Efficacy and safety of corticosteroids in immunocompetent patients with septic shock
Xin Lu, Wei Han, Yan-xia Gao, Shi-gong Guo, Shi-yuan Yu, Xue-zhong Yu, Hua-dong Zhu, Yi Li
1 Emergency Department, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100730, China
2 Department of Epidemiology and Biostatistics, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences/School of Basic Medicine Peking Union Medical College, Beijing 100005, China
3 Emergency Department, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
4 Department of Rehabilitation Medicine, Southmead Hospital, Bristol, United Kingdom
KEYWORDS: Corticosteroids; Septic shock; Immunocompetent patients; Systematic review; Metaanalysis
INTRODUCTION
Septic shock, a life-threatening organ dysfunction caused by the dysregulated host response to infection,is characterized by severe circulatory, cellular, and metabolic abnormalities.[1]It has been regarded as a formidable clinical challenge associated with mortality 30% to 40%.[2,3]Septic shock is a common clinical syndrome, but has pronounced heterogeneity such as variable infection sites and sources, pathogen species,and host comorbidities.[4]There has been an increasing emphasis on evidence-based adjunct therapy beyond hemodynamic support and antimicrobial therapy.[5,6]
Corticosteroids have been used in the treatment of patients with septic shock for more than half a century.[7]Till now, nearly thirty randomized controlled trials (RCTs) have evaluated the efficacy of corticosteroids in these patients but yielded different results, including two well-known RCTs published in the year 2018.[8,9]Twelve systematic reviews since 2018 have been conducted to try to address the discrepancy in these previous trials by classifying the doses of steroids and the severity of shock.[10-21]
However, these studies and reviews have not yet addressed the heterogeneity of the patient population,such as the immunological state of a patient, which is another important clinical aspect and may result in signif icant enrollment bias.
Recently, focusing on immunocompromised patients with septic shock, we performed an observational cohort study, and found that corticosteroid therapy had adverse effects on survival, hemodynamic stability, and hospital duration in the selected population.[22]Therefore, we aim to perform a systematic review which eliminated the impact of immune status to assess the benefits and risks of corticosteroids in septic shock, and to identify the exact group of patients who may benefit from corticosteroid treatment.
METHODS
Search strategy
We systematically performed electronic search of Medline via PubMed, Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, and EMBASE from inception to March 12, 2020. We combined MeSH and title/abstract keywords, such as “steroids”, “glucocorticoids”,“corticosteroids”, “prednisolon”, “methylprednisolon”,“prednison”, “dexamethasone”, “triamcinolon”,“fludrocortisone”, “betamethasone”, “hydrocortisone”,“sepsis”, and “shock, septic” to identify all RCTs comparing corticosteroids with a control group for immunocompetent patients with septic shock.
Study selection
Two authors independently identified the trials for inclusion based on their titles and abstracts, and evaluated the full texts of the papers.
Eligibility criteria
(1) Population. Immunocompetent adult patients with septic shock, defined based on the definition of included trials, were eligible for inclusion. Sepsis patients without circulatory failure were excluded. The immunocompetent patient was def ined as the exclusion of one or more immunocompromised underlying conditions,including immunosuppression, immunodeficiency,immunosuppressive therapy, human immunodeficiency virus positive or acquired immune deficiency syndrome,advanced or end-stage neoplasm, and organ transplant recipients. (2) Intervention. All types of corticosteroids were included, regardless of the formula, dose, start time,and duration of treatment. (3) Control. The control group was allowed for the following interventions: placebo,saline, or no intervention. (4) Outcomes. The primary outcome was short-term mortality during intensive care unit (ICU) or hospital stay. The “short term” was def ined as the mortality on day 28 or day 30. The secondary outcomes included mortality variables, the number of patients with shock reversal (stable hemodynamic status more than 24 hours after withdrawal of vasopressor therapy) within 28 days, and time to shock reversal.The safety outcomes included infection, gastrointestinal bleeding, and hyperglycemia. (5) Type of study. All trials included were RCTs, irrespective of language or publication status.
Data extraction and quality assessment
Characteristics of participants, study design, and outcomes for analyses were extracted following a standardized data extraction form by two reviewers independently. Two investigators independently assessed the risk of bias according to theCochrane Handbook for Systematic Review of Interventionto assign a value of“high”, “low”, or “unclear” for each trial.
We used the Grading of Recommendations Assessment,Development and Evaluation (GRADE) methodology to evaluate the quality of evidence associated with each major outcome and present the results in the summary of findings(SoFs) table.
Statistical analysis
All statistical analyses were performed on Review Manager 5.3 software and trial sequential analysis (TSA)v.0.9.5.10 beta.[23]We presented results as relative risk ratio(RR) for dichotomous data and mean difference (MD) for continuous data, which were pooled using the Mantel-Haenszel (M-H) and inverse variance method, respectively.BothRRandMDwere provided with 95% confidence interval (CI). Heterogeneity was assessed by the Chisquare test with significance set at aP-value of 0.05, and quantitatively by inconsistency (I2) statistics. We reported all results from a more conservative random-effect model taking into consideration clinical heterogeneity. Subgroup analyses were also performed for all outcomes based on the trial quality.
TSA
We performed TSA to assess the increased risk of random errors due to the relatively sparse data and repeated significance testing. The result was displayed on a TSA diagram with a TSA-adjustedCIand an adjusted level of statistical significance. TSA was used to appropriately reduce the risk of a wrong conclusion in a meta-analysis that did not achieve the required information size (RIS). TSA-adjustedCIwas calculated by the random-effect model for diversity (D2) with 5%risk of type I error and a power of 80%. For the estimate of the RIS, we set the intervention effect of a 15%relative risk reduction (RRR), and calculated the control event incidence from the conventional meta-analysis.
RESULTS
Study characteristics
Of the 4,034 records identified in our research, full texts of 207 records were reviewed, and 27 trials initially included were assessed for patients by immune status.Ultimately, nine RCTs were included in our systematic review.[24-32]The results of the search and selection flow diagram were shown in Figure 1. The detailed descriptions of the included trials were presented in Table 1. Nine RCTs with a total of 1,298 participants were finally analyzed, comprising 667 in the corticosteroid group and 631 in the control group.[24-32]
Mortality
The short-term mortality in the corticosteroid and the control groups was 43.8% (292/667) and 45.2%(285/631), respectively. The pooled analysis revealed no statistically signif icant effects of corticosteroids (RR0.95,95%CI0.85 to 1.06,P=0.37,I2=0%, TSA-adjustedCI0.83 to 1.09, moderate-certainty evidence) (Figures 2 and 3, Table 2). TSA withRRR15% produced an incidence of 45.1% and 38.3% in the control and corticosteroid groups, respectively. The cumulativeZ-curves crossed the futility area, which excluded an effect size of 15%RRRor larger (Figure 3).
For the long-term mortality, the pooled estimate ofRRfor 1-year mortality for corticosteroids compared with control was 0.96 (95%CI0.87 to 1.07,P=0.49,I2=0%, high-certainty evidence). Compared with placebo or the control group, corticosteroids lowered the 7-day mortality (RR0.68, 95%CI0.51 to 0.90,P<0.01,I2=0%, low-certainty evidence) in initial meta-analysis.However, the TSA-adjustedCIof the random-effect model was 0.39 to 1.16 without the TSA monitoring boundary being crossed, which was not statistically significant and indicated that the effect was uncertain.
Shock reversal
The conventional analysis revealed a statistically significant shortening of time to shock reversal in favor of corticosteroids (MD-21.56 hours, 95%CI -32.95 to -10.16,P<0.01,I2=0%, TSA-adjustedCI-33.33 to -9.78, moderatecertainty evidence). For shock reversal within 28 days, there was no significant difference between the corticosteroid group and the control group.
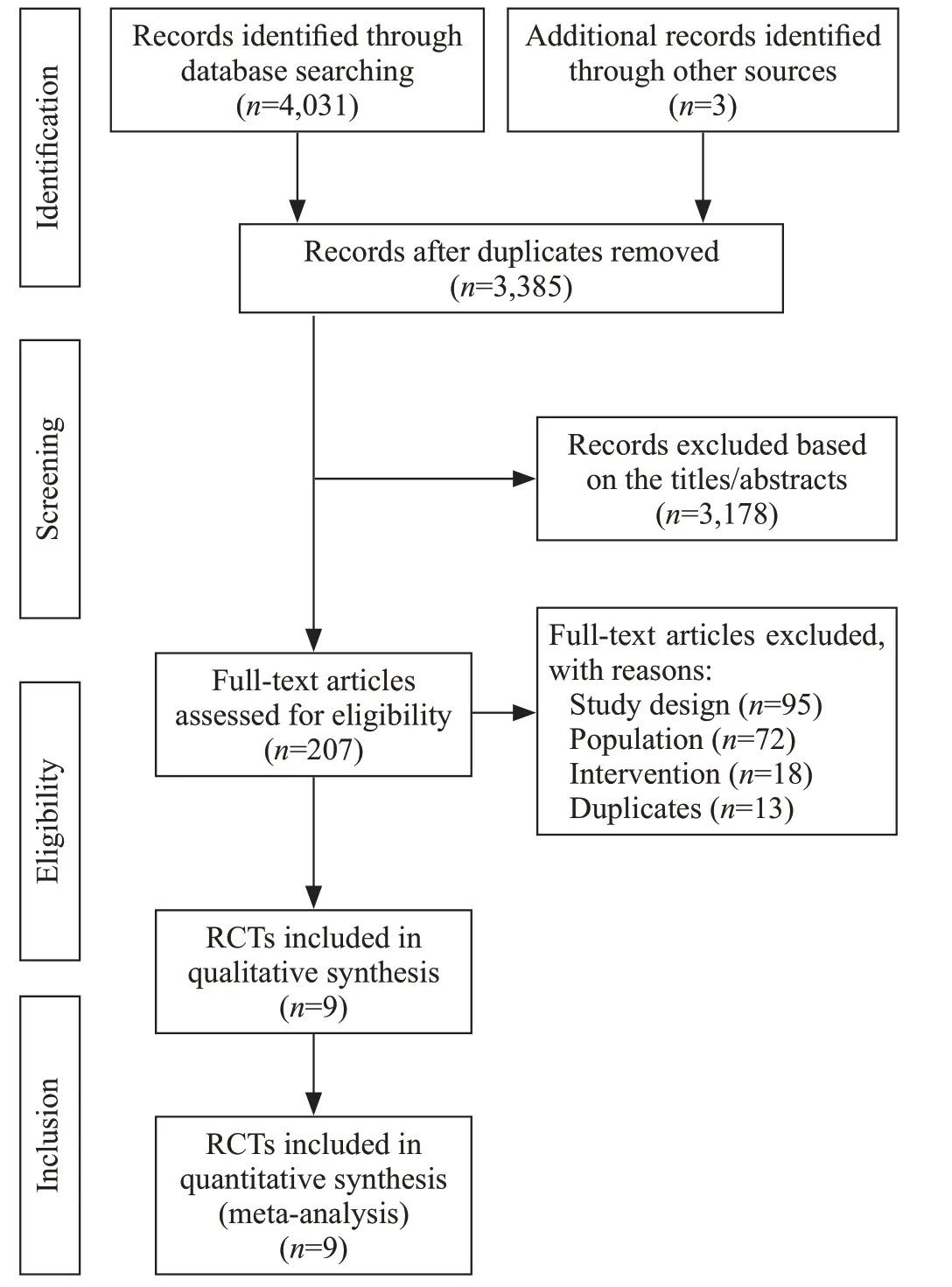
Figure 1. Flow diagram showing results of the search and selection of eligible studies. RCT: randomized controlled trial; study design: not RCT; population: no exclusion of immunosuppression or not septic shock; intervention: not corticosteroids.
Safety outcomes
Corticosteroids likely increased the rates of hyperglycemia (RR1.14, 95%CI1.03 to 1.27,P=0.01,I2=0%, TSA-adjustedCI1.00 to 1.30, moderate-certainty evidence). However, the side effects of corticosteroids on infection and gastrointestinal bleeding were not signif icant.
Subgroup analyses for outcomes based on trial quality
Subgroup analyses for outcomes were performed according to the risk of bias. The results did not demonstrate a beneficial effect of corticosteroids in reducing short-term mortality in the subgroup of high-quality trials (RR0.89,95%CI0.74 to 1.08,P=0.24;I2=0%). For other mortality outcomes, results from trials at the low risk of bias did not substantially differ from the results of all trials.
Study quality
There were two trials classified as low risk of bias,[29,30]four trials as unclear risk of bias,[26-28,32]and three trials as high risk of bias.[24,25,31]Due to the number of studies included in each analysis less than ten,publication bias was not evaluated.
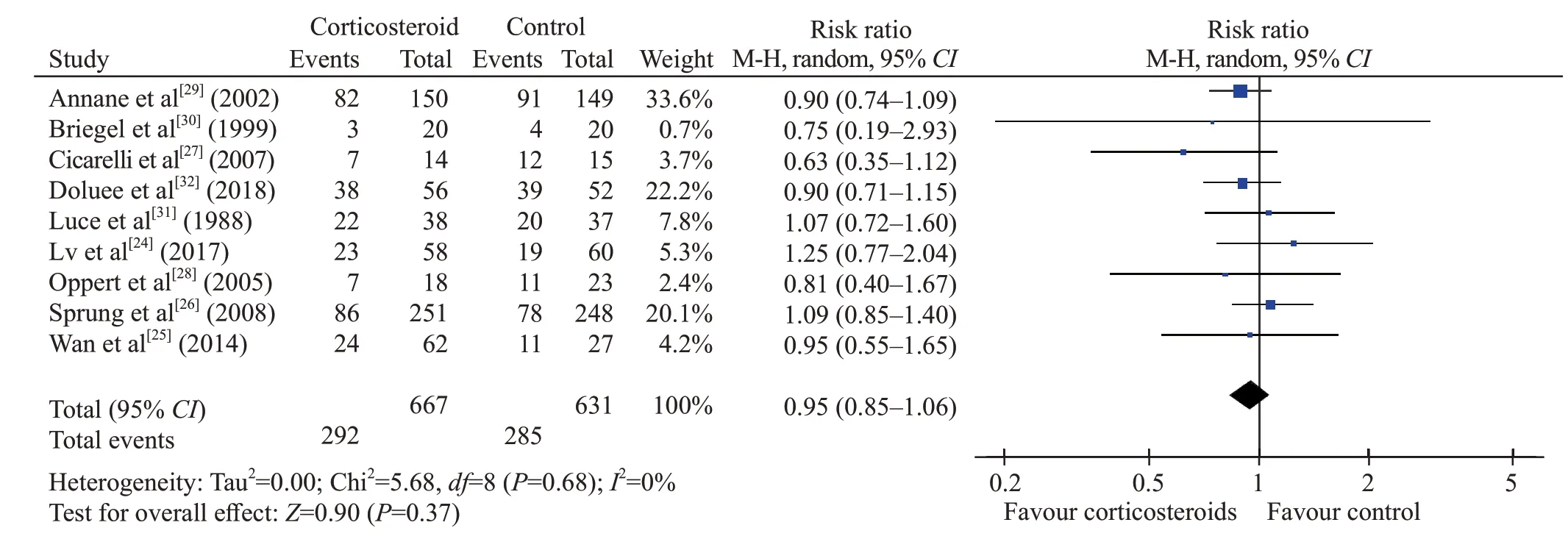
Figure 2. Forest plot of all trials for short-term mortality. CI: conf idence interval; M-H: Mantel-Hansen; df: degrees of freedom.
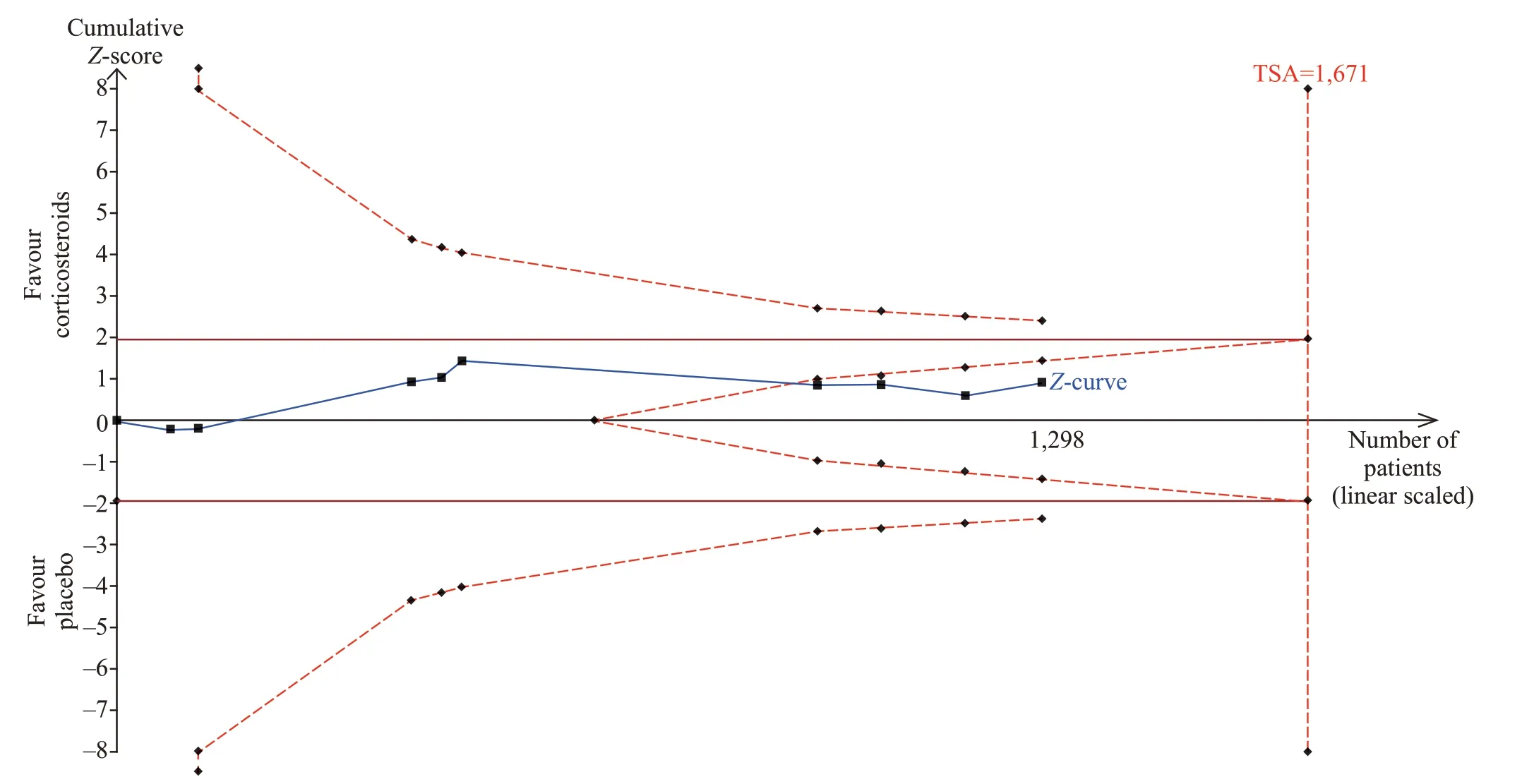
Figure 3. Trial sequential analysis of all trials for short-term mortality. TSA: trial sequential analysis. The required information size was 1,671 patients. The incidence in the control arm of 45.1% with a relative risk reduction of 15.0% produced an incidence of 38.3% in the corticosteroid group. The TSA-adjusted 95% conf idence interval for a relative risk of 0.95 was 0.83 to 1.09 and the cumulative Z-curves crossed futility area.
DISCUSSION
In this meta-analysis of nine RCTs with 1,298 patients with septic shock, we found no benefits of corticosteroids on either short-term mortality or longterm mortality. Our pooled analysis revealed that the administration of corticosteroids resulted in shorter time to shock reversal compared with the control group.
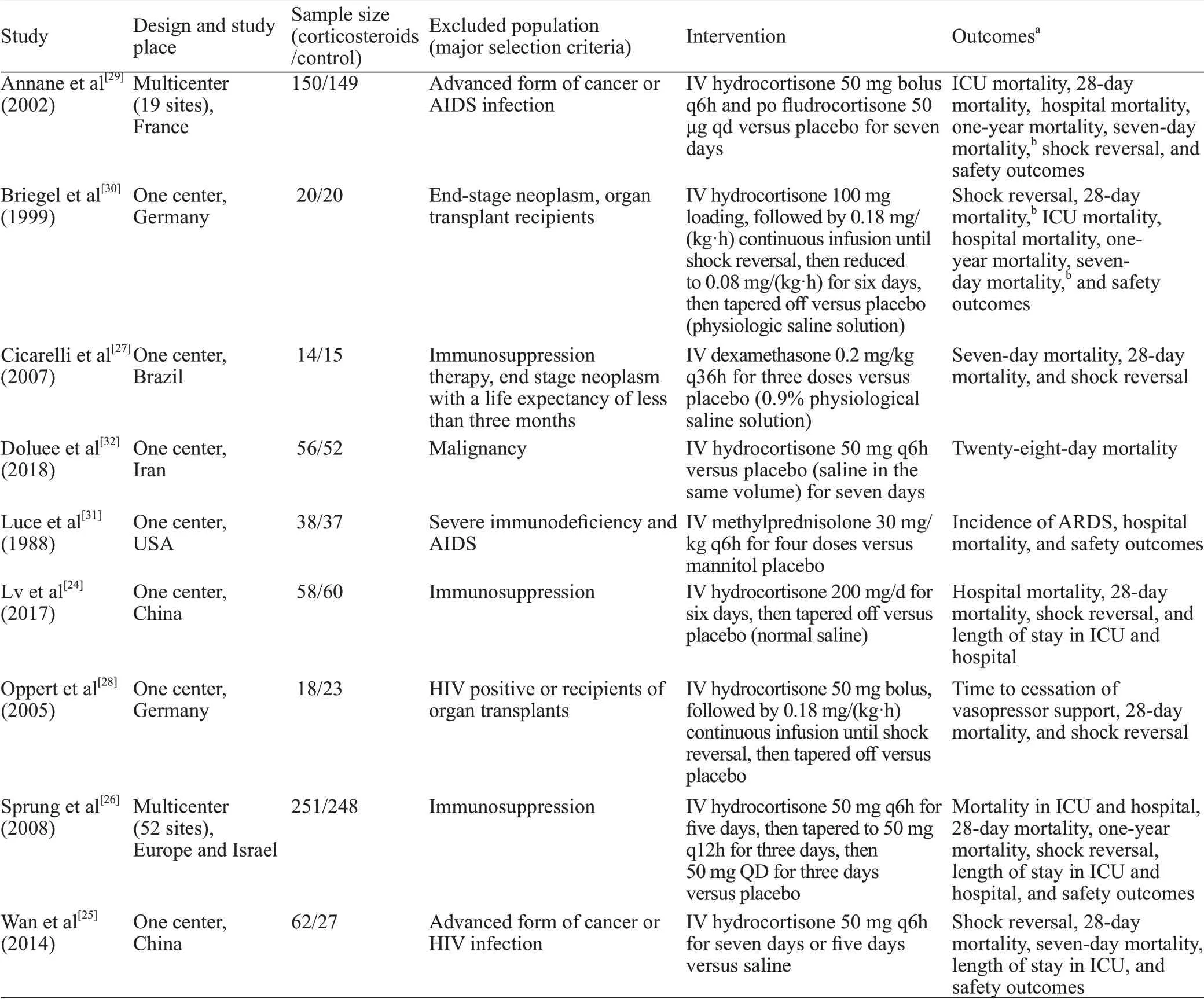
Table 1. Characteristics of included RCTs comparing corticosteroids versus control in immunocompetent patients with septic shock
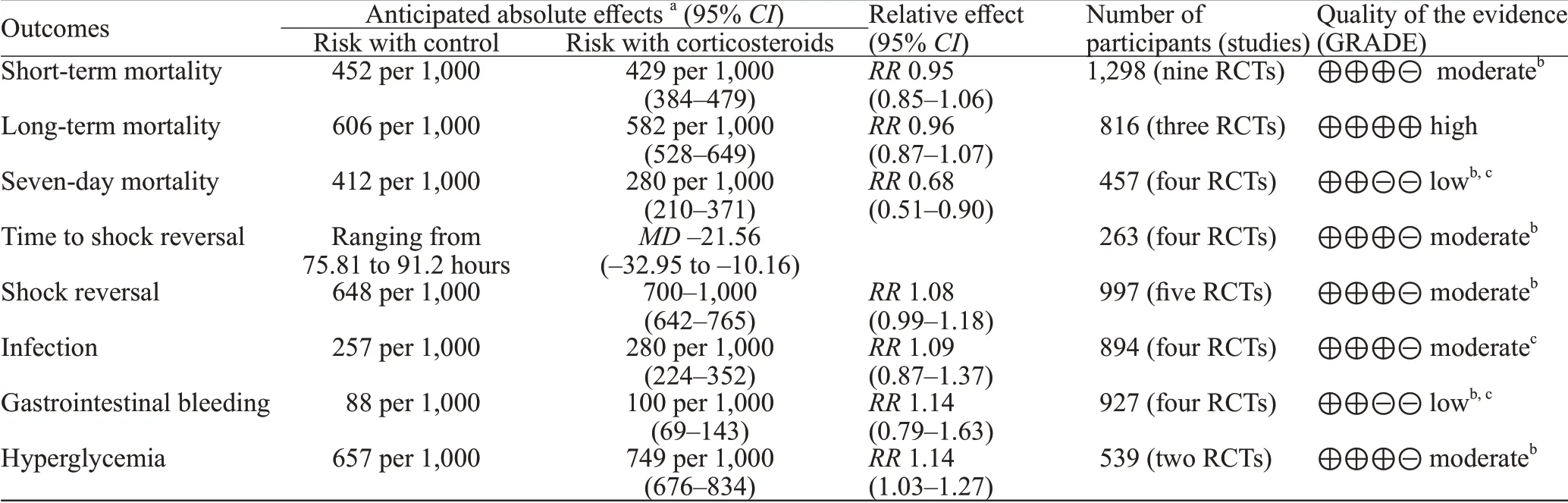
Table 2. Summary of f indings for all included RCTs (grading of recommendations assessment, development, and evaluation)
To the best of our knowledge, this is the first systematic review or meta-analysis to assess the efficacy and safety of corticosteroids in patients with septic shock based on the patients’ immune status. Previous reviews mainly enrolled patients with sepsis or septic shock and performed subgroup analyses based on the trial quality, the doses and regimens of corticosteroids,and the severity of diseases.[10-15]However, there was no differentiation or discussion of the immunological status of patients.
There is a consensus on the definitions for the immunocompetent state and the immunocompromised status: the former is usually defined as the exclusion of the latter. There were some variations in the def inition of“immunocompromised” in each of the aforementioned studies.
The mechanism of corticosteroids in septic shock may be its ability to down-regulate the proinf lammatory response.[33]However, the balance between the immune enhancement and suppression is highly dependent on the immune activation of the host as well as the dose and duration of corticosteroid therapy.[33,34]Immunocompetent patients may exhibit a profound hyper-inflammatory response followed by the cascade of events in the early stage of the disease, when the application of corticosteroids for control of systemic inflammatory response syndrome may be beneficial.The theory might partially account for the findings that corticosteroids significantly reduced the time to shock reversal. While signs of compensated anti-inf lammatory response syndrome may predominate in the whole stages of immunocompromised patients, the assignment of corticosteroids might strengthen the immunosuppression resulting in the accelerated deterioration of septic shock.[34,35]Although corticosteroids did not reduce the short-term and long-term mortalities in immunocompetent patients with septic shock, it was helpful in shock reversal without increasing the risk of infection. Given the findings, the administration of corticosteroids could be considered in immunocompetent patients suffering from septic shock to achieve hemodynamic stability.[36]
Our study has several limitations. Firstly, the systematic review was not registered in the International Prospective Register of Systematic Reviews (PROSPERO), and no protocol has been published. Secondly, we tried to contact the authors of included trials to gather data on immunocompetent persons, but many trials were excluded because of the lack of detailed data on the patients’ immune status. Thirdly, there were many“unclear” ratings for risk of bias assessments, although we attempted to contact trial authors to clarify these ambiguities.
CONCLUSIONS
Corticosteroid therapy is not associated with the lower short- or long-term mortalities compared with placebo in immunocompetent patients with septic shock.However, corticosteroids significantly shorten the time to shock reversal without increasing the risk of infection.The patient’s immune status should also be considered during clinical treatment and clinical trials in future.
Funding:This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2020-I2M-C&T-B-014),CAMS Teaching Reform Research Fund (2018zlgc0101), and CAMS Online Open Course Construction Fund (J2009022861).
Ethical approval:Not needed.
Conf licts of interests:The authors indicated no potential conf licts of interest.
Contributors:XL proposed and wrote the paper. All authors have reviewed and approved the final version of manuscript for publication.
 World journal of emergency medicine2021年2期
World journal of emergency medicine2021年2期
- World journal of emergency medicine的其它文章
- World Journal of Emergency Medicine
- Overlapping public health crises during the coronavirus disease pandemic
- Comparison of intraosseous access and central venous catheterization in Chinese adult emergency patients: A prospective, multicenter, and randomized study
- Empyema associated with vegetable foreign body aspiration
- A red herring: An unusual case of pneumothorax
- A case of a successful post-transcatheter aortic valve replacement His bundle pacing
