Racial disparities in immune-related adverse events of immune checkpoint inhibitors and association with survival based on clinical and biochemical responses
Monica Peravali, Cristiane Gomes-Lima, Eshetu Tefera, Mairead Baker, Mamta Sherchan, Saira Farid,Kenneth Burman, Florina Constantinescu, Irina Veytsman
Monica Peravali, Irina Veytsman, Department of Hematology/Oncology, MedStar Washington Hospital Center, Washington, DC 20010, United States
Cristiane Gomes-Lima, Kenneth Burman, Department of Endocrinology, MedStar Washington Hospital Center, Washington, DC 20010, United States
Eshetu Tefera, Department of Biostatistics and Bioinformatics, MedStar Health Research Institute, Washington, DC 20010, United States
Mairead Baker, Saira Farid, Department of Internal Medicine, MedStar Washington Hospital Center, Washington, DC 20010, United States
Mamta Sherchan, Florina Constantinescu, Department of Rheumatology, MedStar Washington Hospital Center, Washington, DC 20010, United States
Abstract BACKGROUND Immune checkpoint inhibitors (ICPi) cause various immune-related adverse events (irAE) with thyroid dysfunction as a commonly reported abnormality.There is increasing evidence showing positive association with development of irAE and survival. However, prior trials with ICPi had underrepresentation of minorities with < 5% African Americans.AIM To evaluate the association between development of irAE and survival outcomes among a racially diverse patient population.METHODS Data on patients with stage IV solid malignancies treated with programmed cell death-protein 1/programmed death ligand 1 blockers between January 2013 and December 2018 across MedStar Georgetown Cancer Institute facilities were retrospectively reviewed. Patients treated with cytotoxic T-lymphocyte-associated protein 4 inhibitors were excluded. Progression free survival (PFS) and overall survival (OS) were primary endpoints and were calculated using Kaplan-Meier methods and Wilcoxon rank sum test for comparison.RESULTS Out of 293 patients who met eligibility criteria, 91 pts (31%) had any grade irAE;most common AE were endocrine (40.7%) specifically TSH elevation,dermatological (23.1%) and rheumatologic (18.7%). Proportion of irAE was significantly higher in Caucasians vs African Americans (60.4% vs 30.8%), in patients with low programmed death ligand 1, lower LDH, older age, and those who had more treatment cycles with ICPi. Rate of progression was lower in patients with irAE (30.8% vs 46.0%, P = 0.0140). Median PFS (5.8 vs 3.0 mo, P =0.0204) and OS (17.1 vs 7.2 mo, P < 0.0001) were higher with irAE. Statistically significant difference in OS (17.1 vs 8.6 mo, P = 0.0002) but not in PFS (5.8 vs 3.3 mo, P = 0.0545) was noted with endocrine irAE. No differences in survival were observed among other commonly reported irAE. Differences in survival among subgroups of patients with irAE are described.CONCLUSION Development of irAE positively correlated with improved PFS and OS as reported in previous studies. To our knowledge, this is the first study observing differences in OS favoring endocrine AE and Caucasian race. These factors may be potential surrogate markers of prognosis pending replication of these results in large-scale studies.
Key Words: Immunotherapy; Adverse events; Endocrine; Survival; Race; Minority
INTRODUCTION
Emergence of immune checkpoint inhibitors (ICPi) has revolutionized standard of care treatment in a wide spectrum of cancer subtypes resulting in increased survival[1,2].These agents up regulate activity of the immune system by blocking intrinsic down regulators of immunity such as cytotoxic T-lymphocyte-associated protein 4 and programmed cell death-protein 1 (PD-1)[1]. This amplified immune activity can also result in off target autoimmune inflammation of healthy organs resulting in immunerelated adverse effects (irAE)[1,3].
Various mechanisms contribute to the irAE affecting several organ systems, with effect on the endocrine glands noted as one of the most common and often irreversible irAE[1,4-7]. Correlation with the development of these adverse effects on outcomes is controversial, however, there is a growing body of evidence that the development of these adverse events may be associated with improved outcomes[1,8-13].
In this retrospective analysis, we aim to describe the association of development of irAEs to survival outcomes in patients treated with ICPi for advanced stage solid malignancies. We also specifically underscore the effects of endocrine irAEs on survival. More importantly, we sought to address differences in survival outcomes across different racial groups given the significant underrepresentation of minority racial groups in pivotal immunotherapy trials[14].
We hypothesize that development of irAEs will have a positive correlation with progression free survival (PFS) and overall survival (OS) in patients treated with ICPi.We also anticipate that there will be differences in outcomes among various racial groups. There is very limited data on efficacy outcomes in racially diverse patients who receive immunotherapy and develop irAEs making our study unique and clinically relevant.
MATERIALS AND METHODS
This is a retrospective analysis of patients with a diagnosis of stage IV solid malignancy who were treated with PD-1 or programmed death ligand 1 (PDL1)blocker between January 1, 2013 and December 31, 2018 across MedStar Georgetown Cancer Institute facilities. This study was approved by the MedStar institutional review board and a waiver of HIPAA authorization was obtained given its retrospective nature.
Patients
All patients with stage IV solid malignancy in follow-up from January 1, 2013 to December 31, 2018 across MedStar Georgetown Cancer Institute facilities were identified using ICD-10 diagnostic codes. The electronic medical records were reviewed to identify patients who have been initiated on an ICPi with pembrolizumab,atezolizumab, durvalumab, or nivolumab. Patients with hematological malignancies,early-stage solid malignancies and those treated in combination with cytotoxic Tlymphocyte-associated protein 4 inhibitors, such as ipilimumab, were excluded.
Pertinent data collected from the records included age at the time of ICPi initiation,gender, race which was self-identified by patients, date of metastatic disease diagnosis,type of cancer, sites of metastasis, line of treatment, molecular markers, percentage of PDL1 expression, date of start of ICPi, date of first reported irAE, type of irAE, severity of irAE based on Common Terminology Criteria for Adverse Events Version 5.0[15],systemic steroid use, date of death or date of last follow up.
Endpoints
Differences in median PFS and OS and probability of survival among patients who had irAEvsthose without irAE were primary endpoints. Data were further stratified based on age, gender, race, PDL1 expression, line of therapy, median number of cycles and LDH. Secondary endpoints included difference in median PFS and OS and probability of survival among those with endocrine irAE. PFS was defined as the time from initiation of ICPi therapy to radiographic progression. OS was defined as the time from initiation of ICPi therapy to date of death or last follow up.
Statistics
Descriptive statistics were used to summarize demographics, PDL1 expression, line of therapy, number of cycles of immunotherapy. Continuous variables were described by means with standard deviations and medians and first and third quartiles. Twosamplet-test was used to examine differences in the averages between the groups if normality assumption was satisfied and the non-parametric Wilcoxon rank sum test was used when normality assumption was not satisfied. Categorical variables were described by frequencies and column percentages, and Chi-square and Fisher exact tests as appropriate were used to investigate differences between the groups. Kaplan-Meier curves were provided for overall and progression free survival and log rank test was used to compare the curves between the two groups. Statistical significance was defined asP< 0.05.
RESULTS
Patients
464 patients with stage IV solid malignancies were selected for review, and 293 patients met eligibility criteria. 91 patients (31%) had any grade irAE; most common AE were endocrine (40.7%), specifically TSH elevation, dermatological (23.1%) and rheumatologic (18.7%). Patient characteristics and incidence of irAE are described in detail in Tables 1-3. Proportion of irAE was significantly higher in CaucasiansvsAfrican Americans (AA) at 60.4% and 30.8% respectively (P= 0.01). Mean age of patients at initiation of ICPi was 63 years ± 12.8. The incidence of irAE was significantly higher in older patients, with a mean age of 66 years ± 13.2 in comparison to a mean age of 62 years ± 12.4 in those without irAE (P= 0.0108). PDL1 positivity,defined as greater than or equal to 1%, was observed in 71 patients while PDL1 negativity was observed in 70 patients; 192 patients had missing PDL1 status. The incidence of irAE did not differ between the two groups (P= 0.07). However, when these patients were further stratified to PDL1 status < 50% (n= 69) or ≥ 50% (n= 32),there was a statistically significant increase in incidence of irAE in the low PDL1 positive group at 17.6%vs15.4% (P= 0.03). Statistically significant relationship was found between number of cycles of immunotherapy and irAE. The median number of cycles of immunotherapy in patients who had irAE is higher than patients who had no irAE at 8 and 4 respectively (P= 0.001). Median LDH was also lower at 190 in patients with irAEvsthose who did not at 253 (P= 0.04).
Primary endpoints
Rate of progression was lower in patients with irAE at 30.8%vs46.0%, (P =0.01).Median PFS was higher at 5.8 mo in patients with irAEvsthose without at 3.0 mo (P=0.02). Median OS was also higher in those with irAEvsno irAE at 17.1 and 7.2 mo respectively (P< 0.0001). The probability of PFS and OS was statistically significant (P= 0.0088 andP= 0.0004), respectively, based on Kaplan-Meier curves (Figure 1).Statistical significance between groups was defined asP< 0.05.
Secondary endpoints
Statistically significant difference in median OS (17.1vs8.6 mo,P= 0.0002) but not in PFS (5.8vs3.3 mo,P= 0.0545) was noted with endocrine irAE based on Wilcoxon rank sum test. However, these differences were not seen on Kaplan-Meier analysis(Figure 2). No differences in survival were observed among other commonly reported irAE. Differences in survival among subgroups of patients with irAE and endocrine irAE are detailed in Table 4. Median OS was higher with Caucasian racevsAA in patients with irAE (20.6vs12.9 mo,P= 0.0237) and in those who specifically had endocrine irAEs (21.8vs15.8 mo,P= 0.0356). However, once again these did not translate to better survival probability on Kaplan-Meijer analysis (Figure 3).
DISCUSSION
Development of irAE in patients treated with ICPi is not uncommon and is an offtarget effect of increased immune activity[1]. Several studies done in specific tumor types using various anti-PD-1 and anti-PDL1 agents confirmed the association of irAE development with improved outcomes[8-12,16]. Our study similarly concluded a positive correlation of irAE development with increased probability of survival. The inclusion of a racially diverse population of patients in our study is unique and has not been previously reported to the best of our knowledge. Moreover, we also noted important associations with development of endocrine irAE with survival outcomes.
Median PFS and OS were significantly improved in patients who developed irAE in our study. Moreover, positive correlation in median OS was also seen in patients who had endocrine adverse events. A study by Grangeonet al[17]assessing similar survival endpoints in patients with advanced stage non-small cell lung cancer (NSCLC)concluded improved objective response rates, PFS and OS in patients who received ICPi therapy. Moreover, in patients who had thyroid dysfunction PFS and OS were significantly improved. Other studies reported similar outcomes in patients with NSCLC who had thyroid AE following treatment with immunotherapy[9,10,18]. A preliminary study from our group also addressed this issue with a systematic literature review and meta-analysis of patients with lung cancer and head and neck cancer on immune checkpoint inhibitor therapy across twelve randomized-controlled trials comprising of 7060 patients with 3815 patients using ICPi[19]. In that study, at a mean follow-up of 12.2 mo, the OS (hazard ratio: 0.75; 95% credibility interval: 0.70-0.80) and PFS (hazard ratio: 0.77; 95% credibility interval: 0.72-0.81) in the ICPi arm were significantly improved. Endocrine irAE, specifically hypothyroidism, was the most common adverse event with a prevalence of 9% (P= 0.019). However, none of these studies accounted for differences among various racial groups and outcomes in all cancer subtypes is sparse.

Table 1 Patient characteristics
Minority patient enrollment, specifically African American patients, in immunotherapy trials across various tumor types was less than 5%[14]. This raises an important gap in the understanding of clinical outcomes in these patients with the use of ICPi. A study done in 136 metastatic NSCLC patients who received immunotherapy at our institutions did not show a difference in efficacy or safety among Caucasians and AA races (unpublished results). Out of the 293 patients in our study with various stage IV solid malignancies, 41.6% of patients were AA. The incidence of irAE was significantly higher in CaucasiansvsAA 60.4% and 30.8% respectively,P= 0.0141.Moreover, a higher median OS was also seen with Caucasian racevsAA in patients with irAE (20.6vs12.9 mo,P= 0.0237) and in those with endocrine irAEs (21.8vs15.8 mo,P= 0.0356). Although this did not translate to a better probability of survival, our study adds important real-world data in this under-represented group of patients.
A major limitation to our study is the significant heterogeneity in baseline patient characteristics including tumor subtype, type of immunotherapy used, and line of treatment. Despite these limitations, our study confirmed our primary endpoint of improved survival outcomes in patients who develop irAE during treatment. In other subgroups of interest such as Caucasian race and patients with endocrine irAE, there was a positive correlation with median OS however this was not translated to increased rate of survival due to discordant results between the two statistical tests.Prospective studies in similar patients with pre-specified multivariate analyses accounting for tumor type, type of ICPi and line of treatment may further elicit important patterns and could lead to definitive conclusions in these subgroups.
CONCLUSION
Development of irAEs improves PFS and OS in patients with advanced stage solid malignancies treated with ICPi. Our study also underscored two important factors,race and endocrine irAEs, as possible surrogate markers for improved survival given the noted positive correlation with median overall survival.

Table 2 Patient characteristics

Table 3 Patient characteristics
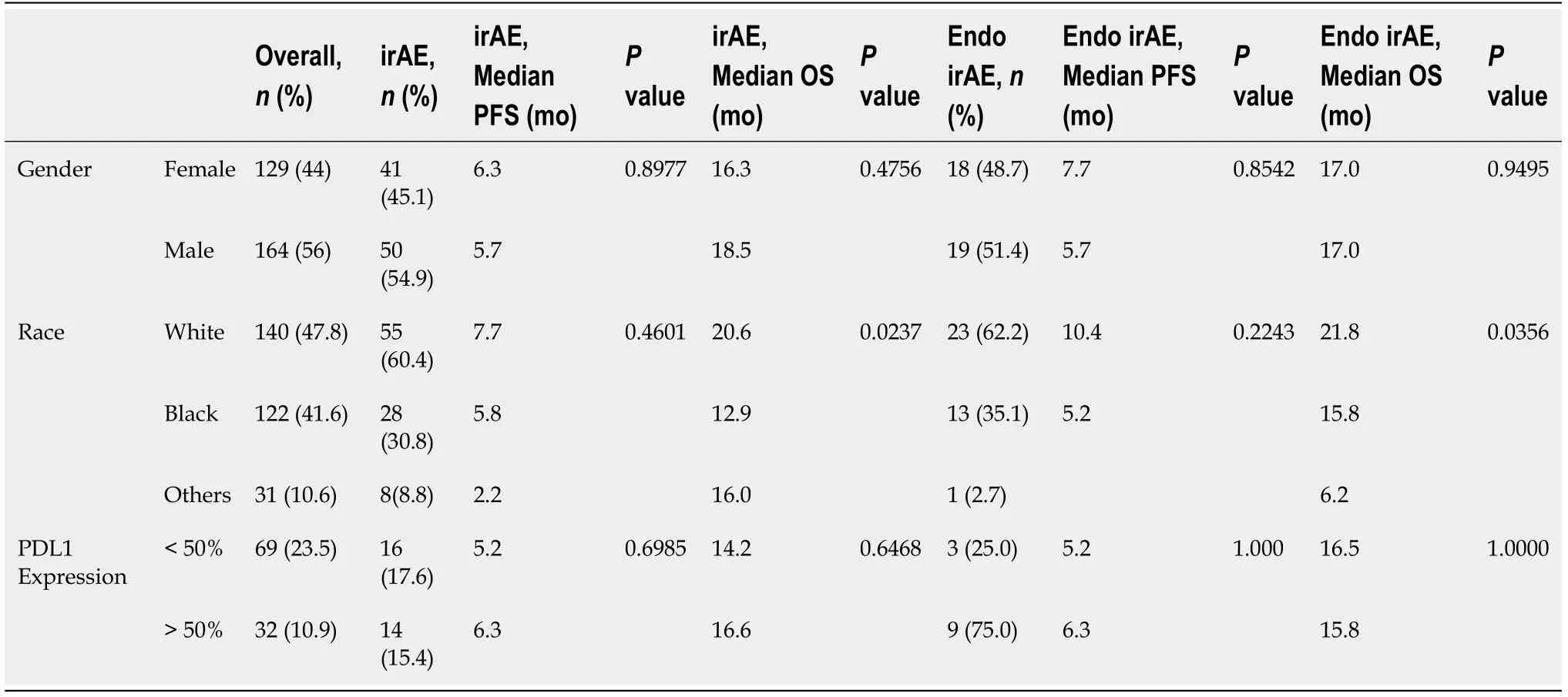
Table 4 Progression free survival and overall survival calculated using Wilcoxon rank sum test for comparison

Figure 1 Kaplan-Meier curves. Progression free survival (A) and overall survival (B) of immune-related adverse events vs no immune-related adverse events using Kaplan-Meier methods with log-rank test for P value. irAE: Immune-related adverse events.

Figure 2 These differences were not seen on Kaplan-Meier analysis. Progression free survival (A) and overall survival (B) of endocrine immune-related adverse events vs no endocrine immune-related adverse events using Kaplan-Meier methods. irAE: Immune-related adverse events.

Figure 3 These did not translate to better survival probability on Kaplan-Meijer analysis. Progression free survival (A) and overall survival (B) based on race using Kaplan-Meier methods.
ARTICLE HIGHLIGHTS
Research background
Immune checkpoint inhibitors (ICPi) constitute a revolutionary therapy for various types of cancer. However, their use is associated with the development of several immune-related adverse events (irAEs), especially related to the endocrine system.
Research motivation
Recently, some studies have suggested that the development of irAEs could result in improved survival outcome.
Research objectives
This retrospective study aimed to describe the association of development of irAEs to survival outcomes in patients treated with ICPi for advanced stage solid malignancies.Moreover, we investigated the effects of endocrine irAEs on survival and we addressed differences in survival outcomes among different racial groups.
Research methods
This is a retrospective analysis of data on 293 patients with diagnosis of stage IV solid malignancy who were treated with programmed cell death-protein 1 or programmed death ligand 1 blocker between 1 January 2013 and 31 December 2018. Progression free survival and overall survival were primary endpoints.
Research results
Out of 293 patients, 91 (31%) had any grade irAEs, amongst which the most common were endocrine (40.7%), followed by dermatological (23.1%) and rheumatologic(18.7%). Caucasians had more irAEs than African Americans (60.4%vs30.8%). Patients with irAE had a lower rate of progression (30.8%vs46.0%,P= 0.0140). Median progression free survival was higher in patients with irAEvsthose without (5.8vs3.0 mo respectively,P= 0.02). Similarly, median overall survival was higher in those with irAEvsno irAE (17.1vs7.2 mo respectively,P< 0.0001).
Research conclusions
This study demonstrates that the development of irAEs is positively correlated with improved survival. It also demonstrates a positive correlation with Caucasian race and overall survival.
Research perspectives
Future prospective studies designed to evaluate cofounding variables for survival such as type of ICPi used, tumor type, and line of treatment are needed to draw definitive conclusions.
 World Journal of Clinical Oncology2021年2期
World Journal of Clinical Oncology2021年2期
- World Journal of Clinical Oncology的其它文章
- Metastatic hormone-sensitive prostate cancer: How should it be treated?
- New Year’s greeting and overview of World Journal of Clinical Oncology in 2021
- Pancreatic cancer in the era of COVID-19 pandemic: Which one is the lesser of two evils?
- New frontiers in focal therapy for prostate cancer: Prostate-specific membrane antigen positron emission tomography/magnetic resonance imaging
- Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review
- Autosegmentation of cardiac substructures in respiratory-gated,non-contrasted computed tomography images
