A combination strategy of functionalized polymer coating with Ta ion implantation for multifunctional and biodegradable vascular stents
Kwng-Hee Cheon ,Cheonil Prk ,Min-Ho Kng ,Suhyung Prk ,Jinyoung Kim ,Seol-H Jeong ,Hyoun-Ee Kim,Hyun-Do Jung,Te-Sik Jng
a Department of Materials Science and Engineering,Seoul National University,Seoul 08826,Republic of Korea
b Department of Chemical and Biological Engineering,Seoul National University,Seoul 08826,Republic of Korea
c Department of Biomedical-Chemical Engineering,Catholic University of Korea,Bucheon-si 03083,Republic of Korea
d Department of Biotechnology,The Catholic University of Korea,Bucheon-si 14662,Republic of Korea
e Department of Materials Science and Engineering,Chosun University,Gwangju 61452,Republic of Korea
Abstract Biodegradable stents made of magnesium(Mg)and its alloys have been developed to minimize persistent inflammation or in-stent restenosis,which are the main problems for permanent stents.However,their rapid corrosion behavior under physiological conditions leads to poor vascular compatibility and premature structural failure,which remains an important unsolved clinical problem.Herein,we demonstrate a new strategy for solving this problem by combining poly(ether imide)(PEI)coating and subsequent tantalum(Ta)ion implantation.The PEI coating covers the whole surface of the Mg stent uniformly via a spray coating technique and provides Mg with superior corrosion resistance and stable sirolimus-carrying ability.Ta ion implantation is conducted by a sputtering-based plasma immersion ion implantation technique only onto the luminal surface of the PEI-coated Mg stent.Its extremely short processing time(< 30 s)permits preservation of the PEI coating’s corrosion protection ability and sirolimus loading characteristics.In addition,a Ta-implanted skin layer that forms on the topmost surface of the PEI coating plays an effective role in not only preventing a rapid release of sirolimus from the surface but also improving the PEI coating’s surface hydrophilicity.Based on in vitro cellular response and blood compatibility tests,Ta ion implantation leads to the improvement of endothelial cell adhesion/proliferation and suppression of platelet adhesion/activation regardless of sirolimus loading.These results indicate that the combination of PEI coating and Ta ion implantation has significant innovative potential to provide excellent vascular compatibility and prevent in-stent restenosis and thrombosis.
Keywords: Magnesium stent;Biodegradability;Tantalum;Plasma immersion ion implantation;Multifunctionality.
1.Introduction
Over the past few decades,magnesium(Mg)and its alloys have received considerable research attention as ideal stent materials because of their attractive biodegradability and biocompatibility [1–6].Traditional bare metal stents,made of non-biodegradable metallic materials,are permanently placed in a blood vessel and act as a foreign body,which may lead to severe chronic inflammatory reactions and long-term endothelial dysfunction [3].In contrast,a biodegradable Mg stent can perform the function of temporary support to open a narrowed blood vessel whereas gradual vascular remodeling takes place[7].In addition,Mg2+itself is considered to be beneficial for endothelial cell migration and subsequent endothelialization via upregulation of actin filament assembly during the protrusion formation process [8–11].However,despite all these fascinating characteristics,the single most challenging problem,which prevents these materials’ successful use in clinical practice,is their extremely high corrosion rates under physiological conditions [3,12–14].Such rapid corrosion behavior may lead to uncontrolled decline of mechanical properties and structural failure,resulting in the collapse of the whole stent segment before vascular remodeling completes [9,10,15,16].Moreover,massive quantities of hydrogen gas and hydroxyl ions generated from the Mg surface can hinder the attachment and proliferation of endothelial cells or,even worse,cause severe inflammatory responses [3,15,17,18].Therefore,it is crucial to develop an efficient way to improve the corrosion resistance of Mg stents to facilitate their practical application in treating vascular disease.
The introduction of a surface coating layer with biopolymers is considered a facile and promising strategy for suppressing the rapid corrosion of Mg,as well as providing additional function to stent–drug elution [19–21].The surface coating layer physically prevents direct contact of Mg with the corrosive biological environment and suppresses the diffusion of corrosion by-products from the substrate to the top surface [19].In addition,incorporated therapeutic agents in the polymeric coating layer are particularly effective for problematic stent-related complications(e.g.,in-stent restenosis)via direct delivery of immunosuppressive drugs into the target lesion [22,23].In previous studies,several polymers,such as poly(ε-caprolactone),poly(lactic acid),poly glycolic acid,and their co-polymers,have proven their promising potential as drug-eluting stents;they possess high stability and loading capacity of drug-in-polymer matrix and extend the duration of release [20].However,those polymers are hardly applicable for the Mg stent because of their inherently poor adhesion stability to the Mg surface and poor corrosion protection ability [19].Under physiological conditions,the corrosive media can diffuse to the weak polymer–Mg interface,reducing the surface coating layer’s ability to adhere to the Mg surface;this process results in impaired corrosion protection during service [19,24].
Poly(ether imide)(PEI)is a potential alternative drugeluting coating material for Mg stents that preserves excellent adhesion to most metal surfaces via acid–base interaction;oxides on the metal surface act as an acid,whereas aromatic imide rings in the PEI as a base in the Lewis sense[25].In addition,PEI possesses excellent coating workability,good mechanical properties,and stable chemical and thermal characteristics,all of which potentially play an important role in providing effective corrosion resistance to the Mg[19,24].However,when PEI is used in-stent applications,it inevitably provokes a safety concern related to blood coagulation and activated platelet function due to its stimulation of reactions with plasma proteins that are hypersensitive to it [26,27].Moreover,the incorporation of immunosuppressive drugs within the surface coating layer is likely to have a negative impact on the neighboring endothelial cells,leading to delayed re-endothelialization via arresting of cell cycle progression [28].Therefore,further adequate surface modification is required to achieve the desired vascular tissue responses while maintaining the corrosion protection ability of the PEI.
Recently,we have developed a simple,rapid technique,called“sputtering-based plasma immersion ion implantation”(S-PIII),to modify the surface characteristics of polymeric substances via massive metal ion implantation [29–31].Numerous metal atoms emitted from a direct current(DC)magnetron sputtering target are ionized by a high-density plasma near its surface and subsequently accelerated onto the polymeric substrate by application of a high negative substrate bias voltage.As these ions are rigorously implanted into the substrate surface,a highly packed,dense metal-implanted layer is formed on the uppermost surface [29].This layer shows superior efficacy in altering the substrate’s physical and chemical properties [29,30].In addition,this layer could provide an effective physical barrier for not only preventing direct contact of loaded drugs with surrounding endothelial cells,but also suppressing initial burst drug release.
Herein,we introduce tantalum(Ta)as the target of S-PIII and allow Ta treatment of only the luminal surface of the PEIcoated Mg stent due to its promising endothelial cell affinity and blood compatibility [30].After S-PIII treatment,the surface structure,chemical composition,and corrosion behavior of the Ta-implanted PEI coating layer on Mg(Ta/PEI-coated Mg)were closely investigated and compared with those of PEI-coated Mg.To measure the Ta treatment’s impact on drug release behavior,sirolimus,one of the most widely used immunosuppresive drugs with proven efficacy in reducing neointimal hyperplasia,was incorporated in PEI coating layers[28].Sirolimus release quantities in the phosphate-buffered saline(PBS)solution were measured with UV spectrometry.Furthermore,in vitrotests using endothelial cells and vascular smooth muscle cells(VSMCs)were performed to assess cell adhesion and proliferation.
2.Experimental section
2.1.Sample preparation
Mg alloy WE43(4.1 wt.% Y,2.1 wt.% Nd,0.56 wt.% Zr,0.028 wt.% Mn,and balance Mg,Yueyang Yuhua Yejin company,Beijing,China)was used as a model Mg substrate for the present work because of its wide use in the evaluation of biodegradable stents [32].Each specimen was prepared with dimensions of 10×10×2 mm and polished using abrasive SiC paper up to 2000 grit prior to cleaning with ethanol in an ultrasonic bath.PEI pellets(Sigma–Aldrich,USA)were dissolved in N-methyl-2-pyrollidone(Sigma–Aldrich,USA)at a concentration of 10% w/v,with a sirolimus(LC laboratory,USA)concentration of 33 wt.%.The solution was stirred at 37 °C overnight.The PEI solution was then spin-coated on the Mg substrate at 3000 rpm for 1 min.The coated samples were dried at 70 °C for 1 day to remove residual solvent and densify the PEI coating layer.Then,the coated specimens were placed in a vacuum chamber.A Tantalum target(diameter 75 mm,thickness 5 mm,purity 99.99%,Kojundo Chemical Lab,Japan)was placed in a DC magnetron sputter gun housing(Ultech Co.,Ltd.,Korea).A vacuum chamber was initially pumped to 5×10−4Pa using rotary and diffusion pumps prior to application of the S-PIII technique.With the coated Mg specimens attached to a stainless steel plate and paralleled with the Ta target,the S-PIII technique was applied with the Ta target at a high negative voltage of 2000 V for 30 s under 7 mTorr of Ar gas pressure.The 50 mA target current was applied,with no additional heat applied.
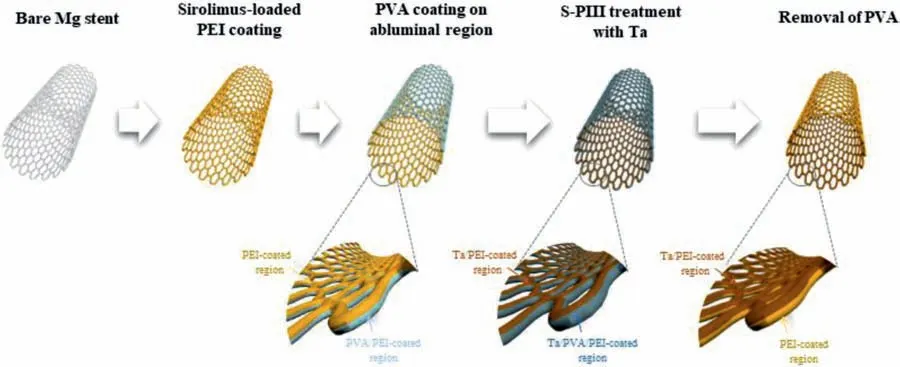
Fig.1.Schematic diagram of experimental methods for fabrication of Ta-implanted PEI coating layer on Mg stent.
A Mg stent with length 25 mm,diameter 2.75 mm,and thickness 150 μm was supplied by GENOSS(GENOSS,Suwon,Korea).The PEI coating was applied using a spray coater(Sonicoater,NOANIX,Korea).The overall coating procedure is shown in Fig.1;the Mg stent was fixed at the end of the jig.A 3% w/v PEI solution with sirolimus was prepared and dispensed four times with N2gas flow onto a moving Mg stent.The drying condition was identical to that of the Mg plate samples.For selective S-PIII,a PVA layer was deposited onto the abluminal side of a Mg stent.PVA(Sigma–Aldrich,USA)powder was dissolved in distilled water to a concentration of 2% w/v in an autoclave.The Mg stent,fixed at the inside of jig by insertion into the jig,was sprayed with N2gas.The S-PIII process was performed with the PVA/PEIcoated Mg stent under identical conditions.Finally,the PVA layer was eliminated using distilled water.
2.2.Characterization of Ta-implanted PEI coating layer
The surface morphologies and thicknesses of the WE43 stent,PEI-coated Mg stent,and Ta/PEI-coated Mg stent were observed using scanning electron microscopy(SEM,JSM-6360,JEOL,Japan)and focused ion beam analysis(FIB,AURIGA,Carl Zeiss,Germany).The Ta distribution on the coating layer was evaluated with energy-dispersive X-ray spectroscopy(EDS)associated with SEM.Transmission electron microscopy(TEM;JEM-2100F,JEOL,Japan)was conducted to obtain the cross-sectional image of the Ta-implanted PEI coating layer.For this evaluation,luminal and abluminal surfaces of the stent were examined using FIB etching technique with carbon protective coating.The elemental linear profile was evaluated by EDS analysis associated with TEM.The mechanical stability of the PEI,the Ta-implanted PEI,and the Ta-implanted PEI coating layer were examined by FE-SEM after stent expansion.The coated Mg stents were mounted on a 5.0 × 25 mm balloon catheter(GENOSS,Korea)and then dilated to their nominal diameters at a pressure of 9 ATM by injecting deionized water into the balloon.The chemical composition of the coating layer was analyzed using X-ray photoelectron spectrometry(XPS;Axis SupraTM,Kratos,England).The hydrophilicity of the PEI-coated Mg sample was evaluated by measuring the contact angle of the distilled water droplet with the Phoenix 300 contact angle analyzer(Surface Electro Optics Co.,Ltd.,Korea).
2.3.Corrosion behavior
The corrosion behavior was characterized by measuring the pH change and the amount of evolved hydrogen gas from each plate shape sample after immersion into accelerated corrosive artificial plasma with bare WE43,PEI-coated WE43,and Ta/PEI-coated WE43 at 37 °C for 10 days.The accelerated corrosive artificial plasma solution was prepared according to a method suggested by previous research [33].Prior to the corrosion test,the other sides of the samples were mounted with epoxy resin.The exposure area was about 1 cm2.The pH value was monitored with a pH meter(Orion 3 star,Thermo Scientific,USA).
The volume change of the Mg stent was analyzed with a fluid circulating system to simulate the environment of blood vessels with a constant blood flow.The circulation of accelerated corrosive artificial plasma solution was conducted with a perfusion pump(SciQ 323,Watson Marlow,UK).The bare WE43,the PEI,and the Ta-implanted PEI-coated WE43 stent were expanded and fixed into a silicone tube with inner diameter of 3 mm using a balloon catheter.The degradation test was conducted with a flow rate of 40–45 ml/min at 37 °C.The 3-D structure of the WE43 stents was monitored usingμ-CT(Skyscan 1173,Konitch,Belgium)at a predetermined time with the following conditions:a resolution of 17 μm using a 1.0 mm aluminum filter,a voltage of 130 kV,and a current of 60 mA.Thereafter,the volume of the stents was calculated by CTan software(Bruker,Kontich,Belgium).
2.4.Sirolimus stability test
The chemical structures of sirolimus,the PEI coating,and the Ta/PEI coating layer without and with the drug were examined using Fourier transform infrared spectroscopy(Nicolet iS50,Thermo Fisher Scientific,USA)at wavelengths from 4000 to 400 cm−1.The stability of the PEI coating layer’s sirolimus loading was analyzed using high-performance liquid chromatography(HPLC,Ultimate3000,Dionex,USA)with UV detection at 278 nm.Prior to HPLC analysis,the PEIcoated Mg and the Ta/PEI-coated Mg with the drug were vortexed in methylene chloride for 6 h to dissolve sirolimus loaded in the coating layer;samples were then dried in a vacuum chamber for 1 day.The dried samples were dissolved using a solution of methanol and distilled water of HPLC grade in a v/v ratio of 80:20 to produce a mobile phase for HPLC analysis.The chromatographic conditions used in the analysis were as follows:C18 column(250 × 4.6 mm,5 μm;Varian,Paloalto,California,USA),flow rate of 1.5 ml/min,injection volume 20 μL,and oven temperature 50 °C.
2.5.Sirolimus release test
Before thein vitrosirolimus release test,a sirolimus standard curve was obtained by preparing sirolimus stock solution.Five milligrams of sirolimus was dissolved in 100 ml of methanol and 0.05% Tween-20 PBS solution(Sigma-Aldrich,St.Louis,MO,USA).One milliliter of the stock solution was diluted with methanol–0.05% Tween-20 PBS(1:1 v/v),and a series of standard solutions were obtained at concentrations of 25,12.5,6.25,3.125,and 1.5625 μg/ml.The absorbances of the working standard solutions were measured by UV/Vis spectroscopy(V-770,JASCO,Japan)at a wavelength of 278 nm.In this analysis,the concentration of sirolimus(mg/ml)was determined to be 13.26 × absorbance value;R2=0.9976.Drug-loaded PEI-coated and Ta/PEI-coated Mg samples was prepared with dimensions of 10×10×2 mm and immersed into 3 ml of 0.05%Tween-20 containing PBS at 37 °C.The drug release medium was collected at predetermined time intervals and was subsequently filtered through 0.2 μm filters(Millex GPfilter unit,Millipore,USA).The optical density of the filtered medium was measured using UV/Vis spectrophotometry at a wavelength of 278 nm after mixing the filtered drug containing PBS medium with anhydrous grade methanol.The release behavior of sirolimus was monitored for 8 weeks.
To calculate the total amount of drug release,the drugloaded samples were immersed in 5 ml of methylene chloride with vortexing for 6 h to dissolve sirolimus loaded in the PEI coating layer.After vortexing,the solution was completely dried in a vacuum,and a mixed solution of methanol and Tween-20 PBS was poured into the drug containing bottle.The absorbances of these solutions were measured using UV/Vis spectroscopy.
2.6.In vitro anti-proliferation test of smooth muscle cell
To examine restenosis inhibitory ability via growth and proliferation of smooth muscle cells,anin vitroantiproliferative test of VSMC was performed on the PEI coating layer without and with the drug.Prior to thein vitrocell test,all samples were sterilized with 70% ethanol and ultraviolet light on a clean bench overnight.The smooth muscle cells(VSMC,CC-2583,Lonza,Switzerland)were cultured in smooth muscle basal medium(SmBM,CC-3181,Lonza,Switzerland)supplemented with smooth muscle basal medium(SmGM-2)and 1% penicillin–streptomycin in a humidified incubator with 5% CO2at 37 °C.
After culturing for 3 and 6 days on the coated Mg sample,the attached cell morphologies were observed with a confocal laser-scanning microscope(CLSM,LSM710,Carl Zeiss,Germany).Before CLSM observation,the samples were washed with PBS twice and stained with Live/Dead assay(L3224,Invitrogen,Carlsbad,USA)for 30 min in the dark.Cell proliferation was evaluated by measuring the quantity of DNA from the attached smooth muscle cells on the PEI-coated sample without and with the drug,using a Cyquant cell proliferation assay kit(C7026,Invitrogen,Carlsbad,USA)after culturing for 3 and 6 days at a density of 3 × 104cells/ml.Before the DNA quantity measurements,the cells on the samples were detached using 0.25% trypsin–ethylenediaminetetraacetic acid(EDTA)and stained in a fluorescent dye solution.The quantity of DNA from detached cells was analyzed using a multiple plate reader(Victor3,Perkin Elmer,USA)at wavelengths of 480/535 nm.
2.7.In vitro endothelialization test
To evaluate fast endothelialization by Ta implantation despite drug loading in the coating layer,anin vitroendothelialization test was conducted with the PEI-coated and Ta/PEIcoated Mg samples with the drug.Before thein vitrocell test,all samples were cleaned with 70%ethanol and dried in a vacuum chamber overnight,followed by sterilization with ultraviolet light on a clean bench for 1 day.Human umbilical cord vein endothelial cells(HUVECs,ATCC,CRL-1730,USA)were cultured in endothelial basal medium(EBM-2)using an endothelial cell growth medium(EGM-2)Single Quot Kit(Lonza,Walkersville,MD)and 1% penicillin–streptomycin in a humidified incubator with 5% CO2at 37 °C.
The overall evaluation assay was similar to that of thein vitroanti-proliferative test of smooth muscle cells.Cell attachment was observed with CLSM after 3 and 6 days of cell seeding at a density of 3 × 104cells/ml on the PEI-coated and Ta/PEI-coated Mg samples with the drug.After culturing,the samples were washed with PBS twice and stained with Live/Dead assay(L3224,Invitrogen,Carlsbad,USA)for 30 min in the dark.Cell proliferation was evaluated by measuring the quantity of DNA from cells on the samples using a Cyquant cell proliferation assay kit after culturing for 3 and 6 days at a density of 3×104cells/ml.Before the DNA quantity measurements,the cells on the samples were detached using 0.25% trypsin–EDTA and stained in a fluorescent dye solution.The quantity of DNA from detached cells was analyzed using a multiple plate reader at wavelengths of 480/535 nm.
2.8.In vitro platelet adhesion test
Platelet-rich plasma(PRP,KOREAN Red Cross Blood Services,Seoul,Korea)was obtained from whole blood of healthy volunteers.For platelet adhesion tests,the PEI-coated and the Ta/PEI-coated Mg samples were sterilized overnight using UV irradiation.Diluted PRP was placed onto the sterilized specimens in a 24-well plate.The specimens were kept in a humidified incubator at 37 °C with 5% CO2for 1 h.After incubation,the platelets were fixed with 2.5% glutaraldehyde for 10 min and dehydrated in 50,60,70,80,90,and 100% ethanol,followed by air-drying.The morphologies of adhered platelets were observed using FE-SEM,and the images were captured randomly to calculate the numbers of adhered platelets.The densities of platelets and the activation stage ratios were quantified from FE-SEM images using ImageJ software(National Institutes of Health,Bethesda,Maryland,USA).Densities were expressed as the number of adherent platelets per mm2.
2.9.Statistical analysis
At least three replicates were conducted for each experimental condition.All the experimental data are shown as the mean±standard deviation as reported by the Statistical Package for the Social Sciences software(International Business Machines,NY,USA).The normality of variables was determined using a Shapiro–Wilk test.One-way analysis of variance was performed for each test,followed by a Tukeypost hoccomparison test.Ap-value below 0.05 was considered significant.
3.Results and discussion
3.1.Characterization of Ta-implanted PEI coating layer on the Mg stent
The surface morphologies of the bare,PEI-coated,and Ta/PEI-coated Mg stents were investigated using FE-SEM analysis,as shown in Fig.2.Under low magnification,there were no obvious differences among the three groups;all stents exhibited good structural connectivity and uniformity,with 150 μm of strut thickness without breaking or cracking at the strut connections.However,when examined at high magnification,only the bare Mg stent showed a relatively rough surface with several micron-sized precipitated particles along the stent struts(Fig.2Aa)due to the electropolishing process used in-stent manufacturing [33].In contrast,the surfaces of the PEI-coated and Ta/PEI-coated Mg stents were considerably smoother(Fig.2Ab and c).In case of the PEI-coated Mg stents,the PEI surface coating layers completely covered the whole surface of each Mg stent(Fig.2Ab and Bb-1).Even at the corner regions of the stent struts,the PEI layers were uniformly coated without any sign of webbing or stringing.According to supplementary cross-sectional observation(Fig.S1),the PEI coating layers on luminal and abluminal surfaces of the Mg stent showed very uniform thickness of 1.2 μm and appropriate adherence to the stent surface.In general,the spray coating technique shows excellent coatability for objects with complex geometry,thanks to its generation of numerous tiny droplets of polymeric solution uniformly transferred by airflow [34].This unique characteristic is mainly attributed to the mechanically stable behavior of the PEI coating layers under the deformation condition;the PEI coating layers observed after the balloon-assisted stent expansion test showed surface structures almost identical to those observed beforehand:no coating delamination,buckling,or fracture,as shown in Fig.2Bb-2 and b-3.In addition,even after S-PIII treatment on the luminal region,the PEI coating still preserved its original smooth surface texture and mechanical stability before and after the stent expansion test(Fig.2Ac and Bc-1).High magnification revealed no microstructural damage or deformation of the PEI coating layer after the stent expansion test(Fig.2Bc-2 and c-3).
To further investigate the surface structures of the PEI coating layer after S-PIII treatment,both the luminal and abluminal surfaces of the Ta/PEI-coated Mg stent were crosssectionally observed using STEM-EDS(Fig.3A).A dense,compact,Ta-implanted skin layer was evident on the topmost luminal surface,exhibiting an approximately 20 nm of thickness with relatively brighter contrast in STEM image.As we intended to treat only the luminal region of the Mg stent with the S-PIII treatment,the abluminal surface showed no evidence of ion implantation.Since energetic metal ion irradiation generally creates gradually accumulated metal nanoclusters within the substrate surface,the Ta-implanted layer showed no apparent interface to the PEI coating;this made possible the excellent structural stability and functionality under a variety of stress and strain conditions [29,30].In fact,according to the EDS line profile analysis along the yellow line from point a to b in STME images(Fig.3A),the depth distributions of Ta and oxygen(O)exhibited parabolic profiles within the Ta-implanted skin layer,showing that their concentrations were increased up to 10 and 40 at.%,respectively,decreasing gradually with increasing distance from the surface.
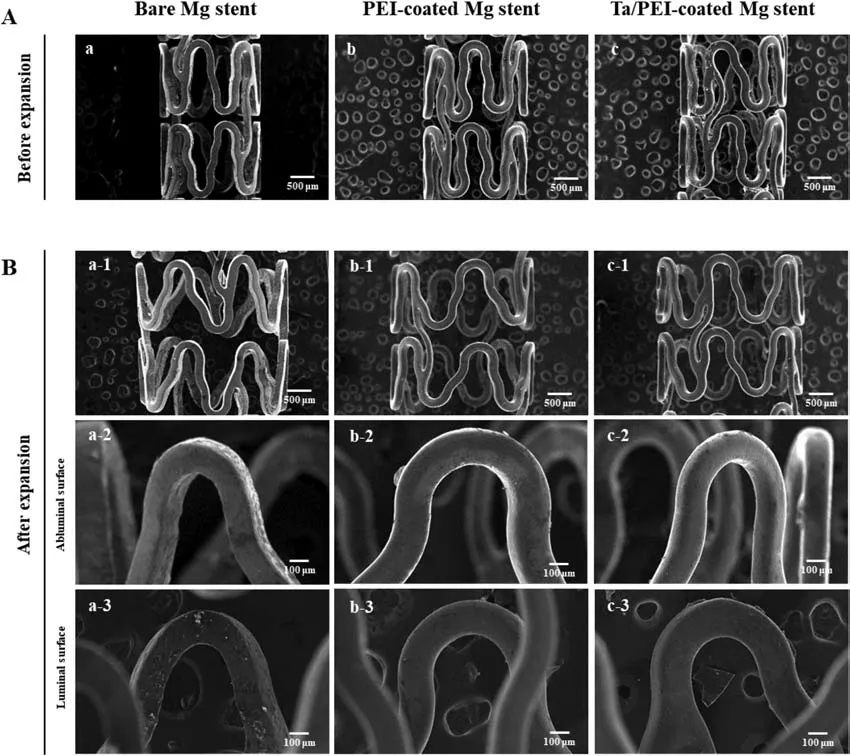
Fig.2.SEM images of(a)bare,(b)PEI-coated,and(c)Ta/PEI-coated Mg stents(A)before and(B)after dilatation.High-magnification images of(a-2,b-2,and c-2)abluminal and(a-3,b-3,and c-3)luminal surfaces of each Mg stent.
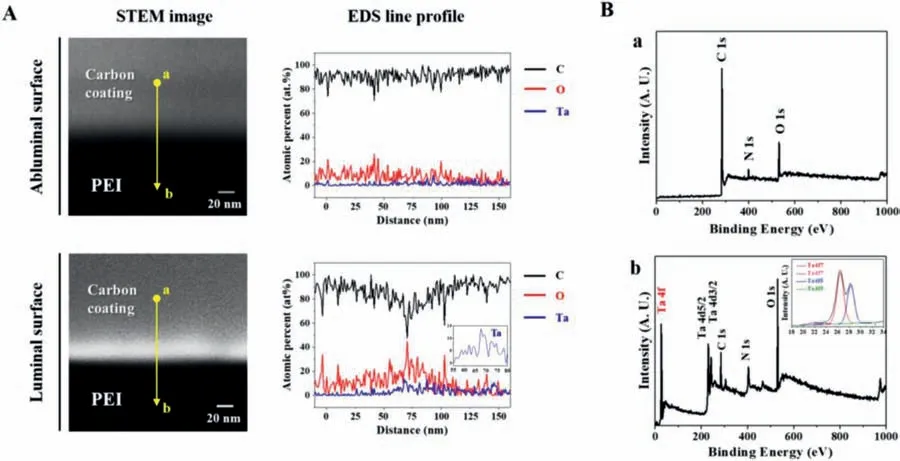
Fig.3.(A)High-resolution cross-sectional STEM images and STEM/EDS elemental profiles of Ta/PEI-coated Mg stent at luminal and abluminal surfaces.Yellow lines in STEM images indicate the region where the EDS analysis was performed.Inset in EDS line profile of luminal surface is the magnified Ta distribution.(B)XPS full spectra obtained from the surfaces of(a)PEI-coated and(b)Ta/PEI-coated Mg samples.Inset in a is the high-resolution spectrum of Ta 4f.

Fig.4.(A)pH change and(B)micro-CT characterization of bare,PEI-coated,and Ta/PEI-coated Mg in accelerated corrosive artificial blood plasma solution:(a)3-D images of each stent at 0,3,6,and 10 d of immersion and(b)their remaining volumes.
XPS analysis was carried out to examine the surface chemistry changes in the PEI coating layer after S-PIII treatment,with results shown in Fig.3B.In the case of PEI-coated Mg,major characteristic components of the PEI(carbon,C;oxygen,O;and nitrogen,N)were detected(Fig.3Ba);C1 andO1s correspond to O=C–N,whereasN1s is attributed to O=C–N-C=O groups [35].In contrast,after S-PIII treatment,the Ta/PEI-coated Mg showed additional peaks for metallic Ta(Ta 4f,Ta 4d,and Ta 4p)with substantial intensities in the XPS spectrum,whereas relative intensities of C and N peaks decreased(Fig.3Bb).Moreover,it was clearly confirmed that the Ta-implanted layer possessed a protective oxide layer of Ta2O5from the high-resolution spectrum of Ta 4f [36],which is generally known to be very stable chemically and biologically favorable in various clinical applications [29,30,37].Therefore,from the point of view of surface structure and chemistry,the Ta/PEI-coated Mg showed the potential for benefits in the prevention of rapid corrosion and treatment of stent-related complications.
3.2.Corrosion protection conferred on the Mg stent by the Ta-implanted PEI coating layer
Corrosion tests with bare Mg,PEI-coated Mg,and Ta/PEIcoated Mg were performed by immersion in accelerated corrosive artificial plasma solution for 10 days.Before immersion,side and bottom surfaces of each sample were sealed with epoxy resin,and variation in pH of each solution was monitored as a function of time.As shown in Fig.4A,the PEI coating layers without and with the Ta-implanted skin layer had a clear effect on the inhibition of rapid Mg corrosion.The bare Mg showed dramatically increased pH levels during the entire monitoring period,reaching pH values as high as 8.7 at 10 days of immersion.In contrast,the PEIcoated and Ta/PEI-coated Mg samples did not become significantly corroded with time;during the test they showed slightly increased pH values of 7.3–7.8 and 7.3–7.65,respectively.PEI-coated and Ta/PEI-coated Mg samples also showed no rapid increase in pH during the initial test stage;their pH profiles with respect to duration were predominantly linear.Ta/PEI-coated Mg exhibited slightly but not significantly better corrosion resistance than PEI-coated Mg.
To evaluate the practical corrosion behavior of each stent,the bare,PEI-coated,and Ta/PEI-coated Mg stents were deployed in silicone tubes with diameter expansion ratios of 171.4%.Accelerated corrosive artificial plasma solution was circulated with speed of 40–45 ml/min to simulate the blood flow in the human coronary artery(Fig.S2)[15].Overall shape changes,and remaining volumes of inserted stents at predetermined time periods were closely monitored by micro-CT and are shown in Fig.4Ba.In the case of the bare Mg stent,overall stent struts were rapidly corroded,fractured,and migrated,showing almost none of the original structure after only 3 days of dynamic corrosion testing.On the contrary,the PEI-coated and Ta/PEI-coated Mg stents seemed to degrade much more slowly and retained their structures longer than the bare Mg stent in circulation conditions without any sudden breakdown throughout the 10 days of dynamic corrosion testing.These microstructural observations match well with the quantitative results of remaining stent volume(Fig.4Bb).Because of its rapid corrosion behavior,the bare Mg stent showed drastically reduced remaining stent volume in 3 days,dropping from 100 to 3%.After 6 days,no volume remained in the micro-CT measurement.In contrast,the PEI-coated and Ta/PEI-coated Mg stents showed slower and more linear temporal trends in remaining stent volume.Although corrosion volumes of both stents gradually increased,their remaining volumes even after 10 days were about 40%,taking almost five times as long as the bare Mg stent to reach 40% remaining stent volume.
As is well known,PEI possesses excellent Mg corrosion protection ability because of its strong adhesion to the Mg surface [25,28].It can minimize the formation of excessive hydrogen gas bubbles at the interface between the surface coating layer and Mg substrate,thus maintaining its protective ability under physiological conditions.In addition,even after stenting procedures,the PEI coating layer retained uniform surface morphologies without coating delamination or fracture(Fig.2B).Since the surface coating primarily plays the role of physical barrier on the Mg surface,the mechanically stable behaviors of the PEI coating layer could effectively prevent direct contact between the corrosive solution and the Mg,thus ensuring the uniform,slow degradation of the Mg stent.In addition,this excellent corrosion protection ability of the PEI coating layer was still maintained after the S-PIII treatment,as is evident from the almost identical corrosion resistance properties of the PEI-coated and Ta/PEI-coated Mg.The extremely short processing time of the S-PIII treatment(≈30 s)combined with the nanoscale Ta ion implantation depth is expected to minimize deterioration of both the mechanical stability and the corrosion protection conferred by the PEI coating layer.
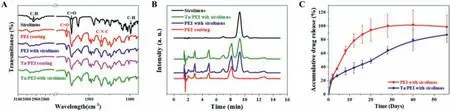
Fig.5.(A)FT-IR transmission spectra of sirolimus and PEI-coated and Ta/PEI-coated Mg samples with and without sirolimus loading.(B)HPLC chromatograms of sirolimus,PEI,and PEI and Ta/PEI with sirolimus loading.(C)Sirolimus release profiles for 8 weeks from PEI-coated and Ta/PEI-coated Mg samples in PBS solution.
3.3.Stability of loaded sirolimus and its release behavior
The loading stability of sirolimus in PEI and Ta/PEI coatings was confirmed by FE-SEM,FT-IR,and HPLC analysis.As shown in Fig.S3,neither surface coating’s surface morphologies showed any apparent differences with and without sirolimus nor did either showing any noticeable recrystallization or agglomeration of the loaded drug.Since recrystallized sirolimus particles of>400 nm diameter provide poor rates of delivery to arterial tissue,uniformly distributed small(<400 nm)drug particles in the PEI and Ta/PEI coating layers are expected to provide the desired efficiency of drug delivery in clinical practice [33].Fig.5A shows the FT-IR spectra of sirolimus-loaded and non-loaded samples of both PEI and Ta/PEI coatings.Free sirolimus was also analyzed for comparison purposes.In the spectra of sirolimus-loaded PEI and sirolimus-loaded Ta/PEI coatings,the characteristic peaks of PEI and sirolimus were clearly detected;the peaks at 2930 and 1746 cm−1correspond respectively to the C–H and C=O bonding of the sirolimus,whereas the peaks at 1778 and 1720 cm−1respectively represent symmetric and asymmetric stretching vibrations of C=O in the PEI;the peak at 1353 cm−1represents C–N–C bonding in the PEI[19,28].No additional or shifted peaks were observed in either coating,indicating that the sirolimus was physically dispersed within the PEI matrix without any secondary chemical bonding or decomposition of the loaded drug [33].The HPLC result also demonstrated the stability of the loaded sirolimus in the PEI and Ta/PEI coatings,as shown in Fig.5B.An essential piece of information required for identifying the molecular structures of drugs in the extracts,the retention time of the free sirolimus was found to be around 9.8 min with excellent peak resolution,whereas the non-loaded PEI coating showed three main peaks at 2.9,4.9,and 8.1 min.In previous studies,sirolimus loaded in polymer matrix was easily affected by ambient environmental factors,such as temperature,acidic/basic conditions,and plasma treatment;this sensitivity leads to the degradation of drug molecules and formation of additional peaks in the HPLC spectra [38].However,in the present study’s HPLC spectra,both sirolimusloaded PEI and sirolimus-loaded Ta/PEI coatings showed only sirolimus and PEI main peaks with strong intensities,demonstrating freedom from issues of compatibility between the loaded sirolimus in the PEI coating and the S-PIII surface treatment.
The release behavior of loaded sirolimus in the PEI and Ta/PEI coatings on Mg plate samples were investigated for 8 weeks.Their accumulated release amounts were measured by UV spectroscopy,as shown in Fig.5C.Prior to the release test,the total amounts of loaded sirolimus in the PEI and Ta/PEI coatings were measured to calculate a relative ratio of drug release,showing exactly the same level of loaded drug:0.75 μg/mm2(Table S1).The PEI coating showed a typical release profile with an initial burst;79% of sirolimus was released with a high rate up to 12 days,whereas a small amount of residual drug was released later.Drug release was complete within around 30 days,after which no further release was observed.On the contrary,the Ta/PEI coating showed an apparent dual release profile of sirolimus involving a very slight burst initially(24% within 2 days)followed by a sustained release at an almost constant rate for the rest of the period.Even after 56 days,the loaded sirolimus was not fully released from the Ta/PEI coating;about 10% of the drug remained in the coating layer.The Ta/PEI coating’s significant extension of the period of drug release is mainly attributed to the Ta-implanted skin layer on the topmost surface of the PEI.Unlike Mg corrosion,which is controlled by diffusion of small water molecules(18.0 Da)on the surface,drug release could be controlled by diffusion of the relatively large molecule size of sirolimus(914.2 Da)at the top surface of the coating layer [39,40].Because the energetic metal ion implantation onto the polymer surface generally leads to compressive stress in the depth direction,the effective spacing between surface polymer chains is reduced[29,30].This results in a denser and thinner metal nucleiimplanted surface skin layer(Fig.3A).Even though small water molecules near the coating surface could relatively easily penetrate to the coating skin layer [41],as exemplified by the almost identical corrosion behaviors of the PEI-coated and Ta/PEI-coated Mg samples,the Ta-implanted surface skin layer acts as an effective physical barrier to drug molecule diffusion and hence shows a sustained release pattern over 56 days.
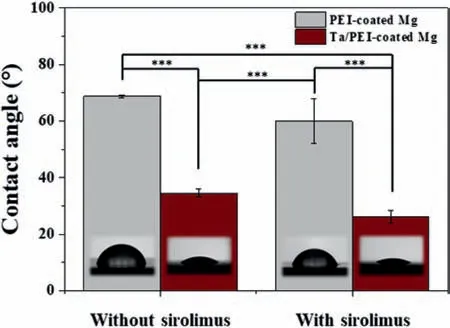
Fig.6.Contact angles of distilled water on PEI-coated and Ta/PEI-coated Mg samples with and without sirolimus loading.Statistical significance of p < 0.001 is indicated by ∗∗∗.
3.4.Surface wettability
Surface wettability is one of the most important surface properties affecting the biocompatibility of materials [29,33].Prior to the investigation of cellular response,surface wettability was evaluated for the PEI-coated and Ta/PEI-coated Mg with and without sirolimus loading;results are presented in Fig.6.In both cases,the contact angles decreased slightly after sirolimus loading due to hydrophilic oxygen-containing functional groups contained in the sirolimus molecules.A contact angle of 60 ° was determined for sirolimus-loaded PEI-coated Mg,whereas a contact angle of 26 ° was determined for the sirolimus-loaded Ta/PEI-coated Mg.However,the amount of incorporated sirolimus was insufficient to produce a statistically significant improvement in surface wettability(p >0.05).On the contrary,the S-PIII surface treatment substantially improved the surface wettability of the PEI coating layer;regardless of drug loading,the Ta/PEIcoated Mg showed markedly and statistically significantly(p <0.001)lower water contact angles(37 °)than the PEIcoated Mg(68 °).This is a typical result of the S-PIII process with polymer substrates,in which massive Ta ion implantation readily breaks the surface polymer networks and generates highly hydrophilic surface species [29,30].Furthermore,as verified above,Ta/PEI–Mg possesses the stable oxide layer of Ta2O5,which is well known for its better wettability than PEI [29],thereby synergistically enhancing surface wettability.
3.5.In vitro cellular response of Ta-implanted PEI coating on Mg stent
Thein vitrocellular responses toward the sirolimusloaded PEI-coated and sirolimus-loaded Ta/PEI-coated Mg were measured in terms of adhesion and proliferation behaviors of smooth muscle cells and endothelial cells.When the stent is deployed in a blood vessel,its abluminal surface is in direct contact with the lesioned vessel wall,whereas the luminal surface is exposed only to blood flow [33].Therefore,the smooth muscle cells approach the abluminal stent surface(Fig.7A),while the endothelial cells approach the luminal surface(Fig.8A).To mimic thisin vivosituation,the VSMCs and HUVECs were separately cultured on different sets of samples.
First,the effect on VSMC behavior of sirolimus loading in the PEI coating layer was examined by CLSM observation and measurement of quantity of DNA.These results were compared with results from sirolimus-nonloaded samples to assessing the function of sirolimus suppressing smooth muscle cell overgrowth in the lumen of a stented vessel(instent restenosis).Fig.7B shows CLSM images of the adhered SMCs on two Mg substrates after 3 and 6 days of culturing.Without sirolimus,the cells adhered well to,and spread out actively on,the PEI-coated Mg surface;they exhibited a progressive formation of filopodia-like protrusions in multiple directions,and the number of cells on the surface also apparently increased during the culturing period.On the contrary,only a few VSMCs adhered individually to the surface of PEI-coated Mg with sirolimus,and their number did not significantly change during 6 days of culturing.In particular,most of the surface with sirolimus was left free of attached cells,and the cells did not display much adhesion to the substrate,showing the limited extent of cell spreading during the culturing period.The results regarding the total quantity of DNA also confirms the significant suppression effect of sirolimus loading on VSMC growth.As shown in Fig.7C,the sirolimus-loaded PEI-coated Mg showed lower quantities of DNA than without sirolimus,and the difference between them became more significant as the cell culture duration increased.
Second,to investigate the endothelial cell responses to the Ta/PEI-coated Mg with sirolimus,designed as the luminal surface of the Mg stent,four different samples were compared for their ability to support HUVEC’s attachment,spreading,and proliferation:PEI-coated Mg with/without sirolimus and Ta/PEI-coated Mg with/without sirolimus.After 3 and 6 days of culturing,adhered HUVEC morphologies on the surfaces of each sample were observed by CLSM and are presented in Fig.8B.Without the Ta-implanted surface layer,the HUVECs were greatly influenced by the existence of sirolimus,as was VSMC;the number and spreading area of the adhered cells were noticeably reduced on the PEI-coated Mg with sirolimus throughout the culturing period,compared with the case of no sirolimus.In contrast,in case of the Ta/PEI-coated Mg,there was no considerable difference in the adhered numbers and spreading morphologies of the HUVECs with and without sirolimus loading.On both surfaces,a large number of cells tightly adhered and progressively spread out during the culturing,showing a typical cobblestone-like monolayer covering almost the entire surface by day 6 of the culture.From the quantitative measure shown in Fig.8C,the Ta/PEI-coated Mg exhibited outstanding rates of cell–surface coverage regardless of sirolimus loading,whereas the PEI-coated Mg with sirolimus showed consistently the lowest values among all treatments.The results regarding total quantity of DNA of HUVECs also indicate a favorable biological effect of the Taimplanted surface layer(Fig.8D).At 6 days of culturing,the quantities of DNA of HUVECs cultured on the Ta/PEI-coated Mg were in some way decreased by the sirolimus,whereas the quantity of DNA showed a substantial increase(59%).In contrast,the HUVECs on the PEI-coated Mg were completely inhibited from proliferation by the sirolimus,showing identical quantities of DNA from culturing day 3 to day 6.

Fig.7.(A)Schematic diagram of VSMC approaching toward PEI-coated surface(abluminal surface)of Mg stent.(B)Representative CLSM images of adhered VSMCs and(C)their DNA amounts on PEI-coated Mg samples with and without sirolimus for 3 and 6 days of culturing.White scale bars indicate 100 μm.Statistical significance is indicated as follows:∗for p < 0.05 and ∗∗for p < 0.01.
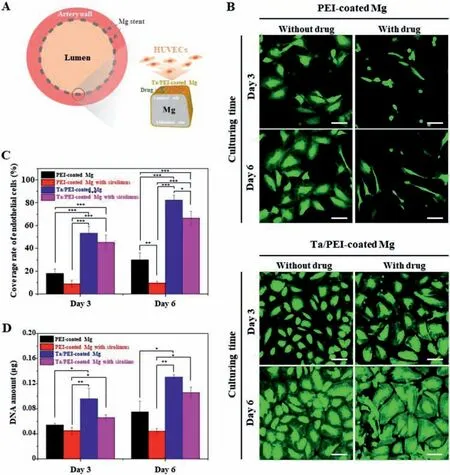
Fig.8.(A)Schematic diagram of HUVEC approaching toward Ta/PEI-coated surface(luminal surface)of Mg stent.(B)Representative CLSM images of adhered HUVECs and their(C)surface coverage rates and(D)DNA amounts on PEI-coated and Ta/PEI-coated Mg samples with and without sirolimus loading for 3 and 6 days of culturing.White scale bars indicate 100 μm.Statistical significance is indicated as follows:∗for p < 0.05,∗∗for p < 0.01,and∗∗∗for p < 0.005.
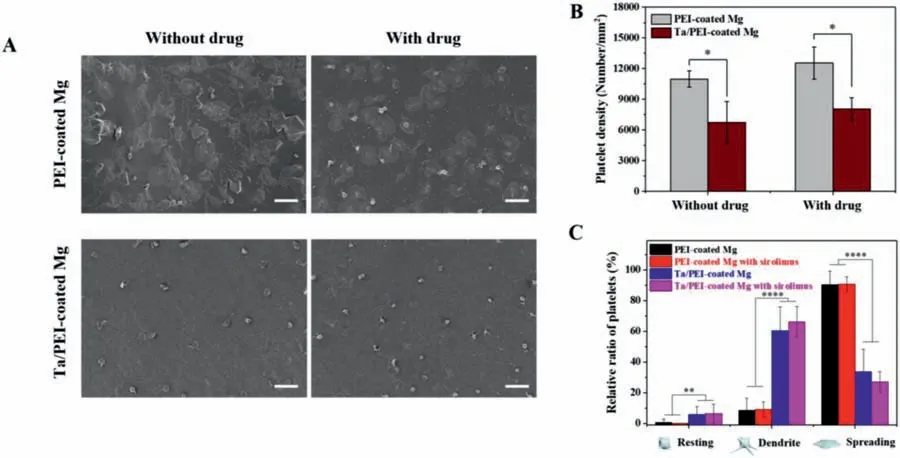
Fig.9.(A)Representative SEM images of adhered platelets on PEI-coated and Ta/PEI-coated Mg samples with and without sirolimus loading.White scale bars indicate 100 μm.(B)Quantitative density of adhered platelets and(C)relative ratio of adhered platelets in resting,dendrite,and spreading on each Mg sample.Statistical significance is indicated as follows:∗for p < 0.05,∗∗for p < 0.01,and ∗∗∗∗for p < 0.001.
Even though anti-proliferative drugs are effective for preventing smooth muscle cell overgrowth,they are also able to retard endothelial cell spreading and proliferation,leading to a limit re-endothelialization on the luminal surface of the stents and to a delay of vascular healing processes[28].Since the monolayer of endothelial cells lining the inner surface of vessels,so-called endothelium,serves crucial vascular functions(e.g.,non-thrombogenic surface and suppression of smooth muscle cell overgrowth),the delayed reendothelialization is directly related to the adverse clinical outcomes of stent placement [30,33,42].However,according to Fig.8B–D,it is noteworthy that the Ta-implanted surface skin layer not only diminished the effect of the sirolimus on the function of HUVECs but also improved cell–surface interactions.Even though the extremely short processing time of S-PIII treatment did not induce any compatibility issue with loaded sirolimus,the nano-thick Ta-implanted layer formed on the topmost surface of the PEI coating played an effective role in preventing direct exposure of the sirolimus to the surface-adhered cells.More importantly,during S-PIII treatment,energetic Ta ion bombardment of the PEI coating surfaces can lead to destruction of polymeric chains,chemical bond cleavage,and creation of reactive oxygen functionalities on the surface of PEI coating [29,30,43].These surface chemical species are well known for its intimate interaction with HUVECs and are closely associated with better HUVEC attachment,spreading,and proliferation rate compared to PEI-coated Mg [30,44].In addition,in previous studies,the implanted Ta itself has shown its unique capacity to facilitate endothelial cell adhesion and proliferation,leading to the rapid formation of an endothelial cell monolayer without affecting VSMC proliferation [45–47].The supplemental VSMC attachment test after 3,6,and 24 h of culturing(Fig.S4)clearly showed that VSMCs adhered and proliferated on both the Ta/PEI-coated Mg and PEI-coated Mg without any significant differences in adhered cell morphology,size,and number.Because excess of VSMC proliferation on the luminal surface of stent is considered to be a major cause of occlusive disorders,the Ta/PEI coating would result in superior vascular healing with complete re-endothelialization by selectively supporting intimate interaction with HUVECs[30,47].
3.6.Blood compatibility analysis
Platelet adhesion and activation analysis were also performed to assess blood compatibility of the stent’s luminal surface.Once adhered platelets become activated,they secrete multiple pro-inflammatory molecules,which are involved in the impaired endothelial function of and vascular inflammation[30,33].Therefore,the same set of samples as used in the HUVEC analysis was tested for the observation of surfaceadhered platelet morphologies.Fig.9A shows the morphologies of adhered platelets on each sample.Without the Taimplanted surface layer,a large number of platelets adhered and extensively spread over the surface of PEI-coated Mg.In contrast,the number of platelets adhering to the Ta/PEI-coated Mg surface was significantly lower,and their morphologies were noticeably changed,showing a compact central body with a few short dendrites.As shown in Fig.9B and C,the Ta/PEI-coated Mg exhibited 70% fewer adhered platelets and a 30% lower relative ratio of platelets in the spread form when compared with PEI-coated Mg.There was no significant difference between PEI-coated Mg samples with and without sirolimus nor between Ta/PEI-coated Mg samples with and without sirolimus.This finding matches those of previous studies,showing the negligible effect of sirolimus on platelet responses.
Adhered platelets were placed into general categories by their shapes:resting,dendritic,and spread forms.Resting is a non-activated platelet state,maintaining a smooth discoid shape,whereas distinct morphological changes occur as platelet activation progresses from short-dendritic to fully spread shapes [48].Even though the platelets on the Ta/PEIcoated Mg showed dendritic morphology,they formed only a few small dendrites and were barely spread,having an essentially rounded shape,so it can be concluded that most of the adhered platelets were in the partial activation state.On the other hand,the PEI-coated Mg exhibited a fully activated state of adhered platelets.Adhesion and activation of platelets is known to correlate with surface morphology,chemistry,composition,and wettability [29,33,48].In particular,more hydrophilic surfaces preclude platelet adhesion effectively,since hydrophilic surfaces apply steric repulsion between proteins that reach the surface [33,49,50].As displayed in Figs.3Bb and 6,after Ta ion implantation the Ta oxide layer that had formed on the topmost surface led to significantly enhanced surface hydrophilicity of the PEI coating.Furthermore,like other metal oxide compounds,Ta oxide is known as an insulator with a broad band gap [29,51].This characteristic can prevent denaturation of fibrinogen by preventing a surface charge transfer between the fibrinogen and the coating surface.Once the fibrinogen is denatured and transformed to fibrinomonomer and fibrinopeptide,the blood coagulation system is activated,and intraluminal thrombus formation is triggered [29,30,52].Thus,the platelet adhesion and activation analysis demonstrates that the Ta/PEI-coated Mg is particularly advantageous for improving the blood compatibility of the stent while minimizing the risk of thrombosis.
4.Conclusion
In this study,we demonstrated a new strategy for improving the corrosion resistance and vascular compatibility of biodegradable magnesium(Mg)stents via combining PEI coating with subsequent tantalum(Ta)ion implantation.The PEI shows excellent spray coating ability and adhesion to the Mg surface,resulting in enhanced corrosion resistance of the Mg stent under physiological conditions.In addition,PEI’s good sirolimus-carrying ability can suppress overgrowth of vascular smooth muscle cells.The introduction of Ta ion implantation onto only the luminal surface of the PEI-coated Mg stent altered neither the corrosion protection ability of the PEI coating nor the loaded drug’s spectral or release characteristics.A Ta-implanted surface skin layer was formed on the topmost PEI surface with a thickness of 20 nm,acting to improve surface hydrophilicity of the PEI coating while preventing a rapid release of loaded sirolimus.In addition,it prevented direct exposure of the sirolimus to the surfaceadhered endothelial cells,leading to remarkable improvements in cell–surface coverage ratio and in proliferation rates.The number of platelets and their activation rates were significantly suppressed in the presence of the Ta-implanted surface layer.These results demonstrate that the combination of PEI coating and Ta ion implantation offers new opportunities to prevent in-stent restenosis and thrombosis while enhancing the clinical outcomes of the Mg stent.
Associated content
Supporting Information.Cross-sectional FE-SEM images of PEI-coated Mg stent at luminal and abluminal surfaces(Fig.S1).Photographs of the fluid circulating system with bare,PEI-coated,and Ta/PEI-coated Mg stents(Fig.S2).Surface morphologies of PEI-coated and Ta/PEI-coated Mg samples with sirolimus loading(Fig.S3).CLSM images of VSMCs on PEI-coated and Ta/PEI-coated Mg samples(Fig.S4).Loading amount of sirolimus in PEI and Ta/PEI surface coatings(Table S1).
Declaration of Competing Interest
The authors of the present work have no conflict of interest to declare.
CRediT authorship contribution statement
Kwang-Hee Cheon:Visualization,Formal analysis,Methodology,Data curation,Writing–original draft.Cheonil Park:Formal analysis,Data curation.Min-Ho Kang:Methodology,Data curation.Suhyung Park:Methodology,Data curation.Jinyoung Kim:Methodology,Data curation.Seol-Ha Jeong:Formal analysis,Data curation.Hyoun-Ee Kim:Conceptualization,Visualization,Data curation.Hyun-Do Jung:Visualization,Data curation,Writing–original draft.Tae-Sik Jang:Data curation,Writing–original draft.
Acknowledgment
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute(KHIDI),funded by the Ministry of Health &Welfare,Republic of Korea(Grant No:HI18C0493).
 Journal of Magnesium and Alloys2021年6期
Journal of Magnesium and Alloys2021年6期
- Journal of Magnesium and Alloys的其它文章
- Rapid-developing Mg-based biodegradable materials:The editorial of the special issue on Mg-based functional materials -biomaterials section
- Magnesium-Based Materials for Energy Conversion and Storage
- A special editor’s issue on Mg-based functional materials:Design and development
- Assessing the microstructure and in vitro degradation behavior of Mg-xGd screw implants using μCT
- Enhanced hydrogen generation from hydrolysis of MgLi doped with expanded graphite
- In vitro corrosion-fatigue behavior of biodegradable Mg/HA composite in simulated body fluid
