Blood eosinophils and mortality in patients with acute respiratory distress syndrome: A propensity score matching analysis
Hao-tian Chen, Jian-feng Xu, Xiao-xia Huang, Ni-ya Zhou, Yong-kui Wang, Yue Mao
1 Department of Emergency Intensive Care Unit, the Second Affiliated Hospital of Zhejiang University School of Medicine,Hangzhou 310009, China
2 Department of Respiratory Therapy, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
3 Department of Respiratory Medicine, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
4 Department of Medical Administration, the Second Affiliated Hospital of Zhejiang University School of Medicine,Hangzhou 310009, China
5 Department of Cardiology, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009,China
KEYWORDS: Critical care; Acute respiratory distress syndrome; Eosinophils; Mortality;Corticosteroid
INTRODUCTION
Acute respiratory distress syndrome (ARDS) represents an important public health problem worldwide, leading to a high mortality rate of approximately 40%.[1]ARDS is associated with excess inflammation contributing to increased endothelial and epithelial permeability and leading to the accumulation of protein-rich alveolar oedema f luid in the lung interstitium.[2]During the process of ARDS, immune effector cells have key activities in defence of the normal lung.
Eosinophils (EOSs) are key innate immune cells in host defence,[3]and they have been found to be associated with mortality in patients with chronic obstructive pulmonary disease (COPD)[4,5]and asthma.[6,7]The blood EOS counts are considered as a potential biomarker for identifying COPD patients at risk and as a reference for the usage of inhaled corticosteroids.[8]For ARDS, EOSs have been considered as an important immune response contributor, and they may be a diagnostic biomarker.[9,10]The accumulation of EOSs in ARDS patients was documented to be a prognostic indicator of patient survival.[11]Recently, a retrospective analysis of 112 patients with ARDS found that ARDS surviving patients have higher blood EOS counts than non-survivors and that EOSs may play a protective role in ARDS independent of corticosteroid use.[12]The prognosis of ARDS patients is closely related to factors such as tidal volume.[13,14]The relationship between blood EOSs and mortality in patients with ARDS needs to be further evaluated with a large sample size after full consideration of confounders.
The purpose of our study is to detect the relationship between blood EOSs and 28-day mortality in patients with ARDS after adjusting for possible confounding factors by Cox regression and propensity score matching(PSM). We also aim to investigate whether this relationship varies by corticosteroid use.
METHODS
Database introduction
Our data source was the Medical Information Mart for Intensive Care III (MIMIC-III, version 1.4), an open international database. The MIMIC-III database includes deidentified health-related data associated with over forty thousand patients who stayed in critical care units (ICUs) of the Beth Israel Deaconess Medical Center between 2001 and 2012. Data were extracted by the author HTC (certif ication number: 37147539).
Inclusion and exclusion criteria
Patients with ARDS who were 16 years or older, used mechanical ventilation during the ICU stay, and stayed in the ICU for at least 48 consecutive hours, were selected for inclusion. To screen the patients with ARDS accurately, the diagnostic information recorded in the MIMIC-III database and the Berlin criteria[15]were considered simultaneously,and the following condition was proposed: the onset of ARDS was acute, patients must have partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio (P/F) ratio ≤300 mmHg (1 mmHg=0.133 kPa) when positive end expiratory pressure (PEEP) was at least 5 cmH2O (1 cmH2O=0.098 kPa) and the free-text radiology reports mentioned bilateral opacities/infiltrates in the first 24 hours after ICU admission. The patients with COPD or asthma and patients without EOS data within the f irst 72 hours after ICU admission were excluded.
Data extraction
Structured query language (SQL) was used to extract the following data: age, sex, weight, body mass index(BMI), heart rate (HR), mean arterial pressure (MAP), P/F ratio, comorbidities (diabetes, sepsis), disease severity score (Simplified Acute Physiology Score II [SAPS II]),laboratory outcomes (white blood cell [WBC] count, red blood cell [RBC] count, platelet [PLT], blood lactate,pH, EOS count), mechanical ventilation (tidal volume),minute ventilation (L/minute), and drugs (corticosteroid,vasopressor, and antibiotics). The extracted data were obtained within 72 hours after ICU admission.
Grouping and def inition
According to the cut-off value of 2%, the maximum value of EOS counts within 72 hours after ICU admission were used to divide the patients into EOS counts ≥2% and EOS counts <2% groups. ARDS severity was classified based on P/F ratio: 200 mmHg<P/F≤300 mmHg (mild), 100 mmHg<P/F≤200 mmHg (moderate), and P/F≤100 mmHg(severe). Corticosteroids can decrease blood EOS t least 50%at the first four hours after administration and then return to baseline within 24 hours.[16]Therefore, we excluded the patients who used corticosteroid 24 hours before the EOS count recording time. In the subgroup analysis, all patients were assigned to two subgroups based on the usage of any corticosteroid drugs except the external administration route within 72 hours after ICU admission, including dexamethasone, hydrocortisone, and methylprednisolone.Vasopressor included norepinephrine, epinephrine,dobutamine, dopamine, vasopressin, and phenylephrine. The mean tidal volume ≤6 mL/kg predicted body weight (PBW)within 72 hours after ICU admission was adopted to def ine adherence to the target of low tidal volume. The primary endpoint was the 28-day mortality, defined as death within 28 days from ICU admission. The secondary endpoints included ICU mortality, hospital mortality, length of ICU stay, and length of hospital stay. For patients with ICU stay more than one time, only the f irst ICU stay of the f irst hospitalization was considered.
Statistical analysis
Continuous variables were summarized as the mean±standard deviation or median (upper and lower quartiles) when appropriate, and categorical data were summarized as proportions. The characteristics of patients with ARDS were compared using Student’st-test,Wilcoxon rank-sum test, and Chi-square test according to the distribution of the data. The Kaplan-Meier method and log-rank tests were used to compare 28-day mortality among the EOS counts ≥2% and EOS counts <2%groups. Cox regression models were used to assess the relationship between EOS counts and 28-day mortality.A backward stepwise method withP<0.05 was used to build the model. Sixteen potential confounders with aP-value <0.10 in the univariate analyses were included in the Cox regression models: age, BMI, weight, HR,P/F ratio, sepsis, ARDS severity, SAPS II, WBC, EOS counts, lactate, pH, tidal volume, minute ventilation,low tidal volume, and vasopressor use. The variance inflation factor (VIF) was used to test multicollinearity,and VIF≥10 indicated multicollinearity between variables.The proportional hazards assumption was tested using Schoenfeld residuals, withP<0.05 constituting evidence for non-proportionality. Subgroup analyses were also performed separately in patients who used corticosteroids and those who did not. PSM was used to balance the cofounders between the EOS counts ≥2% and EOS counts<2% groups. A multivariable logistic regression model was used to evaluate the propensity score by the variables that entered the Cox regression model and that were essential to ARDS prognosis (sepsis and low tidal volume). A 1:1 nearest-neighbour matching algorithm was used with a calliper of 0.05. AllP-values were two-tailed, andP<0.05 was considered statistically signif icant. Statistical analyses were performed using STATA (Version 16; Stata Corp.,College Station, TX, USA).
RESULTS
Characteristics of patients
A total of 2,567 patients were included, and the 28-day mortality rate was 24.19% (621/2,567). The baseline characteristics of enrolled patients are shown in Table 1.
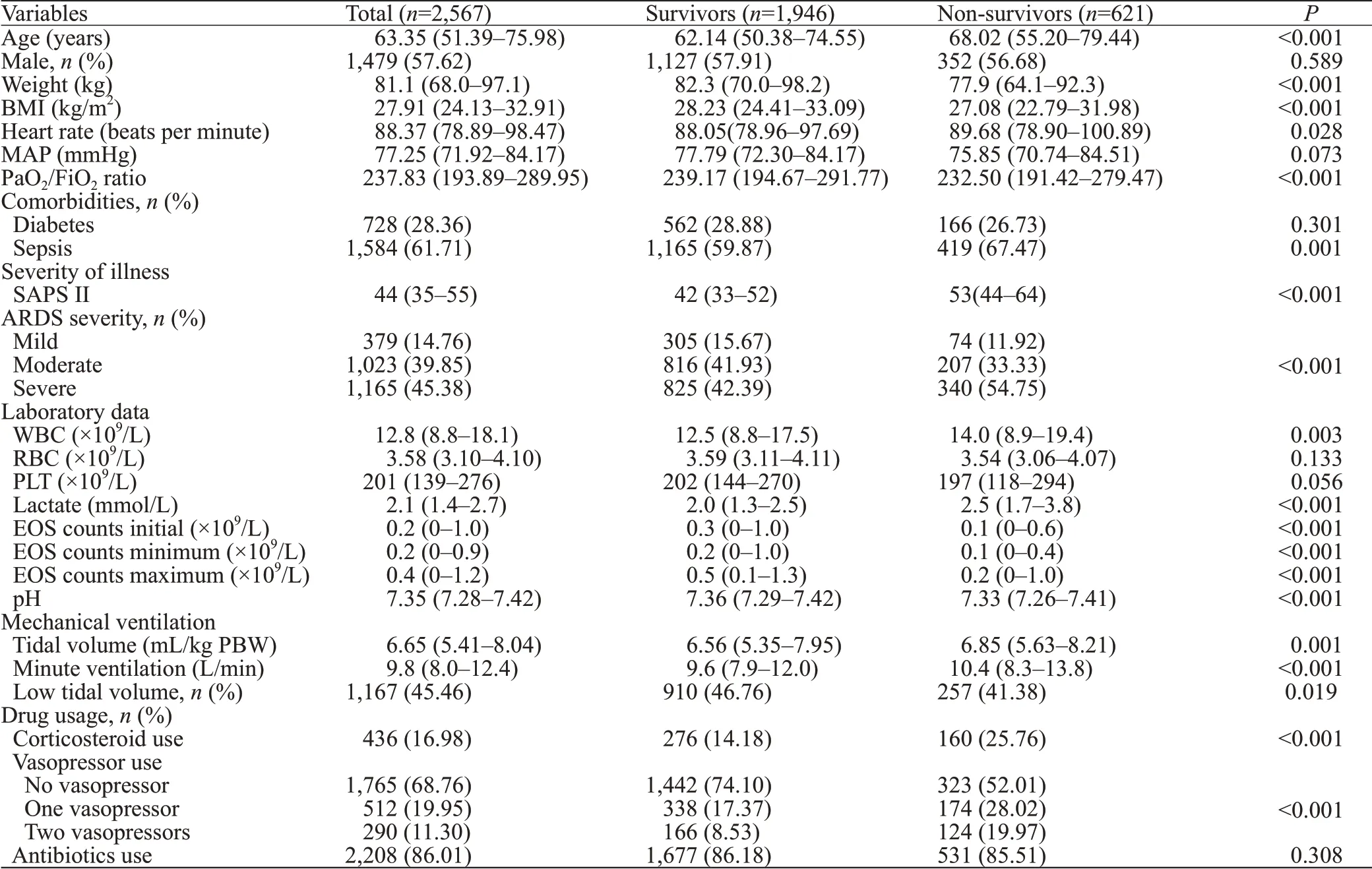
Table 1. Comparisons of baseline characteristics between survivors and non-survivors
Clinical outcomes
Without adjusting for covariates, the EOS counts ≥2%group had a significantly lower 28-day mortality rate, ICU mortality rate, and hospital mortality rate than the EOS counts <2% group (Table 2). In patients who did not use corticosteroids, the result was similar to the crude outcome,but this result was not observed in patients who used corticosteroids. The differences in the median length of ICU stay and length of hospital stay were not signif icant between the EOS counts ≥2% group and the EOS counts <2% group.Kaplan-Meier survival curves depicting the 28-day survival distributions of patients with EOS counts ≥2% or EOS counts <2% are presented in Figure 1, and the comparison between the two groups showed that patients with EOS counts ≥2% had a signif icantly higher survival rate (log-rank test,P=0.026).
Relationship between EOS counts and 28-day mortality
To assess the relationship between EOS counts and 28-day mortality and to test whether the relationship varied by corticosteroid used, three models were developed using Cox regression analyses (Table 3).Model 1 used all patients in our study, and EOS counts≥2% showed a significant association with a decreased 28-day mortality rate (hazard ratio [HR] 0.731; 95%confidence interval [CI] 0.581-0.921,P=0.008) after adjustment for SAPS II, lactate, minute ventilation,vasopressor use, ARDS severity, BMI, age, and P/F ratio.Model 2 used patients who did not use corticosteroids,and the results were similar to those in Model 1, withHRof 0.697 (95%CI0.535-0.909,P=0.008). Model 3 included patients who used corticosteroids, and EOS counts were not included into the model because theP-value was 0.860. We also detected an interactive effect of EOS counts and corticosteroids on the 28-day mortality with an odd ratio of 2.585 (95%CI1.444-4.627,P=0.001).
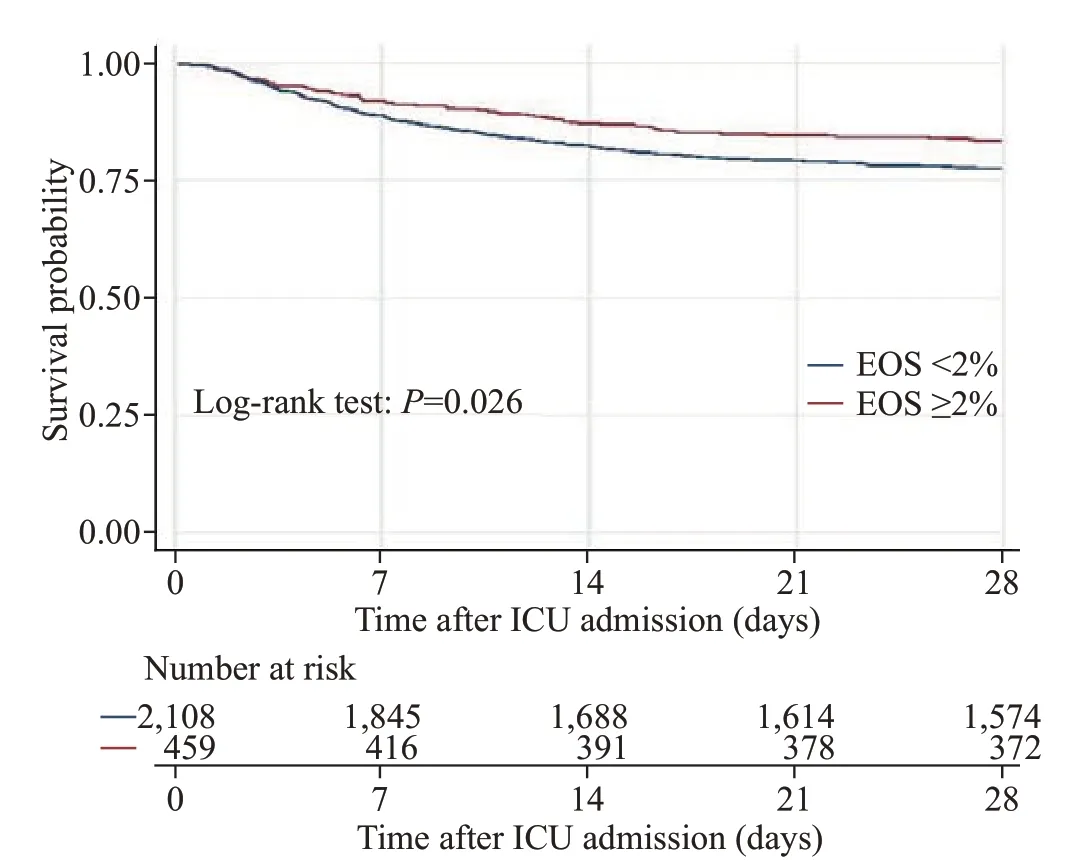
Figure 1. Kaplan-Meier survival curve of the study population. EOS:eosinophil; ICU: intensive care unit.
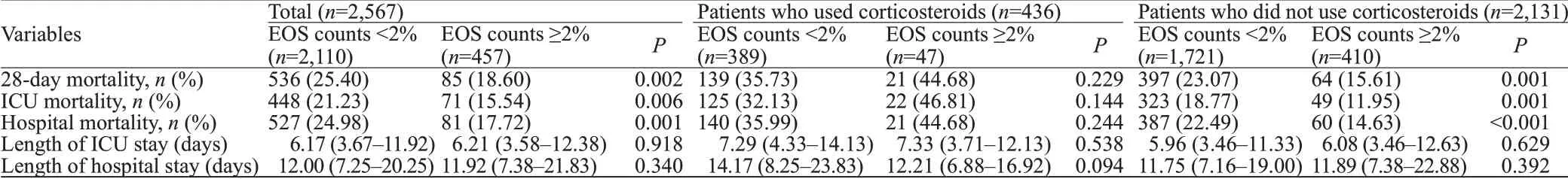
Table 2. Comparisons of outcome characteristics between the EOS counts <2% and EOS counts ≥2% groups
Outcomes after PSM
A total of 457 matched pairs were obtained after PSM . No significant difference was observed in any confounders between the two matched groups, indicating excellent matching among all pairs. Compared with the EOS counts <2% group, the EOS counts ≥2% group had significantly lower 28-day mortality (18.60%[85/457] vs. 26.70% [122/457],P=0.003), ICU mortality(15.54% [71/457] vs. 23.19% [106/457],P=0.003),and hospital mortality (17.72% [81/457] vs. 26.26%[120/457],P=0.002) after matching. The differences in the median length of ICU stay and length of hospital stay were not significant between the EOS counts ≥2%group and the EOS counts <2% group after matching.
DISCUSSION
In our large-sample study, we demonstrated that increased blood EOS counts were related to a significantly decreased risk of 28-day mortality after ICU admission in patients with ARDS. After adjustment for covariates, this result remained consistent in the PSM analysis. However, an interaction was observed between blood EOS counts and corticosteroid use.The relationship between blood EOSs and 28-day mortality was detected only in patients who did not use corticosteroid drugs, whereas this relationship was nonexistent in patients who used corticosteroids.
The ARDS is related to innate immune response.Neutrophil-dependent lung injury is the key pathway.The inf lammatory factors released from endothelial cells can recruit neutrophils and dramatically increase the number of neutrophils migrating to lungs.[17]Neutrophils may cause alveolar damage by forming extracellular traps in response to endothelial injury and histone release and further lead to multiple organ failure or death.[18]Recently, Zhu et al[12]found that EOSs can be grouped into CD101+and CD101-subtypes by the CD101 marker. CD101+EOSs may play a pro-inflammatory role by overexpressing alarmins. CD101-EOSs, the EOS subtype mostly elevated in patients with ARDS,might play a protective role in the inf lammatory process by preventing neutrophil recruitment and stimulating clean-up of neutrophil debris through the production of protectin D1. Our study suggests that EOSs play a possible protective role in ARDS patients, which has rarely been demonstrated previously.
Corticosteroids may improve oxygenation and shorten mechanical ventilation times in ARDS.[19]However, no consistent result has been reported regarding whether corticosteroids should be routinely used in ARDS patients. Meduri et al[20]found that methylprednisolone can signif icantly improve pulmonary and extrapulmonary organ dysfunction in ARDS patients and reduce ICU mortality by downregulating systemic inflammation.Guidelines for corticosteroid insufficiency (CIRCI)also suggest that corticosteroids should be used in early moderate-to-severe ARDS.[21]However, in a randomized controlled trial including 180 patients with ARDS, no benef it of corticosteroids was found in hospital survival;moreover, using methylprednisolone two weeks after the onset of ARDS can signif icantly increase the 60-day and 180-day mortality rates.[22]A similar result[23]was also detected in patients with sepsis-associated ARDS.In our study, 28-day non-survivors had a higher ratio of corticosteroid use when compared with survivors, and the relationship between EOS counts and 28-day mortality was non-existent in patients who used corticosteroids; this suggests that the potential protective role of EOSs can be counteracted by corticosteroid use. Although corticosteroids improve clinical symptoms to some extent, the clinical use of corticosteroids in ARDS should be considered with caution, taking into account both the negative effects and the use time.
The large sample size from the MIMIC III database was our study strength, and it allowed a more indepth analysis under full consideration of confounding variables and ensured robust results; however, the study also has limitations. First, patients in our study were divided based on the maximum value of blood EOS counts within 72 hours after ICU admission. However,the EOS fluctuation and variation tendency may also affect patients’ prognoses, and this needs further study.Second, the best cut-off value of EOSs has yet to be determined. A cut-off value of 2% has been used in a previous study of COPD;[24]therefore, we used 2%for our group standard, but this cannot avoid related bias. Third, there were many important data missed in the MIMIC-III database. Inflammatory markers,such as C-reaction protein, are important indicators of prognosis for ARDS patients. However, the proportion of missed C-reaction protein data was higher than 20%,and thus we did not include it in this study. Fourth, the subgroup analysis was conducted only according to whether corticosteroids were used within 24 hours before ICU admission to 72 hours after. Whether the dose of corticosteroids and the time courses of the corticosteroid treatment affect the relationship between EOS counts and the outcome of patients with ARDS needs to be explored in future studies. Finally, the present study was a retrospective study which only allowed us to deduce the relationships between the blood EOS counts,corticosteroids, and mortality, and a definite causal relationship cannot be established. Further studies, such as randomized controlled trials (RCTs), are needed to verify this relationship.
CONCLUSIONS
Higher EOS counts are related to lower mortality in patients with ARDS. This relationship is not influenced by confounders, such as the characteristics of mechanical ventilation or the disease severity. However, this result is significant only in patients who do not use corticosteroids. To def initively assess the protective role of blood EOS counts in ARDS, larger RCTs are needed.
Funding:None.
Ethical approval:The right of this database was approved by the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) and consent was obtained for the original data collection. Patients’ information in the MIMIC-III database was anonymized; therefore, informed consent was not required.
Conflicts of interests:The authors declare that they have no competing interests.
Contributors:HTC and JFX contributed equally to this study. YM conceived and designed the study. HTC extracted data and performed all statistical analyses together with JFX. XXH and NYZ were involved in drafting the manuscript and the interpretation of the data.YKW was also involved in interpretation of the data and made critical revisions to the discussion section. All the authors gave f inal approval of the version to be published and agreed to be accountable for all aspects of the work regarding questions related to the accuracy or integrity of any part of the work.
 World journal of emergency medicine2021年2期
World journal of emergency medicine2021年2期
- World journal of emergency medicine的其它文章
- World Journal of Emergency Medicine
- Overlapping public health crises during the coronavirus disease pandemic
- Comparison of intraosseous access and central venous catheterization in Chinese adult emergency patients: A prospective, multicenter, and randomized study
- Empyema associated with vegetable foreign body aspiration
- A red herring: An unusual case of pneumothorax
- A case of a successful post-transcatheter aortic valve replacement His bundle pacing
