Dissociative photoionization of methyl crotonate: experimental and theoretical insights*
SUN Ruirui, LI Yanbo, CHEN Jun, MENG Qinghui, WANG Huanhuan, ZHANG Hang, SHAN Xiaobin, LIU Fuyi, SHENG Liusi
(1 School of Physical Sciences, University of Science and Technology of China, Hefei 230026, China;2 National Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, China)
Abstract The photoionization and dissociation of methyl crotonate have been studied by tunable vacuum ultraviolet synchrotron radiation coupled with time-of-flight mass spectrometer in the photon energy region 9.0-14.5 eV. The ionization energy of methyl crotonate and the appearance energies for major fragments C4H5O+,C4H4O+, are determined to be 10.11, 10.73, 10.88, 12.2, 11.93(12.77), and 12.44 eV, respectively, according to the photoionization efficiency curves. Based on the experimental AEs and energies predicted by ab initio G3B3 calculations, possible formation channels for the major fragments C4H5O++CH3O, C4H4O++CH4O, are proposed. Transition states and intermediates involved in the dissociation channels are also located. The majority of the proposed channels occur through isomerization processes prior to dissociations. Hydrogen shift and ring closing/opening are found to be the dominant processes during photoionization and dissociation of methyl crotonate.
Keywords methyl crotonate; synchrotron radiation; mass spectrometry; dissociative photoionization; G3B3 calculations
Methyl crotonate (CH3CH=CHCOOCH3, MC), containing a double bond and functional carboxyl group, is an α-β unsaturated ester widely used as monomers or comonomers in organic synthesis and preparation of spices[1]. They can be used for the preparation of paints and dispersants for paints, adhesives and inks, the manufacture of cleaning products, antioxidants and amphiphilic surfactants, water-based resins and dispersants for textiles and paper as well as be used as precursors of aromatic bases in cosmetics, shampoos, soaps, detergents, and cleaning agents[2-7].
Due to its high-vapor pressure ((1.986±0.027) kPa at 25 ℃), MC would be easily released into environment during industrial processes. In the atmosphere, MC can be oxidated or degraded by OH[4]and NO3[8]radicals and other oxidants like the O3[9-11]molecule or Cl[4,12]atom, and then the products can be transformed into low-vapor pressure organic compounds, which may in turn contribute to the formation of secondary organic aerosol(SOA). Therefore, it is desirable to have a clear understanding of the unimolecular chemistry of MC so as to study its reaction with tropospheric oxidants and assess their impact on the air quality.

In this work, in order to obtain accurate IE of MC and the appearance energies (AEs) of the fragment ions and to explore the mechanism of the complicated photodissociation process, tunable synchrotron vacuum ultraviolet (VUV) coupled with photoionization mass spectrometry (PIMS) was utilized to perform a study of dissociative photoionization of MC in the energy region 9.0-14.5 eV. The IE of MC and the AEs of major fragment ions were acquired by measurements of the photoionization efficiency curve (PIE) spectra. The major dissociative photoionization channels were proposed and discussed in detail on the basis of experimental measurements and theoretical calculations.
1 Experimental methods and computational methods
1.1 Experimental methods
The whole experiments were carried out at the Atomic and Molecular Physics Beamline (BL09U),National Synchrotron Radiation Laboratory, Hefei, China, which have been reported in literatures[14-17], and only a short description is given here. The VUV synchrotron radiation (SR) is provided from an undulator of the 800 MeV electron storage ring which is monochromatized with a 6-m length monochromator. By using three interchangeable spherical gratings, photon energies can be adjusted between the range 7.5-124 eV. In this study, mass spectra and PIE curves of MC were measured in the energy region 9.0-14.5 eV, so only the 370 lines/mm grating was used which covers the photon energy range 7.5-22.5 eV. The absolute photon energy of monochromator is precisely calibrated with the known IEs of inert gases. The energy resolution (E/ΔE) of photons at 15.9 eV is measured by threshold photoelectron-photoion coincidence (TPEPICO) to be 9 meV (full width at half maximum), with an average photon flux of 1012photons/s[18]. The home-made TOF-MS used in this study consists of seven electrostatic lenses that focus and accelerate the ions from the photoionization region, which provides a mass resolutionM/ΔMof ~800.
Liquid MC (Aladdin, 98%) without further purification at room temperature was contained in a home-made sample reservoir. The MC sample was carried by Ar (purity 99.99%) and expanded into the ionization chamber through a nozzle with a diameter of 70 μm and one skimmer. The generated ions were drawn out of the photoionization region by a pulse extraction field triggered with a pulse generator (DG 535 SRS) and detected by a microchannel plate detector. During the experiments, higher harmonics of the undulator were effectively suppressed by using Ar as the filter gas with an operating pressure of 0.773 3 kPa. PIE spectra were obtained by scanning the monochromator with step width of 30 meV. Detailed information about the experimental apparatus has been reported elsewhere[15,18].
1.2 Computational methods
All the energies of the MC molecule and relevant ions involved in this work were calculated by quantum chemistry methods using Gaussian 09 suite of programs[19]on the supercomputing system in the Supercomputing Center of USTC, Hefei, China. The geometries of the parent molecule, fragment ions, intermediates, and transition states were optimized using density functional(DFT) method with the B3LYP/6-31g(d) hybrid functional. The description of the theoretical methods involved and the general calculations procedures were described in the literatures[20-21]. Harmonic vibrational frequencies were computed at the same level of theory, and they provide the zero-point vibrational energies(ZPTE) and can be used for making sure that the local minimum or transition state was located. In order to verify the reactants and products are connected by the computed transition states, the intrinsic reaction coordinate (IRC)[22]calculations at the same level were also performed. Accurate energies were obtained at the G3B3 level[23]. In order to better assess experimental data and to predict possible dissociation mechanisms, enthalpies of formation at 298 K (ΔHf,298) for possible products were obtained by using the calculated enthalpy changes for their equations of formation at the B3LYP/6-31g(d) level and the experimental ΔHf,298for the isolated atoms of C, O, and H[24].
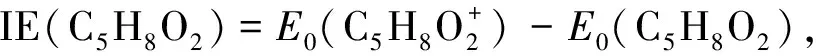
(1)

(2)
2 Results and discussion
2.1 VUV photoionization mass spectra

Fig.1 Photoionization mass spectra of MC at different photon energies

The PIE curves of the MC radical cation and the main fragment ions shown in Fig.2 are obtained by integrating the area of each mass peak as a function of photon energy, followed by background subtraction and normalization to the photon flux.
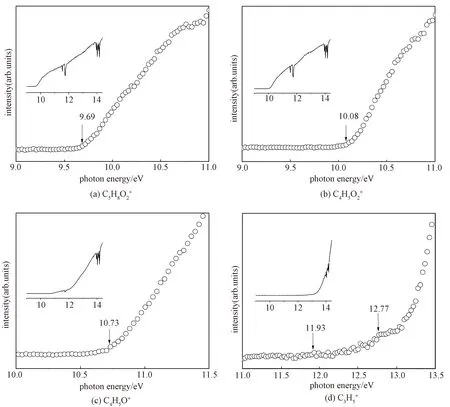
Fig.2 The PIE curves of the parent ion (a) and the fragmentions (b),C4H5O+ (c), and (d)
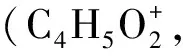
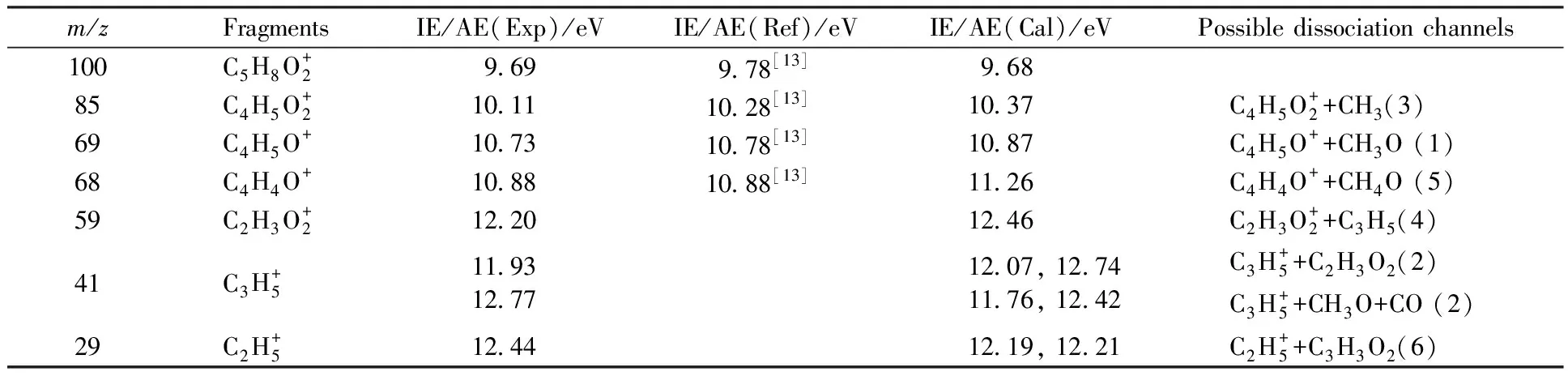
Table 1 Theoretical and experimental values of the ionization energies and appearance energies for main fragment ions from dissociative photoionization of MC
The relative branching ratios of the molecular ion of MC and its major fragment ions as functions of photon energy are derived from data of PIE curves and are plotted in Fig.3. The sum of ion abundances is normalized to 1 at any photon energy. In the energy region 10-12 eV, the fractional abundance of the molecular ion decreases abruptly while those of the fragment ions atm/z85 and 69 rise, implying that these fragments originate from the molecular ion. In the energy region 12-14 eV, the fractional abundances of the molecular ion and ion atm/z85 decrease while those of the fragment ions atm/z68, 59, 41, and 29 rise, implying that these fragments compete with the fragment ion atm/z85 in the dissociation channels.
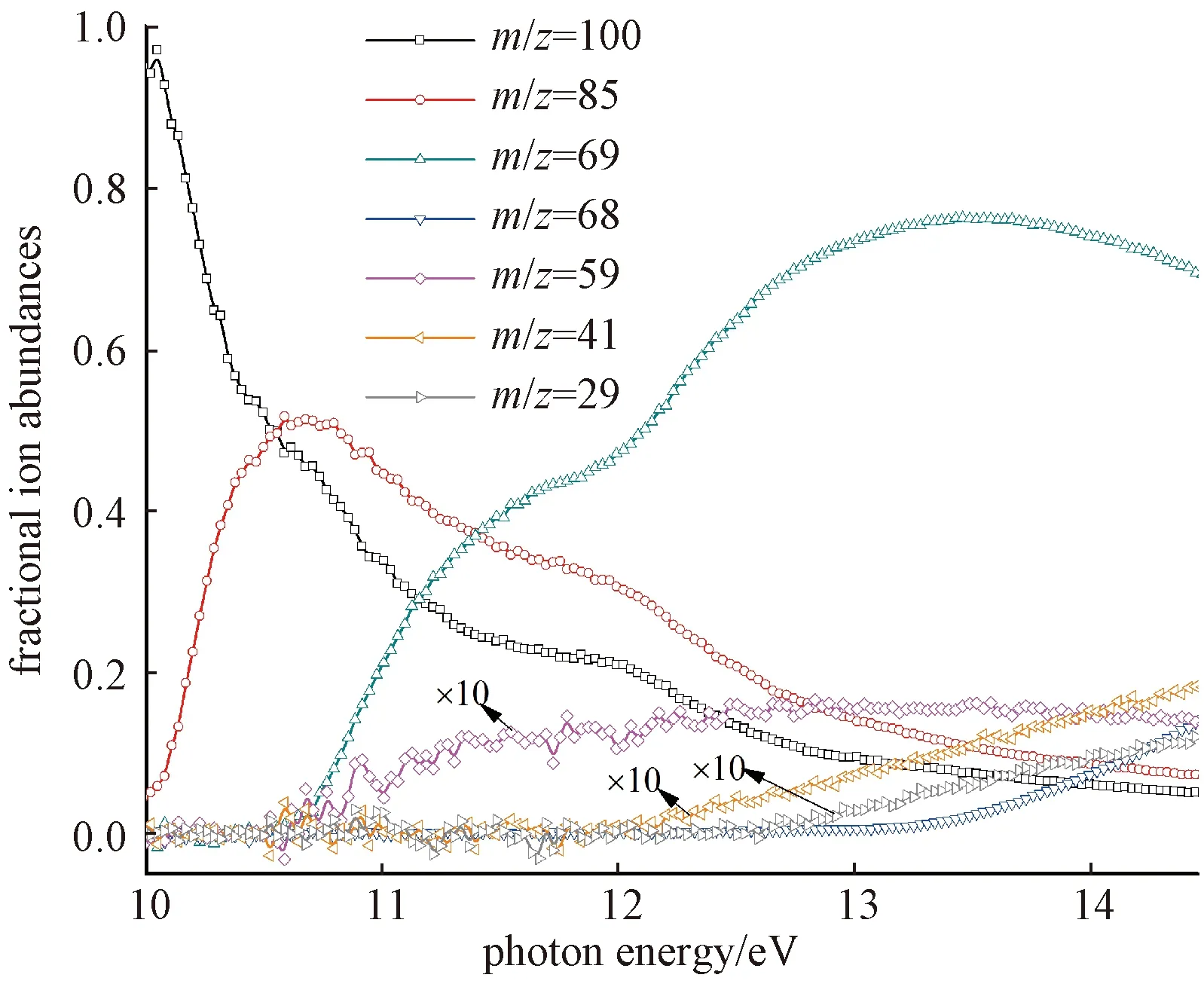
Fig.3 Relative branching ratios of the molecular ion of MC and its major fragment ions as functions of photon energy
2.2 Dissociation mechanisms

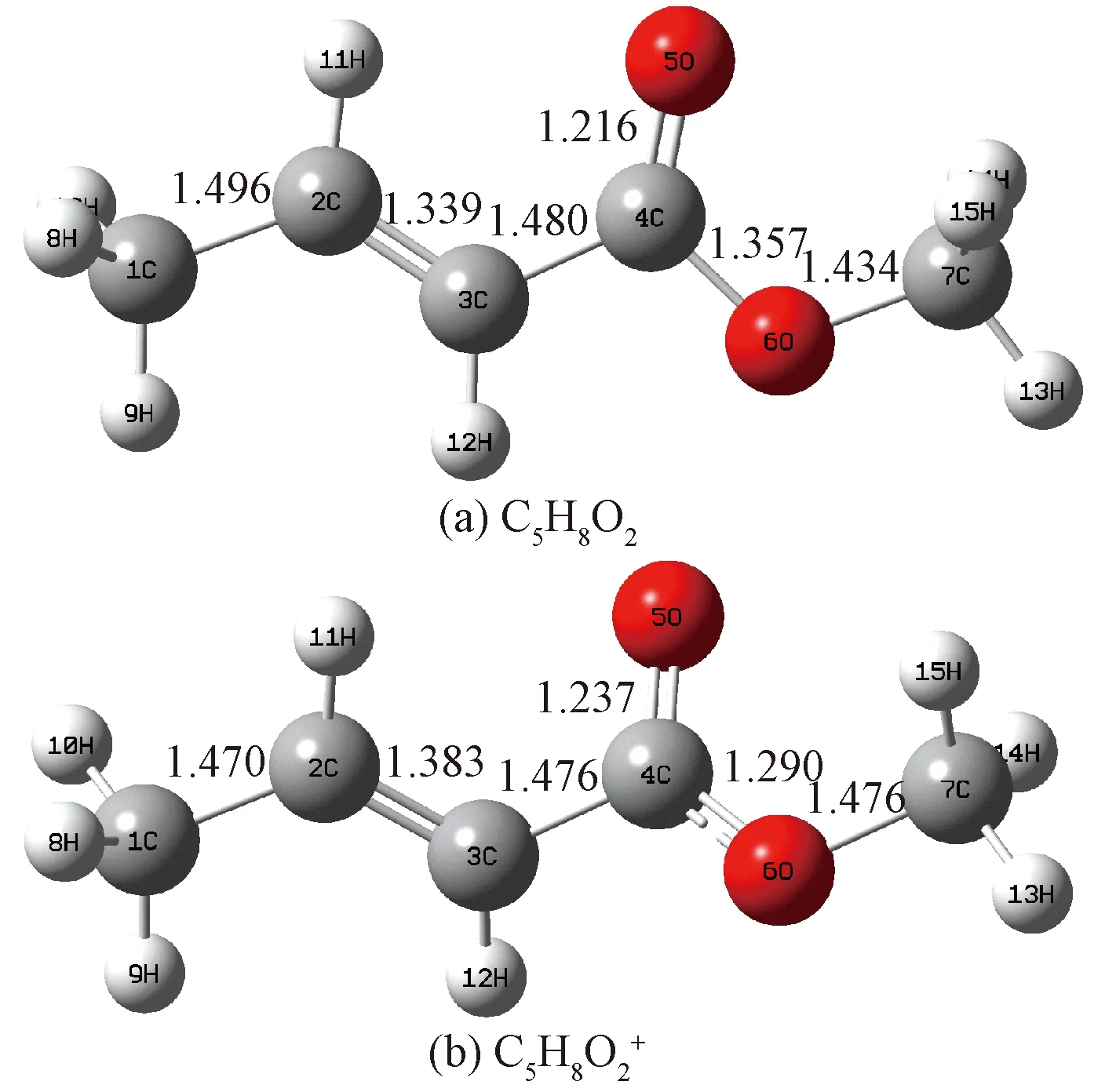
Fig.4 Geometries of C5H8O2 (a) and (b) optimized at the B3LYP/6-31g(d) level of theory

Table 2 Enthalpies of formation at 298 K (ΔHf,298) for possible products of MC photoionization
2.3 Direct dissociation channels Channel 1 C4H5O++CH3O

2.4 Indirect dissociation channels
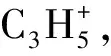

Fig.5 Formation pathways of two possible structures of


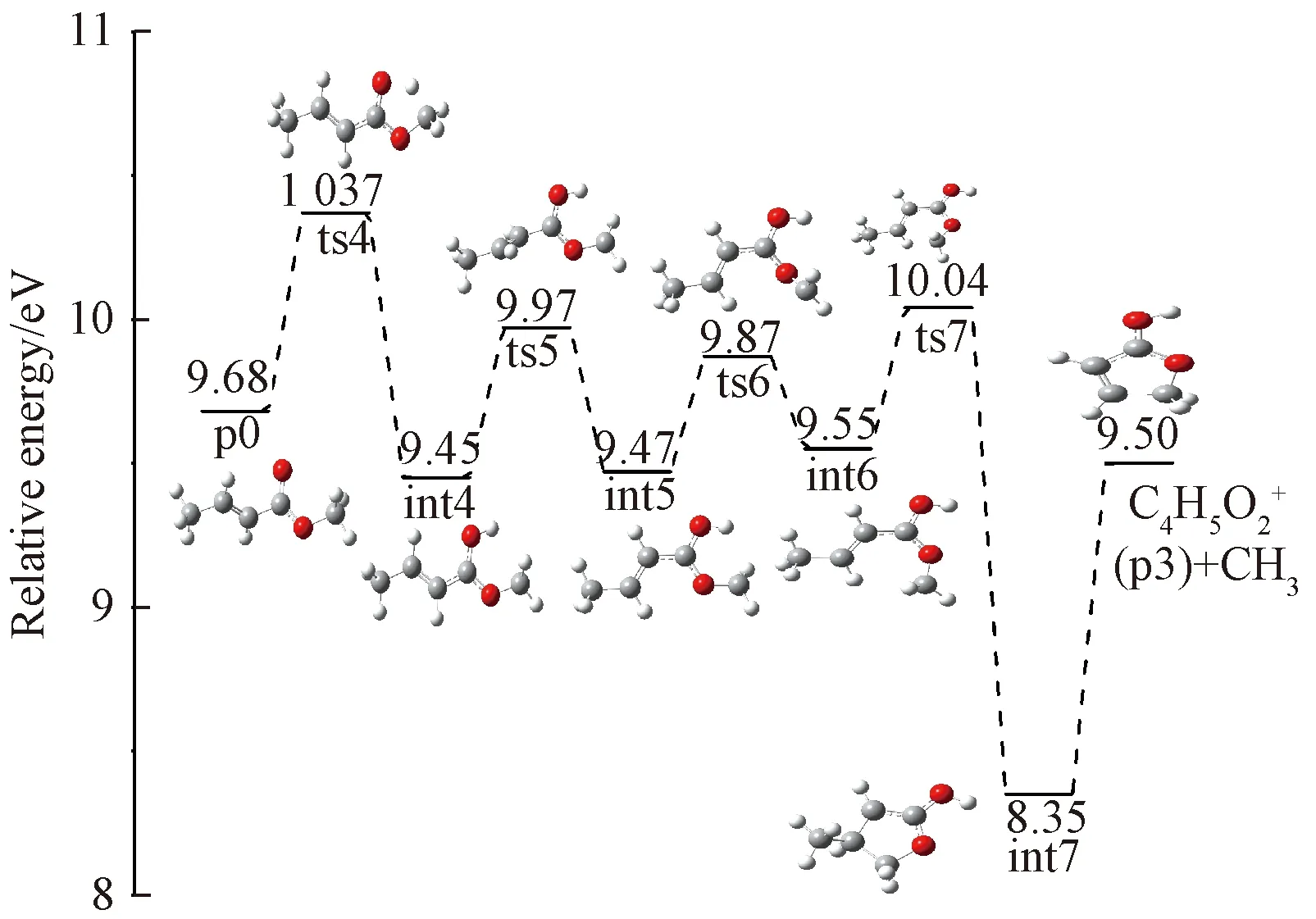
Fig.6 Formation pathway of
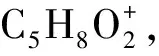

Fig.7 Formation pathway of
Starting from int4, rotation of O5 happens to yield the intermediate int8 overcoming a barrier of 0.25 eV. Then, the H atom shifts to C3 to yield int9. Then, a four-membered ring intermediate int10 is produced via transition state ts10. Then, H atom shifts from C3 to C4 yielding int11, followed by H migration between C1 and C2. The final product p4 is yielded by breaking the C3-C4 bond in int12, accompanied with the loss of C3H5radical. According to theoretical calculations, ts6 has the highest energy along the entire pathway, and the corresponding appearance energy is 12.47 eV, showing good agreement with the experimental AE value of 12.16 eV.
Channel 5 C4H4O++CH4O
A proposed channel for this reaction to form C4H4O+(p5) is shown in Fig.8 which is actually branched from channel 2-b. Starting from int3, H atom shifts from C3 to O6 to yield int13 by overcoming a barrier of 1.17 eV. The final product p5 is yielded by breaking the C-O bond in int13, accompanied with the loss of CH3OH. The reaction path for the bond dissociation is computed at the B3LYP/6-31g(d) level, and no distinct transition states are found in the bond cleavage. The calculated AE for p5 is 11.26 eV, which is a little higher than the experiment value. In addition, as shown in Table 2, the calculated ΔHf,298(C4H4O+) value is 198.71 kcal/mol, which is basically consistent with the value of 195.27 kcal/mol reported by Lias[24].
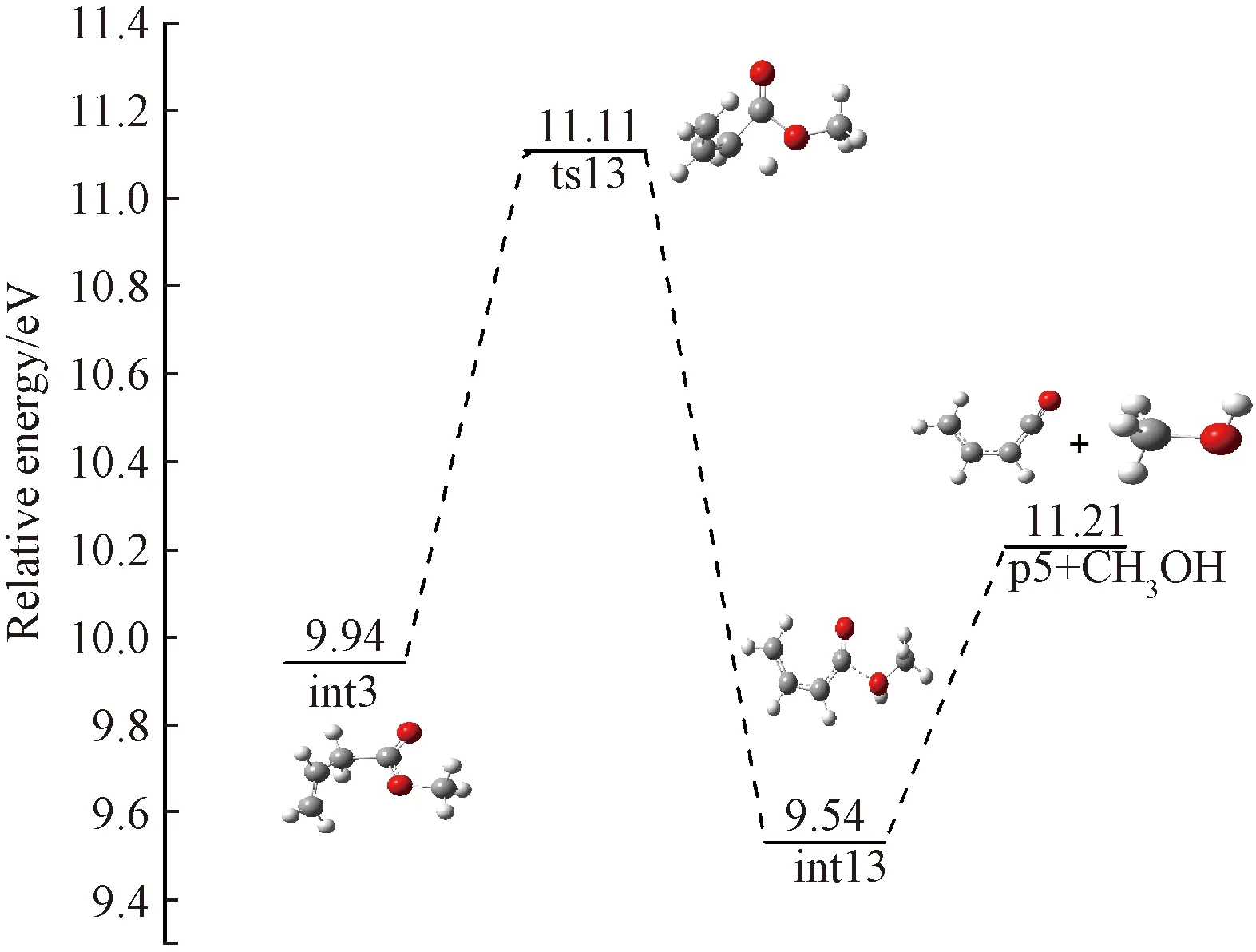
Fig.8 Formation pathway of C4H4O+
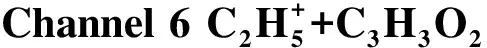
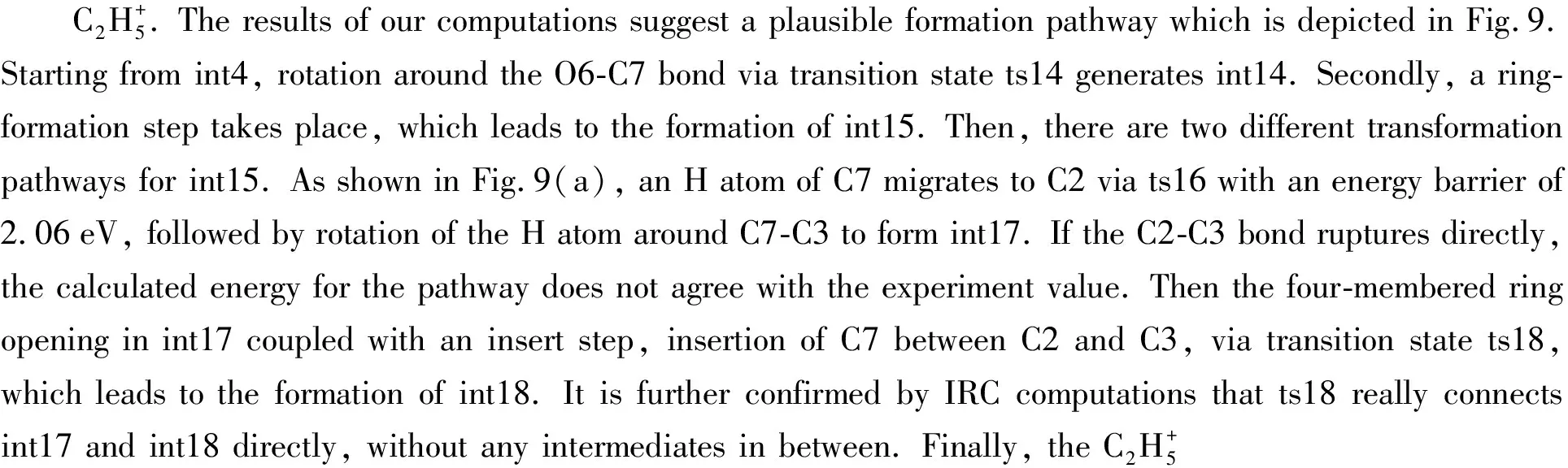
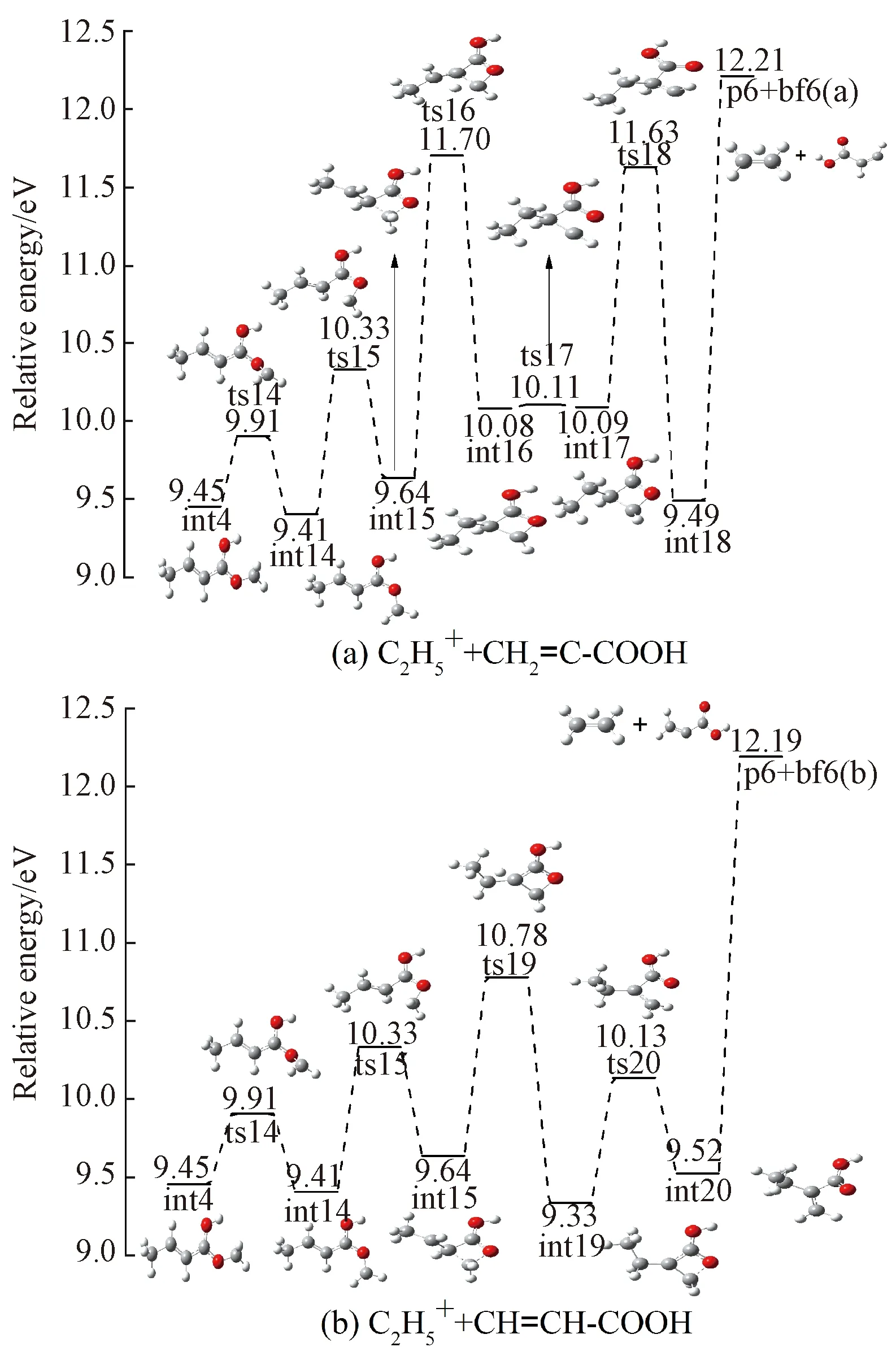
Fig.9 Formation pathways of
3 Conclusions


