颖骨架化合物hypercohin K以及一系列桥环间苯三酚. 活性研究表明,hypercohin A和hypercohin K都表现出一定的乙酰胆碱酯酶抑制活性和细胞毒活性.
川鄂金丝桃(HypericumwilsoniiN. Robson)作为藤黄科金丝桃属植物,主要产地为四川东部及南部、湖北西部,生长于海拔1 000~1 750 m的山坡灌丛、林下或草地上,其果实可入药,具有药用价值,用于治疗目赤[4]. 鉴于从金丝桃属其他植物中分离得到有价值的化合物,而目前关于川鄂金丝桃的化学成分未见报道,本课题组认为川鄂金丝桃有较大的研究价值和前景. 综合利用各种分离提取技术,对采自重庆市的川鄂金丝桃中的化学成分进行提取分离纯化,从川鄂金丝桃的石油醚提取部位分离得到了15种化合物,且化合物1~15首次从该植物中分离得到,包括黄酮类、甾体类、三萜类、饱和脂肪酸酯类、氧杂蒽酮类、多环多异戊烯基取代间苯三酚衍生物等.
1 实验材料与方法
1.1 仪器和材料、试剂Bruker DRX-600 MHz核磁共振仪(德国Bruker公司),Finnigan MAT-95型质谱仪,Q-TOF Micro LC-MS-MS质谱仪,Waters制备型高效液相色谱仪(美国Waters公司),Waters 2998 DAD检测器(美国Waters公司),Waters 2707自动进样器(美国Waters公司),COSMOSIL C18(250 mm×φ10 mm,5 μm)半制备柱(日本COSMOSIL公司),COSMOSIL 5PFP (250 mm×φ10 mm,5 μm)半制备柱(日本COSMOSIL公司),HP20 大孔树脂(日本三菱公司),GF254200-300,0.038 5~0.054 mm薄层硅胶板(烟台江友硅胶开发有限公司),Sephadex LH-20葡聚糖凝胶(美国Amersham公司),色谱级甲醇及色谱级乙腈(美国TEDIA试剂公司). 其余试剂均为市售分析纯.
1.2 样品来源川鄂金丝桃于2018年8月采集于重庆市,经中南民族大学万定荣教授鉴定为藤黄科金丝桃属川鄂金丝桃(Hypericum wilsoniiN. Robson).现存放于湖北省武汉市中南民族大学药学院植物标本库(No.SC0758).
1.3 提取与分离取川鄂金丝桃干燥全草21 kg,粉碎,用80%的乙醇室温浸提(4×20 L, 3 d/次),合并多次提取液,减压浓缩至无醇味即得浸膏1 947 g. 将浸膏加5倍体积热水分散后,依次用石油醚、乙酸乙酯、正丁醇萃取得到石油醚部位(HWPE,452 g)、乙酸乙酯部位674 g、正丁醇部位712 g.取石油醚粗提物(430 g)用大孔树脂柱层析进行粗分,以水-乙醇梯度洗脱(体积分数20%→30%→40%→50%→60%→65%→70%→75%→80%→82%→85%→90%→95%),薄层色谱板检测合并得到9个组分Fr. A~Fr. I,其中组分Fr. H中出现结晶,过滤得到单体化合物3(68.9 mg);组分Fr. B用凝胶柱层析粗分,以甲醇(加入0.1%甲酸)洗脱,合并得12个组分(Fr.Ba~Fr.Bl),Fr.Bf组分结晶析出化合物7(4.6 mg),经高效液相色谱(乙腈-水,体积比30∶70→39∶61,22 min,流动相含有0.1%的甲酸)分离得到化合物8(5.2 mg);Fr.Bg经高效液相色谱(乙腈-水,体积比36∶64→49∶51,8 min; 49∶51→55∶45,14 min,流动相含有0.1%的甲酸)分离得到化合物1(55.7 mg)、2(7.0 mg). Fr. G(115 g)经过正相硅胶柱层析初步分离,以石油醚-乙酸乙酯梯度洗脱(1∶0→0∶1),经TLC检测合并分为Fr.Ga~Fr.Gj共10个组分,其中Fr.Gg中有结晶析出,过滤得化合物6(57.4 mg),Fr.Gi中有结晶析出,过滤得化合物4(1.887 1 g). Fr.Ge经正相硅胶柱层析(石油醚-二氯甲烷体积比1∶1)等度洗脱,得8个组分(Fr.Ge1~Fr.Ge8). Fr.Ge3经高效液相色谱(乙腈-水,体积比84∶16→95∶5, 40 min; 95∶5→100∶0,10 min;流动相含有0.1%的甲酸)进行粗分,得到11个组分,Fr.Ge3~11经高效液相色谱细分得到化合物10(4.2 mg)、12(8.9 mg)、13(2.6 mg). Fr.Ge7经高效液相色谱(乙腈-水,体积比90∶10,等度洗脱,流动相含有0.1%的甲酸)粗分为9个组分,其中Fr.Ge7-2为化合物5(77.9 mg),Fr.Ge7-4为化合物11(133.7 mg),Fr.Ge7-6为化合物9(75.8 mg).Fr.Ge7-1经高效液相色谱分离得到化合物15(5.1 mg),Fr.Ge7-3经高效液相色谱分离得到化合物14(2.2 mg).
2 结果与讨论
2.1 实验结果综合应用大孔树脂柱层析、正相硅胶柱层析、葡聚糖凝胶色谱、高效液相色谱等多种分离方法从川鄂金丝桃石油醚部位(HW-PE)中分离得到15个化合物. 分别为folecitin(1)、表儿茶素(2)、β-谷甾醇(3)、β-胡萝卜素(4)、白桦脂酮酸(5), 单棕榈酸甘油(6)、1,7-二甲氧基-3,6-二羟基氧杂蒽酮(7)、3,4-二羟基-2-甲氧基氧杂蒽酮(8)、27-epifurohyperforin isomer 1(9)、uralione H(10)、attenuatumione E(11)、sampsonione K(12)、attenuatumione C(13)、hypersampsonone C(14)和otogirinin B(15).按结构分为黄酮类、甾体类、三萜类、饱和脂肪酸酯类、氧杂蒽酮类、多环多异戊烯基取代间苯三酚衍生物. 见图1.
2.2 化合物的波谱数据化合物1:C21H20O11,黄色粉末;ESI-MS(m/z):449[M+H]+.1H NMR (600 MHz,CD3OD)δH: 7.34 (1H, d,J=1.9 Hz, H-2′), 7.31 (1H,dd,J=8.3, 1.9 Hz, H-6′), 6.91 (1H, d,J=8.3 Hz, H-5′), 6.37 (1H, d,J=2.0 Hz, H-8), 6.20 (1H, d,J=2.0 Hz, H-6), 5.35 (1H, br s, H-1″), 4.22 (1H, m, H-2″),3.75 (1H, dd,J=3.2, 9.4 Hz, H-4″), 3.42 (1H, m, H-5″), 3.33 (1H, m, H-3″),0.94 (3H, d,J=6.1 Hz, H-6″).13C NMR (150 MHz, CD3OD)δC: 179.6 (C-4),165.9 (C-7), 163.2 (C-5), 159.3 (C-9), 158.5 (C-2),149.8 (C-4′), 146.4 (C-3′), 136.2 (C-3), 122.9 (C-1′), 122.9 (C-6′), 116.9 (C-2′), 116.4 (C-5′), 105.9(C-10), 103.5 (C-1″), 99.8 (C-6), 94.7 (C-8), 73.2(C-3″), 72.1 (C-5″), 72.0 (C-4″), 71.9 (C-2″), 17.7(C-6″). 其波谱数据与文献报道的化合物一致[5],故鉴定化合物为folecitin.
化 合 物2:C15H14O6,白 色 粉 末;ESI-MS(m/z):289[M-H]-.1H NMR (600 MHz, CD3OD)δH: 6.97(1H, d,J=1.6 Hz, 3-OH), 10.48 (1H, s, 6-OH), 7.38(1H, s, H-8), 6.80 (1H, s, H-5), 6.38 (1H, br s, H-4),6.33 (1H, br s, H-2), 3.84(3H, s, 7-OCH3), 3.81(3H, s, 1-OCH3).13C NMR (150 MHz, CD3OD)δC: 172.6(C-9), 162.9 (C-3), 161.7 (C-1), 159.0 (C-4a), 152.9 (C-10a), 150.2 (C-6), 145.6 (C-7), 114.4 (C-8a), 105.8 (C-8), 104.9 (C-9a), 102.3 (C-5), 95.5 (C-2), 94.9 (C-4),55.9 (1-OCH3), 55.8 (7-OCH3). 其波谱数据与文献报道的化合物一致[6],故鉴定化合物为表儿茶素.
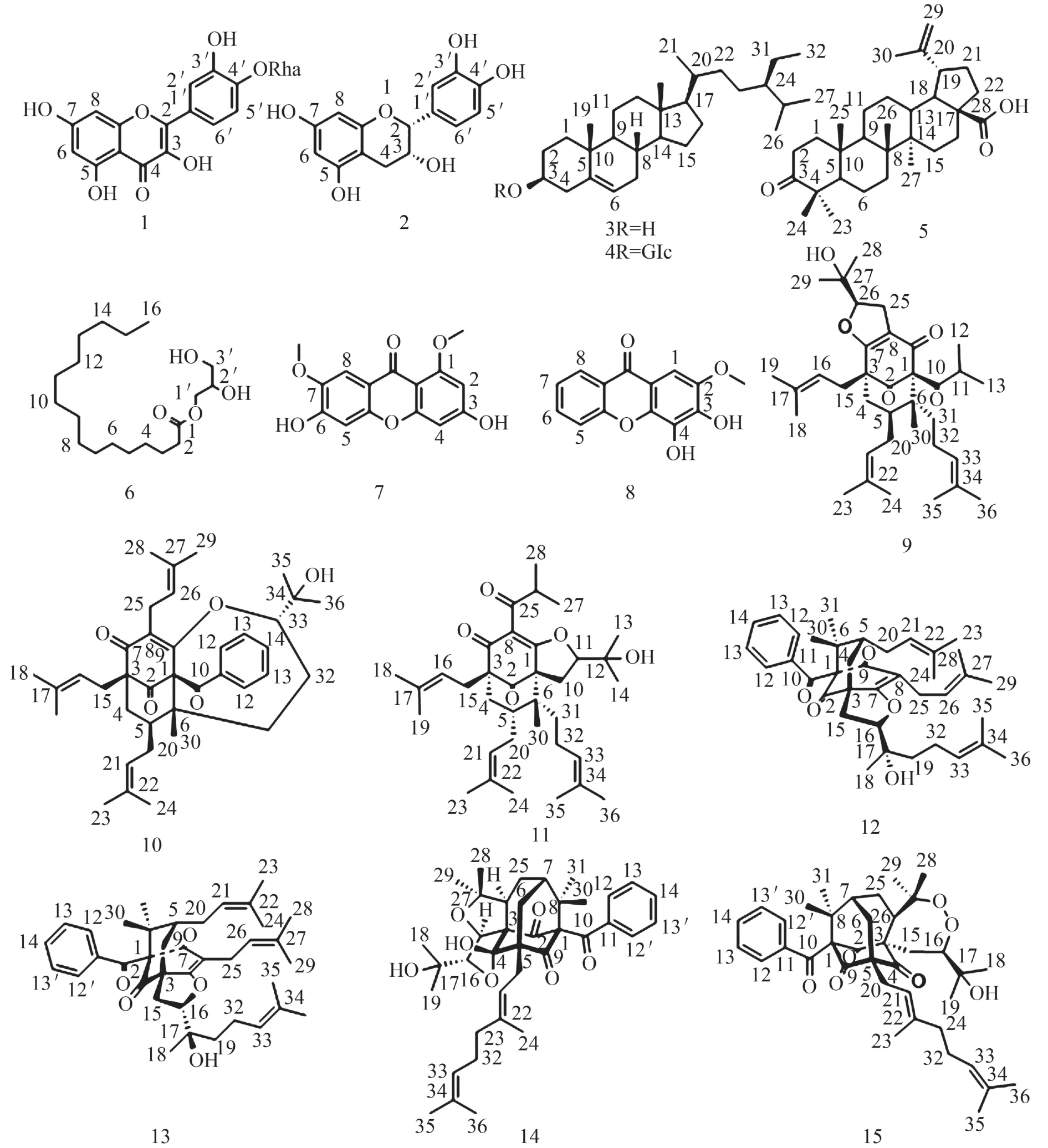
图1 化合物1~15结构Fig. 1 Chemical structures of compounds 1~15
化合物3:C29H50O,白色粉末;EI-MS(m/z):397[M+H]+.1H NMR (600 MHz, acetone-d6)δH: 5.31(1H, br d,J=5.1 Hz, H-6), 3.39 (1H, m, H-3), 2.21(2H, m, H-4), 2.05 (1H, m, H-12a), 1.97 (1H, m, H-7a), 1.88 (1H, m, H-1a), 1.84 (1H, m, H-2a),1.76 (1H,m, H-16a), 1.69 (1H, m, H-26),1.44~1.63 (6H, m, H-2b, H-7b, H-8, H-11, H-15a), 1.20~1.44 (5H, m, H-16b, H-20, H-22a, H-23a, H-25a), 1.03~1.20 (6H, m,H-1b, H-12b, H-15b, H-17, H-23b, H-25b), 1.01 (3H,s, H-19), 0.91~0.99 (5H, m, H-14, 22b, 21),0.79~0.88 (11H, m, H-9, 24, 24′, 28, 27), 0.72 (3H,s, H-18).13C NMR (150 MHz, acetone-d6)δC: 142.4(C-5), 121.6 (C-6), 71.7 (C-3), 57.7 (C-14), 56.9 (C-17), 51.2 (C-9), 46.7 (C-24), 43.4 (C-13), 43.1 (C-4), 40.7 (C-12), 38.3 (C-1), 37.3 (C-10), 37.0 (C-20), 34.7 (C-22), 32.8 (C-7), 32.7 (C-8), 32.6 (C-2),29.9 (C-26), 29.0 (C-16), 26.7 (C-25), 25.0 (C-15),23.8 (C-23), 21.8 (C-11), 20.2 (C-19), 19.9 (C-28),19.4 (C-27), 19.3 (C-21), 12.3 (C-24′), 12.3 (C-18).其波谱数据与文献报道的化合物一致[7],故鉴定化合物为β-谷甾醇.
化合物4:C35H60O6,黄色油状;ESI-MS(m/z):575[M-H]-.1H NMR (600 MHz, DMSO-d6)δH: 5.32(1H, t,J=4.9 Hz, H-6), 4.21 (1H, d,J=7.8 Hz, H-1′),0.95 (3H, s,H-19), 0.90 (3H, d,J=6.4 Hz, H-21), 0.82(3H, d,J=6.8 Hz, H-27), 0.81 (3H, d,J=6.4 Hz, H-26),0.79 (3H, d,J=6.8 Hz, H-29), 0.65 (3H, s, H-18).13C NMR (150 MHz, DMSO-d6)δC: 140.5 (C-5), 121.3(C-6), 100.8 (C-1′), 76.9 (C-5′), 76.8 (C-3′), 76.8 (C-3), 73.5 (C-2′), 70.1 (C-4′), 61.1 (C-6′), 56.2 (C-14),55.5 (C-17), 49.6 (C-9), 45.2 (C-24), 41.9 (C-13),39.1 (C-12), 38.3 (C-4), 36.9 (C-1), 36.3 (C-10),35.5 (C-20), 33.4 (C-22), 31.5 (C-9), 31.5 (C-8),29.3 (C-2), 28.7 (C-25), 27.9 (C-16), 25.4 (C-23),23.9 (C-15), 22.6 (C-28),20.6 (C-11), 19.8 (C-26),19.2 (C-27), 19.0 (C-19), 18.7 (C-21),11.8 (C-29),11.7 (C-18). 其波谱数据与文献报道一致[8],故鉴定化合物为β-胡萝卜苷.
化合物5:C30H46O3,白色粉末;ESI-MS(m/z):453[M-H]-.1H NMR (600 MHz, CDCl3)δH: 4.73 (1H,s, H-29β), 4.61 (1H, s, H-29α), 3.00 (1H, m,H-19),2.48 (1H, m, H-2β), 2.40 (1H, m, H-2α), 2.28 (1H, m,H-16β), 2.21 (1H, m, H-13), 1.99 (2H, m, H-15β, 22β),1.89 (1H, m, H-1β), 1.71 (1H, m, H-12β), 1.68 (3H, s,H-30), 1.62 (1H, m, H-18), 1.54 (1H, m, H-21β), 1.52(2H, m, H-6), 1.47 (1H, m, H-22α), 1.44 (4H, m, H-7,11β, 16α), 1.42 (1H, m, H-15α), 1.38 (2H, m, H-1α, 9),1.35 (1H, m, H-5), 1.34 (1H, m, H-11α), 1.22 (1H, m,H-21α), 1.06 (3H, s, H-23), 1.05 (1H, m, H-12α), 1.00(3H, s, H-24), 0.98 (3H, s, H-27), 0.96 (3H, s, H-26),0.92 (3H, s, H-25).13C NMR (150 MHz, CDCl3)δC:218.6 (C-3), 182.4 (C-28), 150.5 (C-20), 109.9 (C-29),56.5 (C-17), 55.0 (C-5), 49.9 (C-9), 49.3 (C-18), 47.5(C-4), 47.0 (C-19), 42.6 (C-14), 40.7 (C-8), 39.7 (C-1),38.6 (C-13), 37.2 (C-22), 37.0 (C-10), 34.3 (C-2), 33.7(C-7), 32.2 (C-16), 30.6 (C-15), 29.8 (C-21), 26.7 (C-23), 25.6 (C-12), 21.5 (C-11), 21.1 (C-24), 19.7 (C-6),19.5 (C-30),16.1 (C-25), 15.9 (C-26), 14.7 (C-27). 其波谱数据与文献报道一致[9],故鉴定化合物为白桦脂酮酸.
化合物6:C19H38O4,白色粉末;ESI-MS(m/z):331[M+H]+.1H NMR (600 MHz, CDCl3)δH: 4.21 (1H,dd,J=11.7, 4.5 Hz, H-1α), 4.15 (1H, dd,J=11.7, 6.2 Hz,H-1β), 3.93 (1H, m, H-2), 3.70 (1H, dd,J=11.5, 4.0 Hz,H-3α), 3.60 (1H, dd,J=11.5, 5.8 Hz, H-3β), 2.35 (2H,t,J=7.6 Hz, H-2′), 0.88 (3H, t,J=7.0 Hz, H-16′).13C NMR (150 MHz, CDCl3)δC: 174.6 (C-1′), 70.4 (C-2), 65.3 (C-1), 63.4 (C-3), 34.3 (C-2′), 32.1 (C-3′),29.9 (C-5′), 29.8 (C-6′), 29.8 (C-7′), 29.8 (C-8′),29.8 (C-9′), 29.6 (C-10′), 29.5 (C-11′), 29.4 (C-12′),29.3 (C-13′), 25.1 (C-14′),22.8(C-15′), 14.3 (C-16′).其波谱数据与文献报道一致[10],故鉴定化合物为单棕榈酸甘油.
化合物7:C15H12O6,黄色粉末;ESI-MS(m/z):287[M-H]-.1H NMR (600 MHz, DMSO-d6)δH: 10.74(1H, s, 3-OH), 10.48 (1H, s, 6-OH), 7.38 (1H, s, H-8),6.80 (1H, s, H-5), 6.38 (1H, br s, H-4), 6.33 (1H, br s,H-2), 3.84(3H, s, 7-OCH3), 3.81 (3H, s,1-OCH3).13C NMR (150 MHz, DMSO-d6)δC: 172.6 (C-9), 162.9 (C-3), 161.7 (C-1), 159.0 (C-4a), 152.9 (C-10a), 150.2 (C-6), 145.6 (C-7), 114.4 (C-8a), 105.8 (C-8), 104.9(C-9a), 102.3 (C-5), 95.5 (C-2), 94.9 (C-4), 55.9 (1-OCH3), 55.8 (7-OCH3). 其波谱数据与文献报道一致[11],故鉴定化合物为1,7-二甲氧基-3,6-二羟基氧杂蒽酮.
化合物8:C14H10O5,黄色粉末;ESI-MS(m/z):257[M-H]-.1H NMR (600 MHz, acetone-d6)δH: 8.24(1H, dd,J=8.0, 1.6 Hz, H-8), 7.79 (1H, dt,J=8.6, 1.8 Hz, H-6), 7.59 (1H, dd,J=8.6, 1.0 Hz, H-5), 7.43 (1H,dt,J=8.0, 1.0 Hz, H-7), 7.25 (1H, s, H-1), 3.96 (3H, s,2-OCH3).13C NMR (150 MHz, acetone-d6)δC: 176.0(C-9), 156.8 (C-5a), 146.6 (C-2), 143.0 (C-3), 141.7 (C-4), 135.0 (C-6), 134.1 (C-4a), 126.9 (C-8), 124.5 (C-7), 122.2(C-8a), 118.8 (C-5), 114.7 (C-1a), 97.0 (C-1),56.5 (2-OCH3). 其波谱数据与文献报道一致[12],故鉴定化合物为3,4-二羟基-2-甲氧基氧杂蒽酮.
化合物9:C35H52O5,无 色 油 状;[α]D20+16.5(c1.0, CHCl3);ESI-MS(m/z):551[M-H]-.1H NMR(600 MHz, CDCl3)δH: 5.03 (1H, m, H-17), 4.98 (1H,m, H-22), 4.94 (1H, m, H-32), 4.80 (1H, dd,J=10.0,8.4 Hz, H-27), 2.98 (1H, dd,J=13.6, 10.0 Hz, H-26α),2.94 (1H, dd,J=13.6, 8.4 Hz, H-26β), 2.51 (1H, m, H-31α), 2.45 (1H, m, H-31β), 2.12 (1H, m, H-21α), 2.09(1H, m, H-11), 2.04 (1H, m, H-16α), 1.91 (1H, dd,J=13.6, 4.3 Hz, H-5α), 1.87 (1H, m, H-15α), 1.85 (2H,m, 16β), 1.77 (1H, m, H-21β), 1.70 (3H, s, H-24), 1.69(6H, s, H-34, 35), 1.64 (1H, m, H-4), 1.63 (3H, s, H-19), 1.58 (3H, s, H-20), 1.56 (3H, s, H-25), 1.52 (1H,m, H-15β), 1.42 (1H, m, H-5β), 1.29 (3H, s, H-30),1.17 (3H, s, H-29), 1.13 (3H, d,J=6.5 Hz, H-12), 1.05(3H, d,J=6.5 Hz, H-13), 1.00 (3H, s, H-14).13C NMR(150 MHz, CDCl3)δC: 209.7 (C-10), 206.6 (C-1),187.2 (C-9), 176.4 (C-7), 135.0 (C-33), 133.6 (C-23),131.3 (C-18), 124.7 (C-17), 122.5 (C-22), 120.2 (C-32), 119.3 (C-8), 93.3 (C-27), 83.9 (C-2), 71.8 (C-28),54.6 (C-6), 48.5 (C-3), 42.7 (C-4), 42.5 (C-11), 38.6(C-5), 36.6 (C-15), 29.4 (C-31), 27.5 (C-21), 27.0 (C-26), 26.6 (C-30), 26.1 (C-24), 26.0 (C-34), 25.8 (C-19), 24.9 (C-16), 23.2 (C-29), 21.7 (C-13), 20.7 (C-12), 18.3 (C-25), 18.2 (C-35), 17.8 (C-20), 14.1 (C-14). 其波谱数据与文献报道一致[13],故鉴定化合物为27-epifurohyperforin isomer 1.
化 合 物10:C38H50O5,无色油状;[α]D20-92.2(c3.5, CHCl3);ESI-MS(m/z): 587[M+H]+.1H NMR(600 MHz, CDCl3)δH: 7.59 (2H, d,J=7.4 Hz, H-12,16), 7.45 (1H, t,J=7.4 Hz, H-14), 7.28 (2H, t,J=7.4 Hz,H-13, 15), 5.15 (1H, t,J=7.2 Hz, H-35), 4.95 (1H, t,J=7.0 Hz, H-25), 4.86 (1H, t,J=6.6 Hz, H-30), 3.72(1H, d,J=9.1 Hz, H-20), 3.18 (1H, dd,J=14.5, 6.6 Hz,H-29α), 3.04 (1H, dd,J=14.5, 6.6 Hz, H-29β), 2.54(2H, d,J=7.2 Hz, H-34), 2.38 (1H, dt,J=13.5, 2.0 Hz,H-18α), 2.10 (2H, m, H-4, 24α), 2.00 (1H, dd,J=13.5,3.9 Hz, H-18β), 1.91 (1H, dd,J=13.8, 4.7 Hz, H-5α),1.86 (2H, m, H-19), 1.68 (6H, s, H-27, 37), 1.67 (1H,m, H-24β), 1.66 (3H, s, H-38), 1.64 (1H, m, H-5β),1.64 (3H, s, H-33), 1.63 (3H, s, H-32), 1.56 (3H, s, H-28), 1.27 (3H, s, H-17), 0.96 (3H, s, H-22), 0.93 (3H,s, H-23).13C NMR (150 MHz, CDCl3)δC: 206.6 (C-1), 196.7 (C-10), 194.4 (C-7), 167.3 (C-9), 137.2 (C-11), 134.8 (C-36),133.7 (C-31), 133.3 (C-26), 132.7 (C-14), 128.6 (C-12), 128.6 (C-16), 128.2 (C-13),128.2 (C-15), 127.4 (C-8), 122.1 (C-25), 120.9 (C-30), 119.7 (C-35), 87.4 (C-20), 72.3 (C-21), 72.0 (C-2), 64.9 (C-6),46.4 (C-3), 40.1 (C-5), 36.8 (C-4), 33.7 (C-18), 29.3(C-34), 27.2 (C-24), 26.2 (C-37), 26.0 (C-27), 25.7 (C-32), 25.7 (C-22), 25.0 (C-23), 24.2 (C-19), 22.8 (C-29), 18.3 (C-38), 18.1 (C-33), 18.0 (C-28), 17.4 (C-17). 其波谱数据与文献报道一致[14],故鉴定化合物为uralione H.
化合物11 (11):C35H52O5,无色油状;[α]D20+95.02(c1.25, CHCl3);ESI-MS(m/z): 551[M-H]-.1H NMR (600 MHz, CDCl3)δH: 4.97 (2H, m, H-20,32), 4.93 (1H, m, H-25), 4.76 (1H, t,J=10.6 Hz, H-11), 2.98 (2H, dd,J=10.6, 1.9 Hz, H-10), 2.47 (1H, t,J=6.5 Hz, H-16), 2.42 (1H, dd,J=11.6, 7.2 Hz, H-19α),2.05 (1H, dd,J=13.4, 4.9 Hz, H-24α), 2.00 (1H, dd,J=12.8, 6.5 Hz, H-31α), 1.92 (1H, dd,J=12.6, 6.5 Hz,H-31β), 1.82 (1H, dd,J=13.8, 4.3 Hz, H-6α), 1.70 (1H,m, H-24β), 1.68 (1H, m, H-7), 1.66 (3H, s, H-22), 1.65(3H, s, H-27), 1.65 (2H, m, H-30), 1.64 (6H, s, H-23,34), 1.56 (3H, s, H-35), 1.53 (3H, s, H-28), 1.37 (1H,m, H-6β), 1.37 (3H, s, H-13),1.26 (1H, m, H-19β),1.24 (3H, s, H-14), 1.14 (3H, d,J=6.5 Hz, H-17), 1.14(3H, d,J=6.5 Hz, H-18), 1.12 (3H, s, H-29).13C NMR(150 MHz, CDCl3)δC: 208.9 (C-15), 205.9 (C-9),190.8 (C-4), 171.7 (C-2), 134.4 (C-21), 133.6 (C-26),131.8 (C-33), 124.5 (C-32), 122.3 (C-25), 120.7 (C-3),119.6 (C-20), 94.0 (C-11), 74.1 (C-1), 71.2 (C-12),64.0 (C-5), 47.7 (C-8), 42.3 (C-7), 40.9 (C-16),39.6 (C-6), 38.2 (C-30), 29.4 (C-19), 28.2 (C-24), 26.8(C-13), 26.8 (C-10), 26.1 (C-27), 26.1 (C-22), 25.8 (C-34), 25.4 (C-14), 24.4 (C-31), 21.2 (C-17), 21.2 (C-18), 18.2 (C-23), 18.1 (C-35), 17.9 (C-28), 14.6 (C-29). 其波谱数据与文献报道一致[15],故鉴定化合物为attenuatumione E.
化合物12:C38H50O5,无色油状;[α]D20+79.56(c1.00, CHCl3);ESI-MS(m/z): 587[M+H]+.1H NMR(600 MHz, CDCl3)δH: 7.45 (2H, d,J=7.5 Hz, H-28,32), 7.38 (1H, t,J=7.5 Hz, H-30), 7.21 (2H, d,J=7.5 Hz, H-29, 31), 5.12 (1H, t,J=7.1 Hz, H-17), 5.03(1H, dd,J=8.1, 7.0 Hz, H-22), 4.89 (1H, t,J=7.1 Hz, H-36), 4.63 (1H, dd,J=10.5, 5.8 Hz, H-3), 3.08 (1H, dd,J=14.1, 7.0 Hz, H-21α), 2.99 (1H, dd,J=14.1, 8.1 Hz,H-21β), 2.72 (1H, dd,J=13.2, 10.5 Hz, H-2α), 2.30(1H, d,J=14.0 Hz, H-11β), 2.17 (4H, m, H-11α, 15β,35), 2.08 (2H, m, H-16), 1.80 (1H, dd,J=13.2, 5.8 Hz,H-2β), 1.71 (3H, s, H-19), 1.70 (3H, s, H-38), 1.64(3H, s, H-20), 1.63 (3H, s, H-25), 1.62 (3H, s, H-24),1.55 (3H, s, H-39), 1.52 (1H, m, H-15α), 1.50 (1H, m,H-10), 1.48 (3H, s, H-33), 1.41 (3H, s, H-34), 1.36(3H, s, H-14).13C NMR (150 MHz, CDCl3)δC: 205.7(C-12), 194.1 (C-26), 193.6 (C-7), 172.7 (C-5), 137.0(C-27), 133.0 (C-23),132.7 (C-18), 132.7 (C-37),132.1 (C-30), 128.2 (C-29), 128.2 (C-31), 128.1 (C-28),128.1 (C-32), 124.6 (C-36), 123.7 (C-17), 120.0 (C-22), 115.6 (C-6),89.9 (C-3), 77.1 (C-8), 73.0 (C-13),58.9 (C-1), 49.4 (C-9), 47.9 (C-10), 37.1 (C-11), 36.8(C-15), 30.8 (C-2), 29.1 (C-35), 27.1 (C-34), 26.0 (C-24), 25.9 (C-38), 25.9 (C-19), 23.4 (C-14), 22.4 (C-21), 22.4 (C-33), 22.0 (C-16), 17.9 (C-39), 17.9 (C-25), 17.9 (C-20). 其波谱数据与文献报道一致[16],故鉴定化合物为sampsonione K.
化合物13:C38H50O5,无色油状;[α]D20+20.9(c0.15, CHCl3);ESI-MS(m/z): 587[M+H]+.1H NMR(600 MHz, CDCl3)δH: 7.45 (2H, d,J=7.5 Hz, H-28,32), 7.38 (1H, t,J=7.5 Hz, H-30), 7.22 (2H, t,J=7.5 Hz, H-29, 31), 5.15 (1H, t,J=7.0 Hz, H-17), 5.04 (1H,dd,J=7.4 Hz, H-22), 4.88 (1H, t,J=6.9 Hz, H-36),4.67 (1H, dd,J=10.7, 5.7 Hz, H-3), 3.09 (1H, dd,J=14.2, 6.9 Hz, H-21α), 2.99 (1H, dd,J=14.2, 8.2 Hz,H-21β), 2.73 (1H, dd,J=13.0, 10.7 Hz, H-2α), 2.30(1H, d,J=14.1 Hz, H-11β), 2.22 (1H, m, H-11α), 2.18(2H, m, H-35), 2.14 (3H, m, H-15α, 16), 1.80 (1H, dd,J=13.0, 5.7 Hz, H-2β), 1.72 (3H, s, H-24), 1.70 (3H, s,H-38), 1.67 (1H, m, H-15β), 1.65 (3H, s, H-20), 1.64(3H, s, H-19), 1.63 (3H, s, H-25), 1.55 (3H, s, H-39),1.50 (1H, m, H-10), 1.48 (3H, s, H-33), 1.41 (3H, s, H-34), 1.18 (3H, s, H-14).13C NMR (150 MHz,CDCl3)δC: 205.7 (C-12), 194.0 (C-7), 193.6 (C-26),172.7 (C-5), 137.0 (C-27), 133.0 (C-23),132.8 (C-18),132.7 (C-37), 132.2 (C-30), 128.3 (C-28), 128.3 (C-32), 128.1 (C-29),128.1 (C-31), 124.6 (C-36), 123.7 (C-17), 120.0 (C-22), 115.7 (C-6),89.2 (C-3), 77.1 (C-8),73.1 (C-13), 58.9 (C-1), 49.4 (C-9), 47.9 (C-10), 39.6(C-15), 36.6 (C-11), 31.0 (C-2), 29.1 (C-35), 27.1 (C-34), 26.0 (C-38), 25.9 (C-24), 25.9 (C-19), 22.5 (C-16), 22.5 (C-33), 22.4 (C-21), 21.1 (C-14), 17.9 (C-25), 17.9 (C-20), 17.9 (C-39). 其波谱数据与文献报道一致[15],故鉴定化合物为attenuatumione C.
化合物14:C38H50O7,无色油状;[α]D20+9.89(c2.00, CHCl3);ESI-MS(m/z): 617[M-H]-.1H NMR(600 MHz, CDCl3)δH: 7.58 (2H, d,J=7.4 Hz, H-12,16), 7.38 (1H, t,J=7.4 Hz, H-14), 7.26 (2H, t,J=7.4 Hz,H-13, 15), 5.48 (1H, dd,J=10.7, 4.8 Hz, H-23), 5.09(1H, d,J=2.8 Hz, H-17), 5.05 (1H, m, H-28), 4.35(1H, s, 4-OH), 4.01 (1H, d,J=2.8 Hz, H-18), 2.88 (1H,dd,J=14.8, 10.7 Hz, H-22β), 2.52 (1H, m, H-22α),2.50 (1H, m, H-33), 2.47 (1H, m, H-6β), 2.26 (1H, dd,J=14.2, 6.3 Hz, H-6α), 2.12 (2H, m, H-27), 2.10 (2H,m, H-25), 2.08 (2H, m, H-32), 2.03 (1H, m, H-7), 1.73(3H, s, H-26), 1.69 (3H, s, H-31), 1.61 (3H, s, H-30),1.48 (3H, s, H-37), 1.39 (3H, s, H-38), 1.35 (6H, s, H-35, 36), 1.33 (3H, s, H-21), 1.28 (3H, s, H-20).13C NMR (150 MHz, CDCl3)δC: 207.7 (C-9), 203.6 (C-2),194.6 (C-10), 140.8 (C-24), 136.8 (C-11), 132.1 (C-29), 132.0 (C-14), 129.7 (C-12), 129.7 (C-16), 127.9(C-13), 127.9 (C-15), 123.9 (C-28),120.1 (C-23), 106.8(C-4), 89.6 (C-18), 87.0 (C-34), 83.2 (C-17),82.4 (C-1), 76.8 (C-3), 70.0 (C-19), 57.1 (C-5), 52.3 (C-8),50.2 (C-33), 44.8 (C-7), 41.3 (C-6), 40.2 (C-25), 32.2(C-35), 29.0 (C-32), 28.7 (C-22), 27.4 (C-20), 27.0 (C-38), 26.5 (C-21), 26.4 (C-27),26.0 (C-31), 24.3 (C-36),23.1 (C-37), 17.9 (C-30), 16.4 (C-26). 其波谱数据与文献报道一致[17],故鉴定化合物为hypersampsonone C.
化合物15:C38H50O7,无色油状;[α]D20+10.65(c1.67, CHCl3);ESI-MS(m/z): 619[M+H]+.1H NMR(600 MHz, CDCl3)δH: 7.41 (1H, t,J=7.5 Hz, H-28),7.29 (2H, t,J=7.5 Hz, H-27, 29), 7.16 (2H, d,J=7.5 Hz,H-26, 30), 5.15 (1H, t,J=7.0 Hz, H-32), 5.06 (1H, t,J=6.9 Hz, H-36), 4.57 (1H, dd,J=11.6, 2.9 Hz, H-3),3.55 (1H, dd,J=14.9, 11.6 Hz, H-2α), 2.78 (1H, dd,J=10.8, 8.0 Hz, H-7), 2.62 (2H, d,J=7.0 Hz, H-31),2.58 (1H, dd,J=14.3, 6.3 Hz, H-15α), 2.42 (1H, ddd,J=15.5, 10.8, 4.7 Hz, H-8α), 2.10 (1H, m, H-9), 2.03(2H, m, H-35), 1.99 (2H, m, H-34), 1.88 (1H, d,J=14.3 Hz, H-15β), 1.69 (3H, s, H-39), 1.66 (3H, s, H-38), 1.58 (3H, s, H-40), 1.54 (1H, m, H-2β), 1.51 (1H,m, H-8β), 1.48 (3H, s, H-23), 1.36 (3H, s, H-22), 1.30(3H, s, H-20), 1.13 (3H, s, H-18), 1.08 (3H, s, H-21),1.07 (3H, s, H-19).13C NMR (150 MHz, CDCl3)δC:208.1 (C-12), 205.3 (C-13), 204.5 (C-16), 192.3 (C-24), 139.1 (C-33), 134.8 (C-25),132.6 (C-28), 131.7 (C-37), 128.9 (C-26), 128.9 (C-30), 128.2 (C-27), 128.2(C-29), 124.2 (C-36), 118.8 (C-32), 89.0 (C-3), 88.6(C-6), 81.9 (C-11),73.2 (C-17), 68.6 (C-14), 66.7 (C-1), 50.4 (C-10), 44.5 (C-9), 42.7 (C-7), 41.3 (C-15),40.2 (C-34), 31.5 (C-8), 31.2 (C-2), 29.7 (C-31), 28.2(C-20), 26.8 (C-35), 26.0 (C-19), 25.9 (C-38), 25.0 (C-18), 25.0 (C-23), 22.8 (C-22), 18.0 (C-21), 17.8 (C-40), 16.7 (C-39). 其波谱数据与文献报道一致[18],故鉴定化合物为otogirinin B.
3 结论
采用各种波谱技术,以及质谱等对所得化合物进行了结构鉴定,从藤黄科金丝桃属植物川鄂金丝桃中分离并鉴定出15个化合物,包括黄酮类、甾体类、三萜类、饱和脂肪酸酯类、氧杂蒽酮类和多环多异戊烯基取代间苯三酚衍生物. 分离所得的化合物uralione H、attenuatumione C在已有报道中表明具有良好的生物活性:uralione H的细胞实验表明该化合物对PC12细胞的损伤有诱导保护作用[10];attenuatumione C对人体癌细胞系中的SMMC7721和U2OS表现出中等细胞毒性活性[13]. 研究结果为合理有效利用该植物提供了科学依据.

