Pancreatic cancer in the era of COVID-19 pandemic: Which one is the lesser of two evils?
Maitham A Moslim, Michael J Hall, Joshua E Meyer, Sanjay S Reddy
Maitham A Moslim, Department of Surgical Oncology, Fox Chase Cancer Center, Philadelphia,PA 19111, United States
Michael J Hall, Department of Medical Oncology, Fox Chase Cancer Center, Philadelphia, PA 19111 , United States
Joshua E Meyer, Department of Radiation Oncology, Fox Chase Cancer Center, Philadelphia,PA 19111, United States
Sanjay S Reddy, Department of Surgery, Fox Chase Cancer Center, Philadelphia, PA 19111,United States
Abstract Pancreatic adenocarcinoma remains one of the deadliest malignancies affecting the older population. We are experiencing a paradigm shift in the treatment of pancreatic cancer in the era of coronavirus disease 2019 (COVID-19) pandemic.Utilizing neoadjuvant treatment and further conducting a safe surgery while protecting patients in a controlled environment can improve oncological outcomes. On the other hand, an optimal oncologic procedure performed in a hazardous setting could shorten patient survival if recovery is complicated by COVID-19 infection. We believe that oncological treatment protocols must adapt to this new health threat, and pancreatic cancer is not unique in this regard.Although survival may not be as optimistic as most other malignancies, as caregivers and researchers, we are committed to innovating and reshaping the treatment algorithms to minimize morbidity and maximize survival as caregivers and researchers.
Key Words: Pancreatic cancer; COVID-19; Medical oncology; Radiation; Neoadjuvant therapy; Pancreatic adenocarcinoma
INTRODUCTION
Pancreatic adenocarcinoma remains one of the deadliest malignancies affecting the older population. Surgery has been the mainstay of treatment and the only chance for complete oncological cure. Despite the advancements in chemotherapy and radiation oncology, only 20% of cases make it to surgery[1]. As the coronavirus disease 2019(COVID-19) is swiftly evolving, we are at war on two fronts in all patients diagnosed with recent malignancy. These patients are among the most vulnerable. As of the third week of December 2020, there are more than 79 Million cases and more than 1.7 Million deaths Worldwide. Despite the mitigating measures implemented by the local authorities, the federal government and health organizations across the nation, the disease portends severe morbidity and mortality[2].
According to the American Cancer Society, approximately 57600 new pancreatic cancer cases will be diagnosed in the United Sates in 2020. Due to the aggressive biology of pancreatic cancer, robust radiological and pathological diagnosis cannot be hastened enough. Tele-medicine can lessen the impact of restrictive social distancing on multi-disciplinary patient counseling, although it does not resolve the need for infusion visits, radiation simulation and treatment sessions, hospital admissions,imaging studies, laboratory visits and maintaining the minimal requirements for patients involved in clinical trials. Many challenges still persist and include: (1) The role of diagnostic and interventional endoscopy in the setting of low-yield viral testing, unknown dissemination and uncertain hazard for health workers; (2) The scarcity of allocated healthcare resources to meet the supply chain demands at the pandemic epicenters, as well as, implementing social distancing behaviors limiting the accessibility of patients to on-site cancer treatments; (3) The morbidity and mortality emerging from the combined effect of chemotherapy-associated immunosuppression and COVID-19 adverse outcomes; (4) The high mortality of COVID-19 infection on patients in the pre- and post-operative period[3]; and (5) The mental toll on both the patient and family in times of an already overwhelming cancer diagnosis. This is particularly difficult in the COVID-19 era as family members are unable to be with patients in the office setting, before surgery, and during the postoperative recovery.Travel restrictions and visitor limitations intensify this effect.
Caregivers involved in any aspects of cancer patients' care should be absolutely committed to providing evidence-based and peer-reviewed oncological care. It is a mandatory requirement for National Cancer Institute (NCI)- designated cancer centers to review each cancer case at a multi-disciplinary conference as a provision of standard of cancer care. Although these meetings have largely been retooled in the era of COVID-19 to occur both virtually and remotely to assure staff distancing, their value in supporting real-time multidisciplinary communication among clinicians has become increasingly critical.
As cancer care providers, we carry out our responsibilities to maintain the optimal care for pancreatic cancer patients who suffer from poor prognosis at baseline. The limited benefit of treating metastatic pancreatic cancer and the risk of enhancing morbidity and accelerating mortality should be considered on a case-by case basis. In this article, we illustrate our team’s approach to the management of pancreatic cancer at an NCI-designated comprehensive cancer center in the era of the COVID-19 pandemic. As these practices are still evolving, we anticipate more innovative interventions to improve the oncological care of patients with pancreatic cancer and to decrease the risk of COVID-19 dissemination among this vulnerable population.Table 1 demonstrates the evolution of pancreatic cancer staging and workup during the COVID-19 outbreak at our institute.
COVID-19 AND THE RESECTABILITY OF PANCREATIC CANCER
Our surgical and medical management of pancreatic cancer revolves around the current classification of this disease into a resectable, borderline resectable (BLR) and locally advanced (LA) disease. Resectable disease is defined as no tumor contact with the celiac axis (CA), superior mesenteric artery (SMA) and common hepatic artery(CHA), and no or ≤ 180° contact with the superior mesenteric vein (SMV) and portal vein (PV) without venous contour irregularity. LA disease is defined as tumor contact with SMA or CA > 180°, or un-reconstructible SMV/PV due to tumor invasion and occlusion. BLR disease is any tumor and arterial/venous relationship in between of the above[4].
The additional morbidity and mortality of a superimposed COVID-19 infection must be considered in the decision making for these patients. The war at these two fronts is relentless and any strategic planning should take into account the patient as a whole rather than these two high-risk diseases independently. Therefore, in high risk patients and on a case-by-case basis, we consider any degree of abutment of the SMV and PV as an opportunity to introduce BLR treatment approaches rather than surgery as a first-line approach.
UTILITY OF NEOADJUVANT THERAPY IN PANCREATIC CANCER
The use of neoadjuvant therapy in the treatment of those with pancreatic cancer is not novel, and there has been a growing body of literature to support this. This approach's foundation has been extrapolated from the adjuvant therapy data, which was well established from many clinical trials such as the ESPAC-1 and CONKO-001 trials[5-7].The theoretical benefit of neoadjuvant therapy is to treat micro-metastatic/occult disease, down-stage the tumor from BLR or LA to resectable, and measure tumor chemosensitivity[8]. Another advantage is that patients are able to receive more of their intended treatments upfront, addressing the risk of low adjuvant treatment accruals following pancreatoduodenectomy for resectable pancreatic cancer[9].
Two specific chemotherapy regimens have gained enthusiasm in the oncologic practice: 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and gemcitabine/Nab-paclitaxel (Gem/NabP). The use of FOLFIRINOX has been proven in the metastatic setting, with the results from a phase III ACCORD-11/PRODIGE-4 trial demonstrating improved overall survival over single agent gemcitabine[10]. The phase III MPACT trial showed improved survival with Gem/NabP in the metastatic setting as well, when comparing to gemcitabine alone[11]. While there are mounting data to support its use in those with BLR and LA pancreatic cancer, there are also emerging data supporting its use in those with resectable disease. The randomized phase III PREOPANC trial, addressing the role of preoperative gemcitabine-based chemoradiotherapyvsimmediate surgery for resectable and BLR pancreatic cancer in 16 centers, was recently published[12]. Although this study reported no significant overall survival benefit, it demonstrated improved R0 resection, disease-free survival and locoregional failure-free interval in patients who received preoperative chemoradiotherapy. The occurrence of serious adverse events was 52%vs41%,respectively (P= 0.096)[12]. The benefit of preoperative FOLFIRINOX-based chemotherapy is currently being investigated by the PREOPANC-2, NorPACT-1 and PANACHE01-PRODIGE48 trials.
While there is an ongoing debate on how best to sequence the necessary components of pancreas cancer treatment in these patients, the rationale for neoadjuvant chemotherapy, including that advantage of being able to gauge response to systemic treatment, to potentially downstage higher T stage and node-positive tumors, to treat micro-metastatic disease early, and to promote maximal completion of all systemic treatments, all serve as important advantages to this approachvsan approach where newly diagnosed patients are taken immediately to surgery.However, it is important to balance the potential advantages of neoadjuvant chemotherapy in pancreatic cancer with the risks associated with immunosuppressive chemotherapy in the COVID-19 era. The American Society of Clinical Oncology has published recommendations to guide medical oncologists in the current pandemic,although none of the recommended practice adaptions is particularly relevant topatients with a newly diagnosed resectable pancreatic cancer. The American Society of Breast Surgeons has advocated for the use of neoadjuvant therapy among patients with high risk tumors like triple negative breast cancer, as well as the potential for less frequent dosing schedules to reduce infusion room time and therapy visits[13].
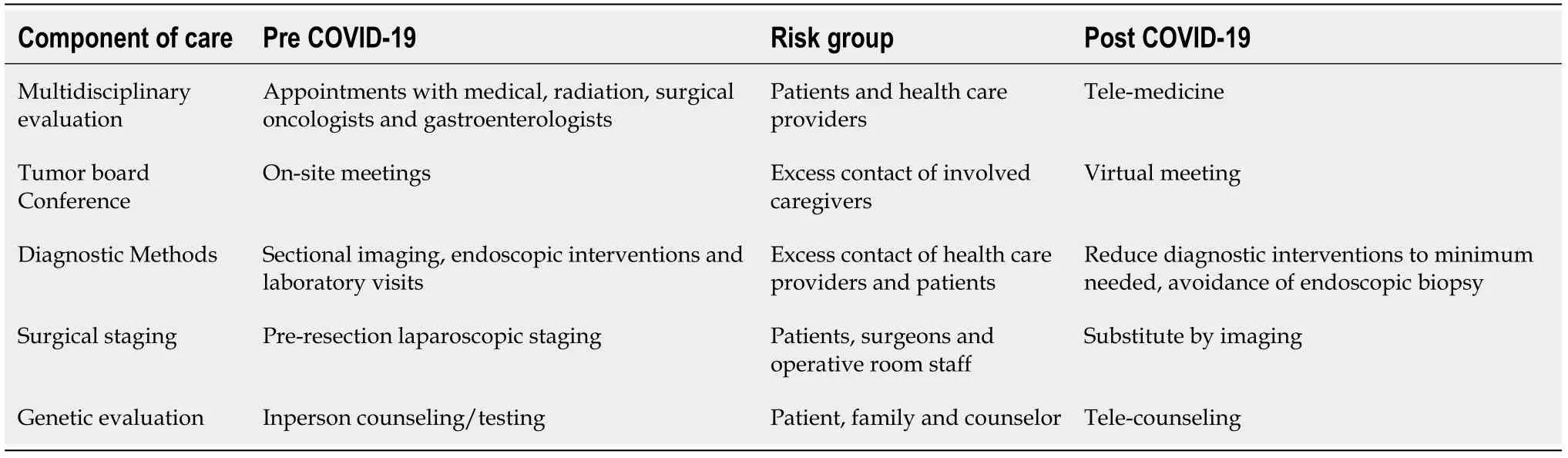
Table 1 Evolution of pancreatic cancer staging and workup in the era of coronavirus disease 2019
Radiation or chemoradiation may be recommended in cases where surgery is being delayed due to COVID-19 concerns. In these cases, it is reasonable to pursue a treatment approach that has been shown to limit local progression rates in an effort to delay surgery. Many radiation oncology departments have been able to limit exposure risks with interventions such reduction in the number of patients coming in for treatment, social distancing in waiting areas, increased time between scheduled patients, more frequent cleaning of high touch surfaces, increased use of masks and in some settings gloves, and strict hand hygiene measures. To further minimize exposure, consideration also needs to be given to the number of visits that are necessary to treat a patient. While there are data supporting longer courses of daily radiation for 5-6 wk, the value of these longer schedules with conventional fractionation should be weighed against the exposure risks of up to 30 visits. By utilizing advanced techniques such as intensity modulated radiation therapy, imageguided radiation therapy, stereotactic body radiotherapy and magnetic resonance imaging-guided adaptive radiation therapy, it may be possible to increase the fraction size and significantly shorten the schedule. Of course, one complicating factor of this move to more precise radiation techniques is that some require placement of a fiducial markerviaan endoscopic procedure, which may increase the exposure risk to both patients and staff. All of these factors are important to weigh for each individual patient. Table 2 summarizes the risks and benefits of immediate resectionvsneoadjuvant treatment followed by resection for resectable pancreatic cancer in the era of COVID-19.
TIMING OF SURGERY
Elective surgery confers a high mortality during the incubation period of COVID-19 infection[3]. This has been attributed to the immune dyscrasia associated with the stress of invasive procedures which culminated into acceleration of symptomatic presentation[14]. Although few studies are available, the perioperative mortality rate has been reported to be as low as 21% in Wuhan, China (47% after intensive care unit admission) and as high as 75% in Iran[3,15]. This is more pronounced in the older population who are typically the affected group in pancreatic cancer[3]. Acute respiratory distress syndrome and acute cardiomyopathy resulting into cardiac shock and fatal arrhythmia were the most common causes of death[3]. The contemporary accepted perioperative mortality rate for pancreaticoduodenectomy at high-volume cancer centers is 2%-4%[16].
The main challenge is considering operative intervention on those who have completed neoadjuvant chemoradiation during the peak of COVID-19 cases. We are evaluating, on a case-by-case basis, the potential of delaying operative intervention for a few weeks until the peak of the curve is flattened in the southeastern Pennsylvania region. Patients who are currently tolerating chemotherapy are being counseled to continue their current regimen until the postulated date of resection. On the otherhand, we must keep in mind that delaying surgery following neoadjuvant radiation therapy can have undesirable consequences. Radiation induced fibrosis can distort anatomical planes and increases surgical difficulty. A recent study conducted at our institute on 44 patients who received neoadjuvant chemoradiation demonstrated that the optimal time to perform pancreatic resection is 6-8 wk after completion of radiotherapy.

Table 2 Risks and benefits of immediate resection versus neoadjuvant therapy followed by resection for resectable pancreatic cancer in the era of coronavirus disease 2019
PREOPERATIVE TESTING AND PREPARATION
All pancreatic cancer cases are scheduled as open procedures to prevent the aerosol exposure of coronavirus suggested by other studies on similar pathogens[17]. The vast majority of the initially available and experimental COVID-19 tests were believed to act as “rule in” rather than “rule out” tests with relatively high false negative rate[18].Also current known cases of COVID-19 might actually be an underestimation of the definite spread of the virus in the community. Hence, all patients presenting to our operative facility are suspected to incubate COVID-19 until we have more accurate tests available. Despite their current limited distribution and scarcity, many effective vaccines have the potential benefit of protecting and prepare this vulnerable population for their medical and surgical treatment.
Operative room, staffing and recovery unit precautions are implemented according to the continuously updated American College of Surgeons and Center for Disease Control and Prevention recommendations. At our institute, we review the weekly oncological procedures being performed on the preceding Thursday during a departmental meeting. This approach ensures the validity of performing such procedure during the current crisis. Procedures that deemed not necessary or can be postponed without compromising the oncological outcomes are followed closely for future operative considerations. Due to the scarcity of resources, the usage of personal protective equipment and the sterilization process of N95 masks are monitored very tightly by the surgical operations. This ensures maximal protection, minimal wasting and effective involvement of the least number of operative personnel needed to conduct an efficient and safe operation.
INSTITUTIONAL MEASURES IMPLEMENTED TO MINIMIZE COVID-19 DISSEMINATION
The course of COVID-19 is long and associated with high transmissibility from asymptomatic patients going through a prolonged incubation period[19]. As a designated comprehensive cancer center, many measures were implemented to protect our vulnerable patients. All employees have their temperature taken upon their arrival to the health campus. Employees with temperatures below 100 °F are given surgical masks to be worn all the time. Those whose temperature is 100 °F or higher are asked to put on masks and self-isolate at home while waiting for occupational health evaluation.
Our visitor policy has been revised to prohibit inpatient visits on campus unless necessary in the setting of end-of-life discussions. Patients who present for surgery or on-site treatments are not allowed to be accompanied by visitors and subsequent transportations are provided by the hospital. Tele-medicine technology has begun to replace regular outpatient visits; these include primarily consultative visits and any visits that do not require on-site treatments or radiological investigations. Quarantine units have been arranged and protocols have been created in the case of a local outbreak. Inpatients who test positive for COVID-19 infection are isolated from other patients in a separate facility and being cared for by a specialized dedicated team.
CONCLUSION
Which is the lesser of two evils is an open-ended question with no true answer. Both pancreatic cancer and COVID-19 are diseases that require utilization of substantial healthcare and human resources. Therefore, we are experiencing a paradigm shift in the multidisciplinary management of pancreatic cancer that must balance the risks of the disease and the risks of treating this deadly disease while the specter of COVID-19 Looms. Utilizing neoadjuvant treatment and further conducting a safe surgery while protecting patients in a controlled environment can improve oncological outcomes,but may also permit delay of high risk, resource heavy pancreatectomy while hospitals funnel valuable resources to address the surge of COVID-19 cases in their communities. Even an optimal oncologic procedure performed in a hazardous setting could shorten patient survival if recovery is complicated by factors such as COVID-19 infection.
The era of COVID-19 is the new normal, for now. We cannot make the mistake of under-estimating the severity and potential duration and long-term impact of this crisis on cancer care and public health. Therapeutic measures are being tested to fight the virus, but there are no guarantees for when these may allow a safe resumption of everyday living as well as previously adopted practices for managing cancer patients.Oncological treatment protocols must adapt to this new health threat, and pancreatic cancer is not unique in this regard. Although long-term survival may not be as optimistic as most other malignancies, as caregivers and researchers, we are committed to innovate and reshape treatment algorithms to minimize morbidity and maximize the chances of survival for our sickest patients.
ACKNOWLEDGEMENTS
We acknowledge all caregivers, all over the world, who put their lives on front lines as they fight to provide the best care for their patients.
 World Journal of Clinical Oncology2021年2期
World Journal of Clinical Oncology2021年2期
- World Journal of Clinical Oncology的其它文章
- Metastatic hormone-sensitive prostate cancer: How should it be treated?
- New Year’s greeting and overview of World Journal of Clinical Oncology in 2021
- New frontiers in focal therapy for prostate cancer: Prostate-specific membrane antigen positron emission tomography/magnetic resonance imaging
- Chronic myeloid leukemia-from the Philadelphia chromosome to specific target drugs: A literature review
- Autosegmentation of cardiac substructures in respiratory-gated,non-contrasted computed tomography images
- Racial disparities in immune-related adverse events of immune checkpoint inhibitors and association with survival based on clinical and biochemical responses
