Role of urine studies in asymptomatic febrile neutropenic patients presenting to the emergency department
Hady Zgheib, Aline El Zakhem, Cynthia Wakil, Mohamad Ali Cheaito, Rola Cheaito, Antoine Finianos,Ralphe Bou Chebl, Rima Kaddoura, Nader Al Souky, Imad El Majzoub
1 Department of Emergency Medicine, American University of Beirut Medical Center, Beirut 11-0236, Lebanon
2 Department of Internal Medicine, American University of Beirut Medical Center, Beirut 11-0236, Lebanon
KEYWORDS: Adult; Cancer; Emergency department; Febrile neutropenia; Urine testing
INTRODUCTION
Febrile neutropenia (FN) is a life-thre atening condition and an oncologic emergency with overall mortality ranging from 5.0% to 9.5% in solid tumor patients and up to 11.0%to 14.0% in liquid tumor patients.[1-4]The infection, which is mostly bacterial, is the leading cause of death in febrile neutropenic patients.[5]In fact, the infection-related mortality is as high as 2.3% and 5.0% in solid and liquid tumor patients, respectively.[4]
Urinary tract infection (UTI) is identified in 5% to 30% of adult oncology patients with FN.[6-8]As opposed to gastrointestinal or respiratory infections, the clinical presentation of UTI can be subtle, including only fever,in the absence of any symptoms such as polyuria,dysuria, and/or urgency.[9]In light of low clinical suspicion, urine tests might not be obtained from patients within the emergency department (ED), and therefore UTI might be overlooked. In addition, the isolation rate of urinary pathogens in cancer patients is very low, partly due to the widespread use of prophylactic antimicrobial therapy.[10-12]Accordingly, a previous study supported the inclusion of urine studies, namely urinalysis (UA) and urine culture (UC), in the diagnostic workup of oncology patients presenting to the ED with FN,[9]while another study questioned their utility and cost-effectiveness.[13]
According to the 2010 clinical practice guidelines of the Infectious Diseases Society of America (IDSA)on FN in adult and pediatric patients, UC is indicated only if signs or symptoms of UTI are present (a urinary catheter is in place or UA results are abnormal).[3,14,15]Nonetheless, it is important to note that this recommendation is of level III evidence, given the absence of randomized controlled studies.[16,17]Additionally, the accuracy of UA findings in detecting UTI was reported to be limited in febrile neutropenic patients,[3]as their UA may display only a little or no pyuria at all given the reduction in neutrophil granulocytes.[18]Yet, specialists from Japan,the United States of America, and some European countries recommend urine testing in the diagnostic evaluation of any febrile neutropenic patients before administrating antibiotics.[19]Relevant prospective studies are particularly rare, of small sample size, or done on pediatric populations.[9,20]This topic remains a controversy in our clinical practice.
The study aims to assess the usefulness of urine studies in detecting UTI in adult cancer patients presenting to the ED with FN but having no urinary signs or symptoms.
METHODS
Study design and setting
This was a retrospective cohort study conducted on adult cancer patients who presented to the ED of the American University of Beirut Medical Center (AUBMC), between January 2013 and September 2018, with FN but without any urinary signs or symptoms. AUBMC is an over 350-bed tertiary care center and a major referral center in Lebanon and the region, receiving more than 55,000 ED visits annually.
Study population
We included all adult patients (>18 years) who presented to the ED of AUBMC with FN but without any urinary signs or symptoms and had their urine tested as part of ED diagnostic workup prior to admission.Only the f irst presentation for each patient was included.We excluded patients who were not admitted, received antibiotics (other than prophylactic antibiotics)within two weeks of presentation, or were clinically/hemodynamically unstable.
Statistical analysis
Descriptive and binariate statistics were conducted on the two groups (positive and negative UCs) with continuous variables presented as mean±standard deviation (SD) or medians and interquartile range (IQR) and categorical variables expressed as frequencies and percentages.Student’st-test and Wilcoxon rank-sum test were used for continuous data, while Chi-square and Fisher’s exact tests were used for categorical data. All tests were interpreted at alpha of 0.05.
The analysis was performed to determine the value of urine studies in diagnosing UTI in asymptomatic adult cancer patients with FN, with UC being considered as the golden diagnostic tool. Two cut-offs were used for UC positive results: ≥105cfu/mL and ≥104cfu/mL. The threshold of ≥105cfu/mL is widely accepted and agreed upon.[14]The other cut-off we used in our study (i.e., ≥104cfu/mL) was in accordance with evidence from studies that suggested the use of a lower threshold in a vulnerable population such as ours.[21]Although the threshold of ≥104cfu/mL is not acknowledged by all practicing physicians, a recent study has considered it in special clinical scenarios (fever, pyuria,bacteremia, etc.);[21]thus, we adopted it in an attempt to evaluate its value. The analysis was conducted using STATA MP Version 13.p (StataCorp LP, USA).
RESULTS
Characteristics of patients
A total of 924 patients were screened, and 284 patients were included in this study (Table 1). The mean age of our population was 48.5±18.5 years. Slightly less than half of the study populations were females (48.9%) with underlying malignancies almost equally distributed between hematological and solid malignancies (49.5% and 47.0%,respectively). Only 3.5% of patients had received stem cell transplants. More than two-thirds (68.7%) of the study population had profound neutropenia. Overall, the mean Charlson Comorbidity Index (CCI) was 3.7±2.1, and the median length of stay (LOS) was 4 days.
Only 11 patients (3.9%) had a positive UC at the cutoff ≥105cfu/mL, whereas 28 patients (9.9%) had a positive UC at the cut-off ≥104cfu/mL. Overall, patients with a positive UC were significantly older and were more likely to be females compared with patients with a negative UC.At the cut-off ≥105cfu/mL, patients with positive UCs were more likely to have solid tumors and profound neutropenia compared with patients with a negative UC. There was no significant difference in the CCI or LOS between both groups. At the cut-off ≥104cfu/mL, patients with positive UCs were more likely to have solid tumors, severe neutropenia, and a higher CCI compared with patients with a negative UC. They were almost equally likely to have profound neutropenia, and there was no significant difference in the LOS between the two groups. The most common organisms wereEscherichia coli(E. coli), followed byKlebsiella,Enterococcus,Lactobacillus,Proteus, andStaphylococcus aureus.
UA results
Patients with a positive UC were more likely to have positive UA findings (Table 2). For both cut-offs (≥105cfu/mL and ≥104cfu/mL), patients with a positive UC had higher rates of positive UA f indings of leukocyte esterase(LE) (36.4% vs. 7.0%,P=0.007 and 28.6% vs. 5.9%,P<0.001, respectively), nitrite (18.2% vs. 0.4%,P=0.004 and 7.1% vs. 0.4%,P=0.026, respectively), pyuria (45.5%vs. 12.8%,P=0.011 and 35.7% vs. 11.7%,P=0.001,respectively) and bacteriuria (63.6% vs. 15.8%,P=0.001 and 57.1% vs. 13.3%,P<0.001, respectively), than those with a negative UC. All in all, for both cut-offs, UC was mostly positive amongst patients with abnormal UA f indings (72.7% vs. 24.9%,P=0.002 and 67.9% vs. 22.3%,P<0.001, respectively).
On the other hand, UA was negative in 27.3% and 32.1% of patients with a positive UC at the cut-offs ≥105cfu/mL and ≥104cfu/mL, respectively. More specif ically,at cut-offs ≥105cfu/mL and ≥104cfu/mL, positive UC groups had no UA finding of LE in 63.6% and 71.4% of cases, nitrite in 81.8% and 92.9% of cases, pyuria in 54.5%and 64.3% of cases, and bacteriuria in 36.4% and 42.9%of cases, respectively. Moreover, bacteria were detected in the UA of 15.8% and 13.3% of patients with a negative UC at ≥105cfu/mL and ≥104cfu/mL, respectively.
UA sensitivity analysis for UTI diagnosis
To diagnose UTI at UC cut-offs ≥105cfu/mL and≥104cfu/mL, bacteriuria was found to be the most sensitive UA finding (63.6% and 57.1%, respectively), followed by pyuria (45.5% and 35.7%, respectively), LE (36.4% and 28.6%, respectively), and nitrite as least sensitive (18.2%and 7.1%, respectively) (Tables 3 and 4). Positive predictive value (PPV) was the highest for nitrite (66.7%) followed by LE (17.4% and 34.8%, respectively), bacteriuria (14.0%and 32.0%, respectively), and pyuria (12.5% and 25.0%,respectively). UA was found to be 72.7% sensitive and 75.1% specific in diagnosing UTI in febrile neutropenic adults at the UC cut-off ≥105cfu/mL, and 67.9% sensitive and 77.7% specific at the UC cut-off ≥104cfu/mL, with PPVs of 10.5% and 25.0%, respectively.
DISCUSSION
FN is a medical emergency in oncology patients.International guidelines have put forth specif ic protocols for therapy and basic workup at the initial evaluation of these patients.[3,14]Urine testing, although commonly performed, is not well-validated, as there is ambiguity regarding its utility as a routine investigation.
Our study, to the best of our knowledge, is the largest in the region to evaluate the utility of urine studies in asymptomatic (i.e., no urinary signs or symptoms) adult oncology patients presenting with FN to the ED.
In this study, UC was positive in only 3.9% of patients at a cut-off ≥105cfu/mL and 9.9% at a cut-off ≥104cfu/mL. This low positive culture rate was not surprising but rather consistent with f indings from previous studies, where infections were reportedly documented in only 20%-30%of all FN episodes,[13]and the rate of UTI ranged between 5% and 30% in oncology patients with FN.[6-9,13]Moreover,the low rate of UTI in this patient population can be further attributed to the rarity of a typical clinical picture of UTI leading to a missed diagnosis in many occasions.
One study on adult cancer patients with FN showed that urine studies were more likely to be positive in symptomatic episodes compared with asymptomatic episodes (relative risk [RR]=7.4,P<0.001).[22]Nevertheless,other studies reported higher rates of documented UTI (18.5% to 47.0%) in FN patients without signs or symptoms suggestive of UTI.[13,23]As a matter of fact, in one of those studies, only 2.2% of the patients presented with urinary symptoms, and none of those had signif icant bacteriuria.[13]
Herein, we conclude that the incidence of UTI in adult cancer patients with FN is low due to a constellation of factors. We also conclude that the presence of signs or symptoms of UTI may or may not be associated with signif icant bacteriuria and is thus an unreliable parameter.[9,13]
In our study, patients with positive UCs were found to be significantly older and more likely to be females compared with patients with negative UCs. This is in line with f indings in the general population, where the prevalence of asymptomatic bacteriuria is known to increase with age and female gender.[24]These f indings are also coherent with results from previous studies on febrile neutropenic oncology patients, showing that the majority of patients with UTI were females.[6,9]
UCs were more likely to be positive in patients with abnormal UA findings. In our study, more than two-thirds of patients with positive UCs had a positive UA. A previous study reported an abnormal UA in 14.5% of patients with a positive UC and severe neutropenia,[13]and 43.0% of patients with a positive UC and moderate to severe neutropenia.[22]The rates reported in our study are similar to those of a study on pediatric oncology patients with confirmed UTI, where 69% of UA samples were abnormal, and 85% had an absolute neutrophil count (ANC) >500 cells/mm3.[23]These differences in the presence of a UA abnormality may be attributed to the severity of neutropenia as well as the urine collection technique; a higher ANC and bladder catheterization are signif icantly associated with the presence of pyuria.[18]
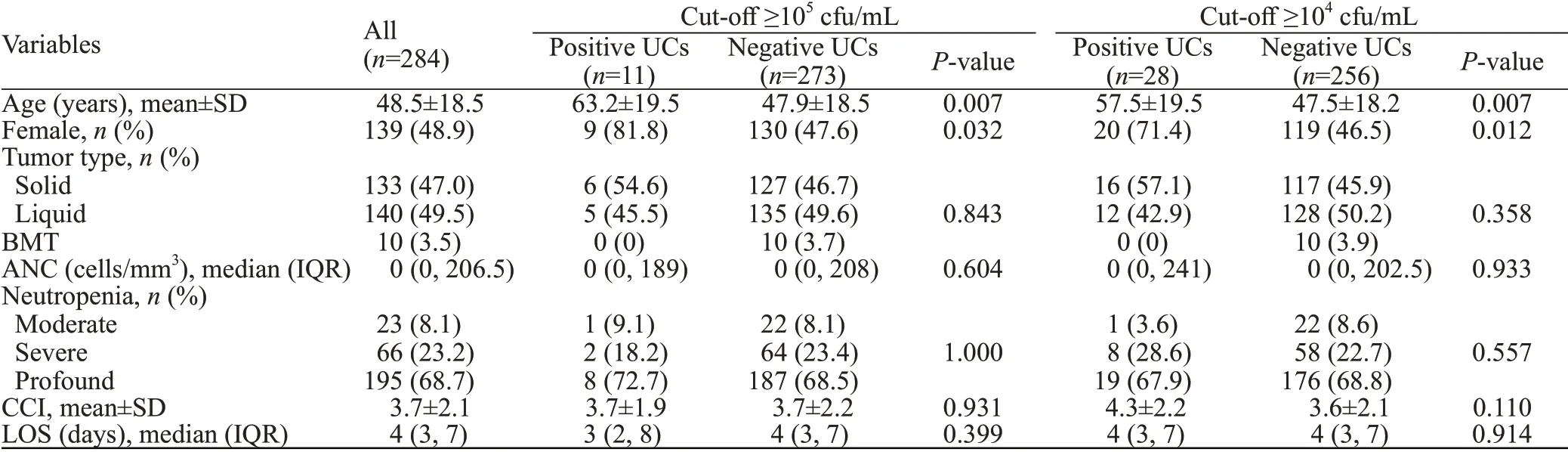
Table 1. Baseline characteristics of patients with positive and negative urine cultures at cut-offs ≥105 cfu/mL and ≥104 cfu/mL
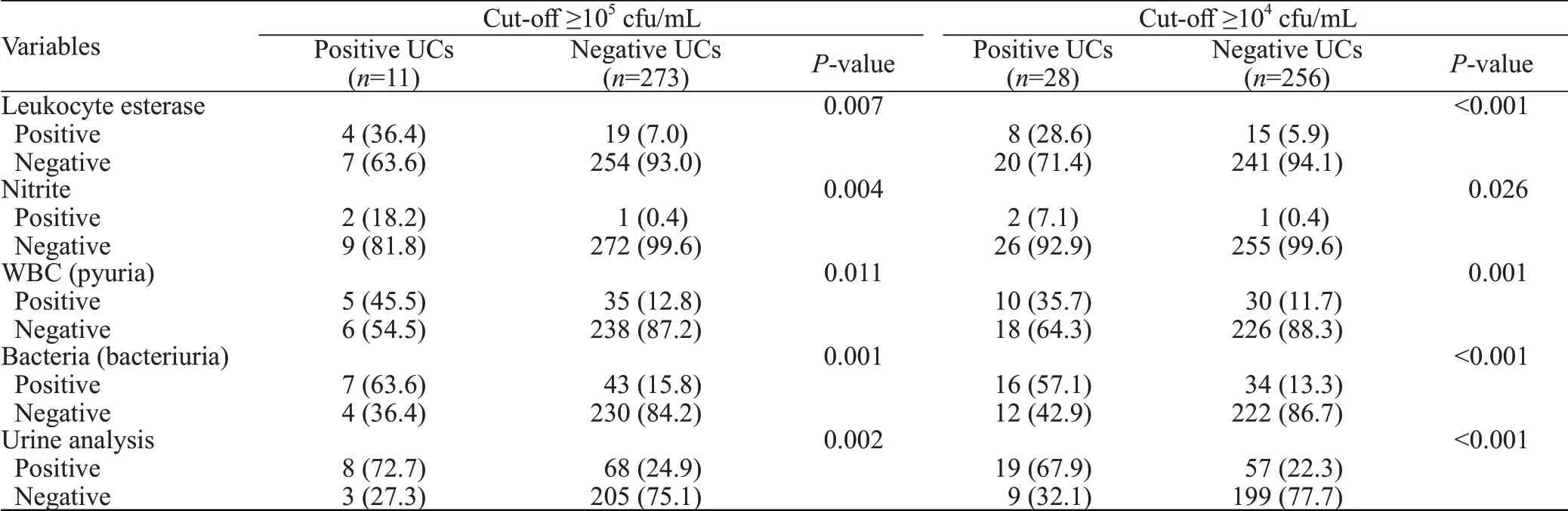
Table 2. UA results of patients with positive and negative urine cultures at cut-offs ≥105 cfu/mL and ≥104 cfu/mL, n (%)
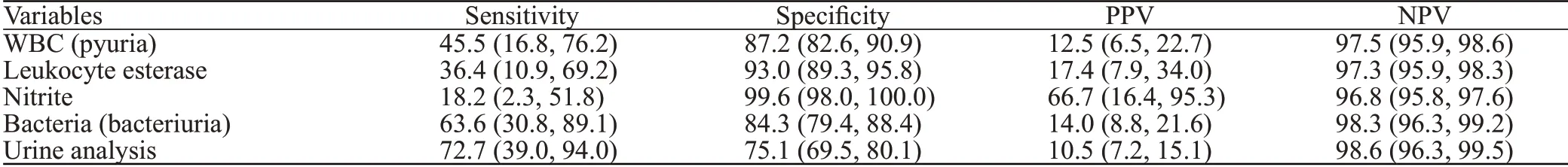
Table 3. Diagnostic performance of urinalysis f indings at a urine culture cut-off ≥105 cfu/mL, % (95% CI)
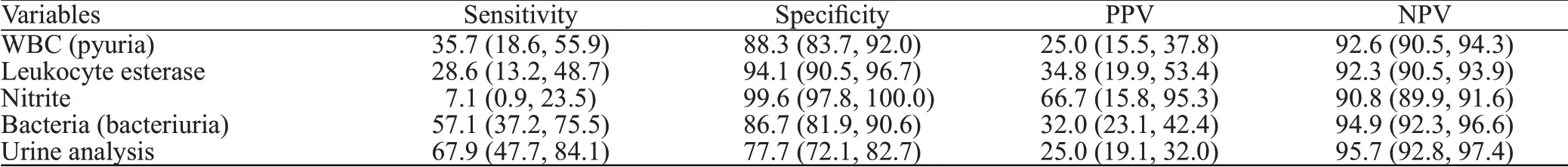
Table 4. Diagnostic performance of urinalysis f indings at a urine culture cut-off ≥104 cfu/mL, % (95% CI)
Moreover, in healthy patients, a negative UA result generally has a high negative predictive value (NPV). In our study, however, almost one-third of UTI patients had normal UA f indings. UA was negative in 27.3% and 32.1%of patients with a positive UC at the cut-offs ≥105cfu/mL and ≥ 104cfu/mL, respectively. Hence, negative UA f indings should be interpreted with caution in febrile neutropenic patients due to the high false negative rates.
The most common UA abnormality in our study was bacteriuria. This was similarly described in a previous study on adult patients with FN.[13]Here, it is worthwhile to note that 13.3% to 15.8% of negative UCs in our study were found to be associated with bacteriuria. Consequently, it might be argued that signif icant bacteriuria in the absence of pyuria ref lects contamination, particularly in patients where urine was not collected by catheterization.[18]
It is well-known that patients with FN have leukopenia and a depressed inflammatory response,limiting the number of white blood cells (WBCs)excreted into the urine. LE is generally produced by neutrophils and may signal the presence of urine WBCs in patients with UTI.[25]Klaassen et al[18]reported the presence of pyuria in 4% of neutropenic children with UTI as compared with 68% of non-neutropenic children.Likewise, in a study on pediatric oncology patients with confirmed UTI, pyuria and LE were reported in 39%and 51% of all samples but only in 15% and 23% of neutropenic patients’ samples, respectively.[23]For this reason, findings of pyuria and LE may be difficult to interpret in a neutropenic patient as more than half of the patients with UTI may show no pyuria or LE.
The presence of nitrite was the least sensitive UA finding for the diagnosis of UTI in our study, followed by LE, pyuria, and bacteriuria. Similar f indings were conveyed in a previous study done on pediatric cancer patients, where pyuria had a higher sensitivity (80.0%) compared with nitrite(60.0%).[26]Additionally, the sensitivities of UA findings in our study population seemed to be lower compared with the general population. In fact, the sensitivity of LE was 28.6% (UC cut-off ≥104cfu/mL) and 36.4% (UC cut-off≥105cfu/mL) compared with 72.4% to 77.0% in the general population,[27]nitrite were found to be sensitive at 7.1%to 18.2% compared with 16.1% to 19.9% in the general population,[27]and pyuria was sensitive at 35.7% to 45.5%compared with 84.0% to 84.4% in general population.[28]Therefore, we can conclude that neutropenia affects the sensitivity of UA f indings in predicting UTI.
Although nitrite was found to be the least sensitive,the presence of nitrite had a high PPV (66.7%). A positive nitrite test serves as a strong predictor of UTI but needs to be conf irmed through a positive UC. The presence of nitrite was also the most specif ic f inding (99.6%). However, in view of sensitivity, the nitrite test alone cannot be used to rule out UTI. In fact, even in the general population, a nitrite test may be negative if the causative organism is not nitrate-reducing(e.g.,Enterococci,S. saprophyticus,Acinetobacter).[27]In contrast, the PPV of LE (17.4% to 34.8%) and WBC(12.5% to 25.0%) in urine was comparatively lower than that of nitrite, similar to reports by Grigg et al.[22]This further consolidates that UA findings of pyuria and LE are less accurate markers in neutropenic patients.
In our study, at a UC cut-off ≥105cfu/mL, UA was 72.7% sensitive and 75.1% specific for the diagnosis of UTI. At a UC cut-off ≥104cfu/mL, sensitivity decreased to 67.9% and specificity increased to 77.7%. Lowering the cut-off increased the PPV from 10.5% to 25.0% with a small decrease from 98.6% to 95.7% in NPV. UA findings of bacteria, nitrite, LE, and pyuria were all less sensitive but more specific. As such, a positive UA result would be interpreted more accurately as signif icant bacteriuria at a UC cut-off ≥104cfu/mL.
Limitations
The results of our study should be considered its limitations. First, this study was single centered with small sample size (a total of 39 positive UCs at both cut-offs).This could affect the external validity and generalizability of our results to other patient populations. Second, it was retrospective in nature and was thus associated with resource constraints and data unavailability, including data on the method of urine specimen collection (clean catch vs. bladder catheterization) and the time a urine specimen was sampled with respect to the time of antibiotics initiation. Third, data on hematuria and transient proteinuria were not collected,although an association between those and UTI had been established.[27,29]
CONCLUSIONS
The incidence of UTI in adult cancer patients with FN is low. The presence of signs or symptoms of UTI may or may not be associated with significant bacteriuria and is thus an unreliable parameter. Pyuria and LE have limited sensitivities in detecting UTI in febrile neutropenic patients.Additionally, a positive UC in cancer patients with FN and without localizing signs or symptoms of UTI may not be associated with UA abnormalities. Therefore, a routine urine test is often unwarranted and inefficient in diagnosing UTI in this population. Prospective large-scale studies are needed to conf irm our results. Current recommendations suggesting a pivotal role of urine studies in the initial workup of these patients can be revised.
Funding:This study did not receive any funding.
Ethical approval:Ethical approval was obtained from the Institutional Review Board at AUBMC under the protocol number(BIO-2018-0455).
Conflicts of interests:The authors declare that they have no competing interests.
Contributors:HZ determined the concept of the study and was a major contributor to the study design, data analysis, interpretation and manuscript production. All authors read and approved the f inal manuscript.
 World journal of emergency medicine2021年2期
World journal of emergency medicine2021年2期
- World journal of emergency medicine的其它文章
- World Journal of Emergency Medicine
- Overlapping public health crises during the coronavirus disease pandemic
- Comparison of intraosseous access and central venous catheterization in Chinese adult emergency patients: A prospective, multicenter, and randomized study
- Empyema associated with vegetable foreign body aspiration
- A red herring: An unusual case of pneumothorax
- A case of a successful post-transcatheter aortic valve replacement His bundle pacing
