Positive selection analysis reveals the deep-sea adaptation of a hadal sea cucumber ( Paelopatides sp.) to the Mariana Trench*
Ruoyu LIU , Jun LIU Haibin ZHANG
1 Institute of Deep-Sea Science and Engineering, Chinese Academy of Sciences, Sanya 572000, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
Abstract The Mariana Trench, the deepest trench on the earth, is ideal for deep-sea adaptation research due to its unique characters, such as the highest hydrostatic pressure on the Earth, constant ice-cold temperature, and eternal darkness. In this study, tissues of a the hadal holothurian ( Paelopatides sp.) were fixed with RNA later in situ at ~6 501-m depth in the Mariana Trench, which, to our knowledge, is the deepest in-situ fixed animal sample. A high-quality transcript was obtained by de-novo transcriptome assembly. A maximum likelihood tree was constructed based on the single copy orthologs across nine species with their available omics data. To investigate deep-sea adaptation, 113 positively selected genes ( PSGs) were identified in Paelopatides sp. Some PSGs such as microphthalmia-associated transcription factor (MITF) may contribute to the distinct phenotype of Paelopatides sp., including its translucent white body and degenerated ossicles. At least eight PSGs (transcription factor 7-like 2 [TCF7L2], ETS-related transcription factor Elf-2-like [ELF2], PERQ amino acid-rich with GYF domain-containing protein [GIGYF], cytochrome c oxidase subunit 7a, [COX7A], type I thyroxine 5′-deiodinase [DIO1], translation factor GUF1 [GUF1], SWI/SNF related-matrix-associated actin-dependent regulator of chromatin subfamily C and subfamily E, member 1 [SMARCC] and [SMARCE1]) might be related to cold adaptation. In addition, at least nine PSGs (cell cycle checkpoint control protein [RAD9A], replication factor A3 [RPA3], DNA-directed RNA polymerases I/II/III subunit RPABC1 [POLR2E], putative TAR DNA-binding protein 43 isoform X2 [TARDBP], ribonucleoside-diphosphate reductase subunit M1 [RRM1], putative serine/threonine-protein kinase [SMG1], transcriptional regulator [ATRX], alkylated DNA repair protein alkB homolog 6 [ALKBH6], and PLAC8 motif-containing protein [PLAC8]) may facilitate the repair of DNA damage induced by the high hydrostatic pressure, coldness, and high concentration of cadmium in the upper Mariana Trench.
Keyword: sea cucumber; Mariana Trench; deep-sea adaptation; positive selection analysis; translucent white body; ossicle degeneration
1 INTRODUCTION
More and more investigations suggest that life is abundant in the deep sea (Jamieson, 2015; Downey et al., 2018), which upends the conventional view regarding the deep sea as the ‘life desert’ (Anderson and Rice, 2006). The deep sea is a unique environment due to its high hydrostatic pressure, low temperature and darkness (Anderson and Rice, 2006). High hydrostatic pressure changes the intra or inter-molecular interactions that tend to induce macromolecular irreversible unfolding and aggregation (Balny et al., 1997; Wilton et al., 2008; Lan et al., 2017). Another limitation is the ice-cold temperature that tends to cause nucleic acids to adopt unfavorable structures and hinders enzyme activities (Anderson and Foote, 1975; Feller and Gerday, 2003).
High throughput sequencing can provide a vast amount of information with just a small piece of tissue. At present, multi-omics investigations have greatly advanced our understanding of deep-sea adaptation, such as adapting vision abilities across three deep-sea fishes (Musilova et al., 2019), enhancing levels of organic osmolytes in hadal (the ocean deeper than 6 000 m) snailfish and hadal amphipods (Simonato et al., 2006; Lan et al., 2017; Wang et al., 2019), adjusting the membrane lipid composition in a hadal snailfish (Wang et al., 2019), chemosymbiotic clams (Lan et al., 2019) , and mussels (Sun et al., 2017), positively selecting cytoskeleton related genes in bathyal (the ocean at depth from 1 000 to 4 000 m) fish (Lan et al., 2018), positively selecting the DNA repair genes in hadal amphipod (Lan et al., 2017) and bathyal fish (Lan et al., 2018), and adapting the symbiosis system in chemosynthetic ecosystems, including for animals such as limpets (Liu et al., 2020), snails (Sun et al., 2020), clams (Lan et al., 2019), squat lobsters (Cheng et al., 2019), mussels (Sun et al., 2017; Zheng et al., 2017), shrimps (Zhang et al., 2017a), polynoids (Mehr et al., 2015; Zhang et al., 2017b), and crabs (Hui et al., 2017) from hydrothermal vents or cold seeps.
A hadal trench is the deepest point on the Earth. It is ideal for deep-sea adaptation research because of its unique characters, such as the highest hydrostatic pressure on the Earth and a constant low temperature (Jamieson, 2015). However, sample collection is diき cult in such a unique environment. At present, only a hadal snailfish genome (Wang et al., 2019) and a hadal amphipod transcriptome (Lan et al., 2017) have been reported. Scarce omics data in hadal trenches limit our knowledge to further understand the molecular mechanism for deep-sea adaptation.
Sea cucumbers (common name for holothurians) are the dominant invertebrates in hadal trenches (Beliaev and Brueggeman, 1989; Jamieson, 2015). Here, we present a transcriptome of a holothurian Paelopatides sp. (Holothuroidea, Synallactida) from the Mariana Trench, which, to our knowledge, is the deepest in-situ fixed macrofauna specimen from the hadal deep. To investigate the molecular mechanism of adaptation to the hadal environment, a positive selection analysis was performed by comparing the transcriptome of the hadal holothurian with its shallow-water relatives.
2 MATERIAL AND METHOD
2.1 Sample collection, ossicle preparation, and RNA extraction and sequencing
During a cruise by DY37-II Dive116 on 16 June 2016, a sea cucumber Paelopatides sp. was captured by the human-occupied vehicle (HOV) Jiaolong on a muddy bottom of the Mariana Trench (141°56.1719′E, 10°57.1693′N) at a depth of ~ 6 501 m (Fig.1a), where the seawater temperature was about 1.68 °C. The body wall of the hadal specimen was crushed into pieces and fixed with RNAlater in situ at the same time as collection. After landing on the deck, the dissected tissues with RNAlater were stored at -80 °C until used for sequencing. Two shallow-water holothurians were also collected in November 2016. Stichopus horrens was sampled on a muddy patch of a seagrass bed in Sanya, China. While Apostichopus japonicus was sampled from a muddy coast in Qingdao, China (Fig.1a). They were captured at a depth of no deeper than 10 m, where the seawater temperature was about 22 °C and 15 °C respectively. The body wall of these two shallow-water samples were dissected in RNAlater and then stored at -80 °C.
For scanning electron microscope (SEM) examinations of ossicles, standard protocols of Smirnov et al. (2000) were followed: first, small pieces of the body wall were dissolved in 6% sodium hypochlorite solution, then rinsed five times in ultrapure water, finally the water with ossicles were transferred to aluminum stubs and dried in a draught drying cabinet. The dried ossicles were observed under SEM Phenom ProX (Thermo Fisher Scientific, Waltham, MA, USA).
The total RNA of each specimen ( Paelopatides sp., Stichopus horrens, and Apostichopus japonicus) was separately extracted from its inner body wall using a RNeasy Plus Universal Kit (QIAGEN, Hilden, Germany). The quantity and quality of the RNA were examined by agarose gel electrophoresis and a NanoDrop 2000 (Thermo Fisher Scientific) as well as Liang et al. (2020). A pair-end library (150 bp) was first prepared with an NEBNext Ultra RNA Library Prep Kit for Illumina (New England BioLabs Inc, Ipswich, MA, USA) following the manufacturer’s recommendations, then purified with an AMPure XP system (Beckman Coulter, Brea, CA, USA), at last assessed on the Agilent Bioanalyzer 2100 system (Agilent Technologies, Inc., Santa Clara, CA, USA). The library was clustered with a TruSeq PE Cluster Kit v3-cBot-HS (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol, and sequenced on an Illumina HiSeq 4000 (Illumina). Library preparation and sequencing were completed by NovoGene (Beijing, China).
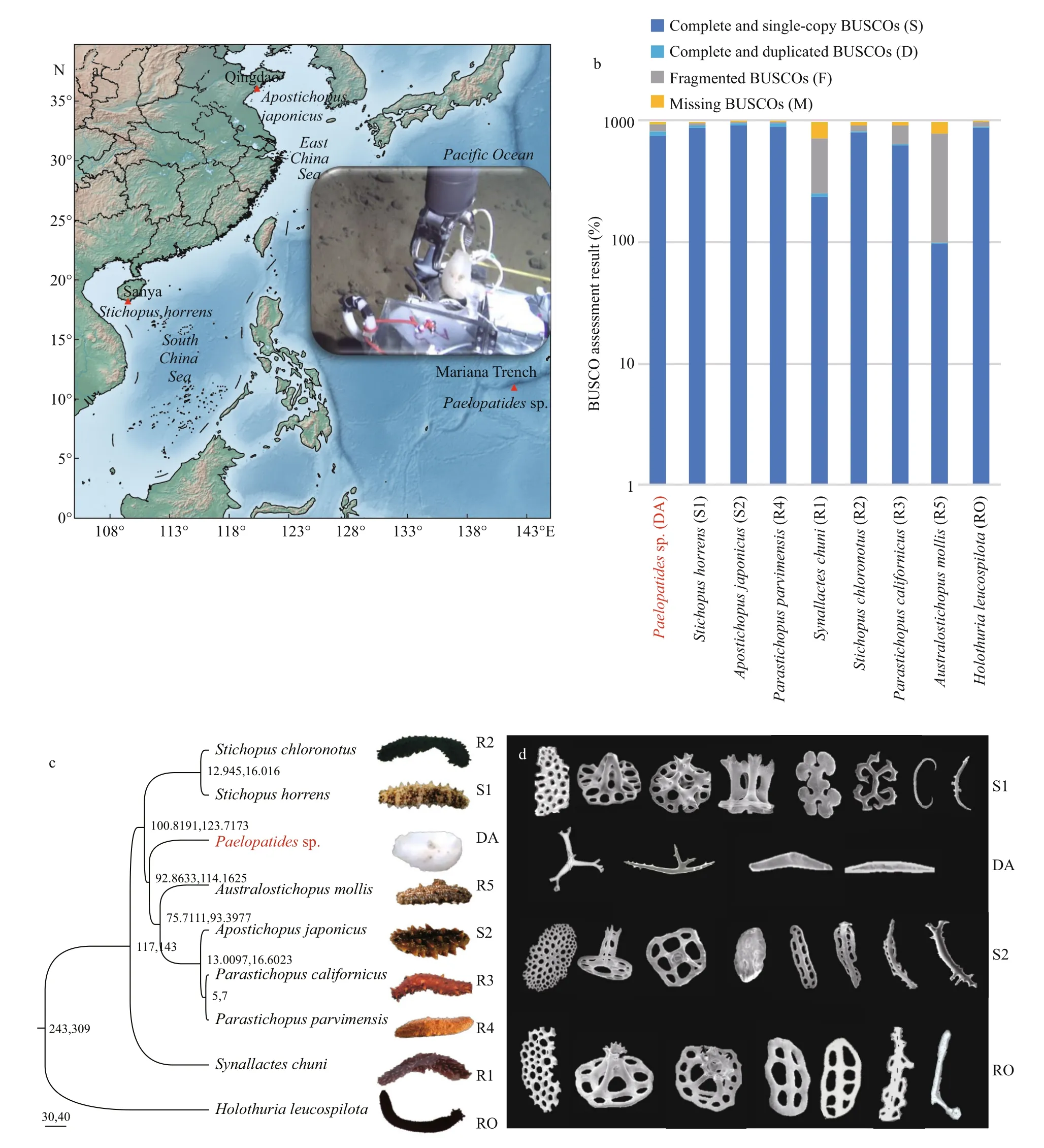
Fig.1 Information of sampling and comparative analyses of Paelopatides sp.
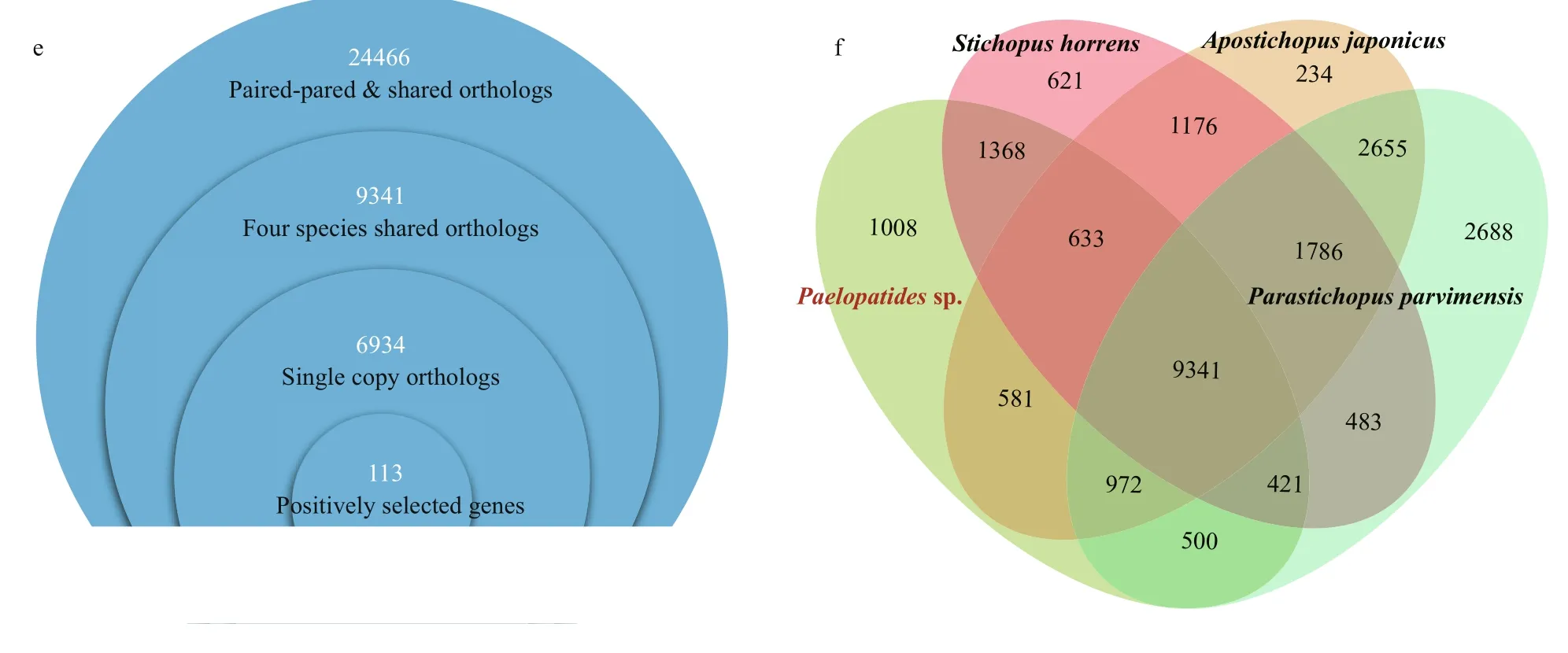
Fig.1 Continued
2.2 Data filtering, assembly, and coding genes prediction and annotation
The raw sequencing reads were evaluated by FASTQC (v0.11.6; Andrews, 2010) and cleaned by fastp (v0.20; Chen et al., 2018) with default parameters. The clean reads were de novo assembled using TRINITY (v2.4.1; Haas et al., 2013) with min_kmer_cov 2 to get the assembled transcriptome. RSEM (Li and Dewey, 2011) was employed to estimate the gene/isoform expression. Only the isoforms with the highest abundance within the same gene were retained (Li and Dewey, 2011). The CD-HIT-EST (v4.6.8; Li and Godzik, 2006) with a threshold of at least 95% similarity was employed for further cleaning (Lan et al., 2017). Finally, only genes longer than 200 bp were regarded as the valuable non-redundant transcripts for subsequent analyses. Benchmarking Universal Single Copy Orthologs (BUSCO v3.0.2b, metazoa_odb9 database; Waterhouse et al., 2018) was employed to evaluate the quality of the transcripts. Coding sequences of the non-redundant transcripts were predicted and translated with TransDecoder (v5.0.2; Haas et al., 2013) with a minimum amino acid length of 50 bp. The non-redundant transcripts were first aligned to the SWISS-PROT database using blastp in BLAST (v2.2.28; Altschul et al., 1997) and the PFAM-A database using the HMMER 3.0 package Hmmscan (Finn et al., 2011) with an E-value <0.01. Then, the non-redundant transcripts were annotated using DIAMOND (v0.8.22; Buchfink et al., 2015) with an E-value <1e-5against the NCBI NR, NT and KOG database. Finally, the domains of the predicted proteins were recovered using InterProScan 5 (v 5.8-51; Jones et al., 2014) with the default parameters.
2.3 Reference species determination
All available genomic and transcriptomic data of the sea cucumbers in Synallactida were screened, including the three species ( Paelopatides sp., Stichopus horrens, and Apostichopus japonicus) sequenced in this study, and five reference species from previously reported studies (Table 1): Synallactes chuni (SRR2895367 in NCBI SRA; Janies et al., 2016), Stichopus chloronotus (SRR2846098; Janies et al., 2016), Parastichopus californicus (SRR1695477; Cannon et al., 2014), Parastichopus parvimensis (SRR2484238; Reich et al., 2015) with transcriptome data, and Australostichopus mollis (PRJEB10682; Long et al., 2016) with genome data. The proteins and the coding sequences of Australostichopus mollis genome were adopted from http://ryanlab.whitney.ufl.edu/genomes/Amol/. The transcriptome of Holothuria leucospilota (Holothuroidea, Holothuriida; DRR023763; Chieu et al., 2018) was used as the outgroup in the further phylogenetic analysis. All reference transcriptomes were re-assembled, translated, and assessed with the same pipelines as described above.
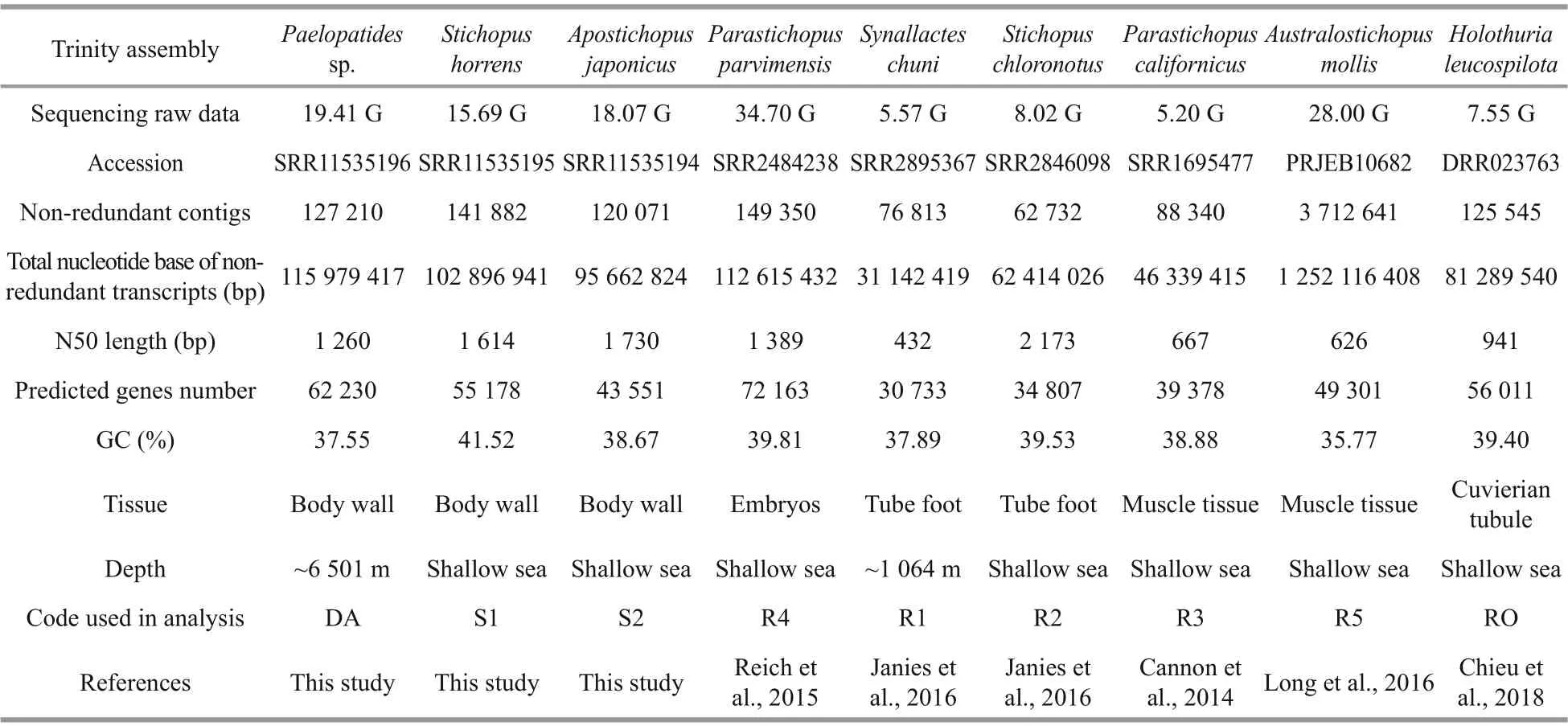
Table 1 De-novo assembly statistics for Paelopatides sp., Stichopus horrens, Apostichopus japonicus, Parastichopus parvimensis, Stichopus chloronotus, Parastichopus californicus, Synallactes chuni, Australostichopus mollis, and Holothuria leucospilota
2.4 Identification of orthologs and phylogenetic analysis
Single copy orthologs of nine species ( Paelopatides sp., Stichopus horrens, Apostichopus japonicus, Parastichopus parvimensis, Synallactes chuni, Stichopus chloronotus, Parastichopus californicus, Australostichopus mollis, and Holothuria leucospilota) were first inferred by orthomcl (v2.0.9; Li et al., 2003), and then were multiple aligned with MAFFT (v7.221; Katoh and Standley, 2013). The poorly aligned positions were trimmed by Gblocks (v0.91b; Talavera and Castresana, 2007). ProtTest (v3.4; Darriba et al., 2011) was used to determine the best model. RaxML (v8.2.11; Stamatakis, 2014) was employed to construct a maximum likelihood (ML) tree with 1 000 bootstraps. The divergence time among species was estimated via r8s (v1.7; Sanderson, 2003). Four calibration nodes based on the Miller et al. (2017) were used as time priors: the most recent common ancestor of Pneumonophora (243-309 million years ago, [Ma]), the most recent common ancestor of Synallactida (117-143 Ma), and Parastichopus californicus - Parastichopus parvimensis (5-7 Ma).
2.5 Positive selection analysis
Transcriptome data combined with a branch site model can effectively identify genome-wide positive selection (Yang and dos Reis, 2011; Yang et al., 2015), and therefore were popular tools for molecular adaptation research (Lan et al., 2017, 2018, 2019). The quality of all the transcripts (Fig.1b) were screened in BUSCO v3.0.2b (metazoa_odb9 database; Waterhouse et al., 2018), and the species with BUSCO missing values higher than that of the hadal species were not used for the positive selection analysis. Finally, three shallow-water species, Stichopus horrens, Apostichopus japonicus, and Parastichopus parvimensis, as well as Paelopatides sp. were retained. The phylogenetic relationship across these four species was reconstructed as mentioned above. ParaAT (v2.0; Zhang et al., 2012) with a -g parameter was used to align the coding DNA sequences of each ortholog according to their amino acid sequence alignment. A modified branch-site model in the codeml module of the phylogenetic analysis by maximum likelihood (PAML, v4.9h; Yang, 2007) was employed to detect the positively selected genes ( PSGs) in deep-sea animals as previously reported (Hui et al., 2017; Lan et al., 2017, 2018, 2019; Sun et al., 2017; Zheng et al., 2017; Cheng et al., 2019). The hadal Paelopatides sp. was designated as the foreground phylogeny; the remaining three shallowwater holothurians were designated as the background phylogeny. An alternative branch site model (Model=2, NSsites=2, and fix_omega=0) and a neutral branch site model (Model=2, NSsites=2, fix_omega=1 and omega=1) were configured. P-values were first computed by a chi-squared test (Zhang et al., 2005) and then corrected by a multiple testing correction (Benjamini and Hochberg, 1995). Genes with Bayesian Empirical Bayes (BEB) sites exceeding 90% and corrected P-values lower than 0.05 were considered as PSGs (Lan et al., 2017). In addition to the annotation information of the seven databases mentioned above, the PSGs were also imported into InterProScan 5 (Jones et al., 2014) to obtain more annotation information.
As to the identified PSGs in the hadal Paelopatides sp., we also compared their expression level with their two shallow-water relatives ( Stichopus horrens and Apostichopus japonicus). The transcripts per million (TPM) was used as the measure of expression, as it has been recommended to be more comparable across samples than fragments per kilobase of transcript per million mapped reads (FPKM; Li et al., 2010). The heatmap was generated by pheatmap packages (https://cran.r-project.org/web/packages/pheatmap) in R (v3.2; The R Core Team, 2016).
3 RESULT
In this study, 129 376 486 raw paired end reads (150 bp) of Paelopatides sp. were generated. After trimming, 125 583 692 clean reads (97.07%) were retained and used for the de novo assembly (Table 1). After removing redundant isoforms, 127 210 nonredundant contigs were produced. The non-redundant contigs ranged from 201 to 15 346 bp with a total size of 115 979 417 bp. The contig N50 of the assembled transcripts was 1 260 bp (Supplementary Fig.S1a & b; Table 1). These assembled transcripts hit 935 (95.60%) of the single copy orthologs in the BUSCO metazoan database (Fig.1b).
In addition, 62 230 protein-coding sequences were obtained from the non-redundant transcripts of Paelopatides sp. (Table 1). The annotation rate of the hadal Paelopatides sp. was slightly lower when compared to that of the two shallow-water holothurian species (Supplementary Fig.S1c; Supplementary Table S1). Interestingly, in the annotation of GO, KEGG, and KOG databases, the annotation percentage of genes related to some pathways was consistently higher in the hadal Paelopatides sp. when compared with their two shallow-water holothurians, including replication and repair; translation; folding, sorting and degradation; cell cycle control, cell division, and chromosome partitioning; chromatin structure and dynamics; and energy production and conversion. Furthermore, the percentage of genes related to rhythmic process and extracellular transport was consistently lower in the hadal Paelopatides sp. (Supplementary Figs.S2-S4; Supplementary Tables S2-S4).
In this study, 706 single-copy orthologs were identified across Paelopatides sp., Stichopus horrens, Apostichopus japonicus, Parastichopus parvimensis, Synallactes chuni, Stichopus chloronotus, Parastichopus californicus, Australostichopus mollis, and Holothuria leucospilota. An ML tree (Fig.1c) was constructed based on these single copy orthologs with 39 930 amino acids. Synallactes chuni showed the farthest phylogenetic relationship to Paelopatides sp. (Fig.1c). Stichopus chloronotus, Parastichopus californicus, and Australostichopus mollis had a higher missing number of the single copy orthologs than Paelopatides sp. (Fig.1b). We did not use these four species in the following positive selection analysis to increase the numbers of single copy orthologs. Moreover, 24 466 paired-pared and shared orthologs were identified across Paelopatides sp., Stichopus horrens, Apostichopus japonicus, and Parastichopus parvimensis. As shown in Fig.1e & f, 9 341 gene families were shared across all four species. Among them, 6 934 were the single copy orthologs (Fig.1e & f). In these single copy orthologs, 113 genes in Paelopatides sp. were detected to be positively selected (Supplementary Table S5). According to KEGG ortholog (KO, Supplementary Tables S6) and enrichment analysis, among these 113 PSGs, at least 10 PSGs (such as microphthalmiaassociated transcription factor [MITF], transcription factor 7-like 2 [TCF7L2], tartrate-resistant acid phosphatase type 5 [ACP5], lysosomal acid phosphatase [ACP2], disks large protein 1 [DLG1], putative fibrillin-2-like isoform X1 [FBN2], presenilin 1 [PSEN1], large subunit ribosomal protein L38 [RPL38], collagen type II alpha and type V/XI/XXIV/XXVII alpha [COL2A & COL5AS]) are related to the distinct phenotypic characteristics of Paelopatides sp. And at least 24 PSGs (ETS-related transcription factor Elf-2-like [ELF2], PERQ amino acid-rich with GYF domain-containing protein [GIGYF], cytochrome c oxidase subunit 7a [COX7A], SWI/SNF related-matrix-associated actin-dependent regulator of chromatin subfamily C [SMARCC], SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily E [member 1 [SMARCE1], type I thyroxine 5′-deiodinase [DIO1], translation factor GUF1 [mitochondrial [GUF1], cell cycle checkpoint control protein RAD9A [RAD9A], replication factor A3 [RPA3], DNA-directed RNA polymerases I/II/III subunit RPABC1 [POLR2E], putative TAR DNA-binding protein 43 isoform X2 [TARDBP], ribonucleoside-diphosphate reductase subunit M1 [RRM1], putative serine/threonineprotein kinase SMG1 [SMG1], transcriptional regulator ATRX [ATRX], alkylated DNA repair protein alkB homolog 6 [ALKBH6], PLAC8 motifcontaining protein [PLAC8], putative 46 kDa FK506-binding nuclear protein-like [FKBP], peptidemethionine (S)-S-oxide reductase [MSRA], prefoldin alpha subunit [PFDN5], BCL2-associated athanogene 2 [BAG2], abhydrolase domain-containing protein 6 [ABHD6], beta-1,4-galactosyltransferase 6 [B4GALT6], cholesterol 24-hydroxylase [CYP46A1], and AT-rich interactive domain-containing protein 2 [ARID2]) may contribute to the deep-sea adaptation, especially for the cold adaptation and cell homeostasis maintenance that mainly include repairing the damaged DNA induced by high hydrostatic pressure, ice-cold temperature, and some heavy metal such as cadmium (Cd), and sustaining structure of protein, membrane and chromosome (Table 2).
4 DISCUSSION
Investigations into deep-sea adaptation have drawn considerable attention for decades, and comparative omics studies have been proven a powerful way to reveal adaptive mechanism (Foote et al., 2015; Hui et al., 2017; Zheng et al., 2017; Liu et al., 2020). Genome-wide positive selection analysis is an effective tool for adaptation research as it can connect ecological change with molecular change (Yang et al., 2015; Lan et al., 2017, 2018, 2019; Wang et al., 2019). In this study, a positive selection analysis was performed across four closely related holothurians. Compared with its three shallow-water relatives, 113 PSGs (Supplementary Table S5) were identified in the hadal holothurian Paelopatides sp. Combined with environmental factors, some PSGs are supposedly related to the distinct phenotypic characteristics of Paelopatides sp., and some supposedly contributing to cold adaptation and cell homeostasis maintenance (Table 2, Supplementary Table S6) in the hadal deep surroundings.
4.1 Translucent white body
Many animals that dwell in dark surroundings are translucent white, such as some cave animals (McGaugh et al., 2014), hadal snailfish (Wang et al., 2019), and some hadal holothurians (Jamieson et al., 2011; Martinez et al., 2019). Our hadal holothurian Paelopatides sp. is also translucent white (Fig.1c). Compared with its colored shallow-water relatives, the genes MITF and TCF7L2 are positively selected in Paelopatides sp. It is well known that mutations of MITF may cause the white phenotype in many organisms (Schmutz et al., 2009; Hauswirth et al., 2012). In a translucent white hadal snailfish, MITFA is also thought responsible for its unpigmented skin (Wang et al., 2019). Consistently, previous investigations have also shown less melanocytes and lower expression levels of MITF in the transparent white holothurians (Xing et al., 2018). Regarding the MITF in Paelopatides sp., positively selected amino acid sites mainly distribute in and around the active site in the transactivation region of MITF (Fig.2a). These mutations might influence the activity of MITF and affect the expression profiles of its target genes (Fig.2e-g). Furthermore, the positively selected amino acids of MITF tend to adopt longer branched amino acids (Fig.2b). Higher hydrostatic pressure tends to reduce biomacromolecules into smaller volumes and tighter structures (Mozhaev et al., 1996). The PSGs in deep-sea creature substitute with longer branched amino acids that might be involved in sustaining protein structure and coping with high hydrostatic pressure. Additionally, the gene TCF7L2 has been reported to show a lower expression level in non-melanoma skin cells (Dehcheshmeh et al., 2018). These two genes may not only participate in the regulation of melanocytes to produce melanin that gives skin its coloration (Moore, 1995), but also help to protect cells from DNA damage induced by UV (Nguyen and Fisher, 2019). The hadal zone is far from sunlight and lack of UV radiation, there is no need to keep melanogenesis as active as the shallowwater species. Here, two melanogenesis PSGs (MITF and TCF7L2) were also expressed at a lower level in hadal Paelopatides sp. compared to their shallowwater relatives (Fig.3), which might be an economic strategy to live in darkness. Therefore, it suggests that these two PSGs might be involved in darkness adaptation besides may contribute to the pigment loss and translucent white skin.
4.2 Ossicle degeneration
The endoskeleton ossicles dispersed in the dermis of the body walls could protect sea cucumbers. In addition, the characteristics of ossicles also serves as a taxonomic index in Holothuroidea (Liao, 1997; Miller et al., 2017). Based on our observations (Fig.1c & d), the body of the hadal holothurian Paelopatides sp. was smooth in low density, small, and conciseossicles in its dermis, and we found no plate ossicle. In contrast, the shallow-water holothurians were covered with rough papillae where high density, large and complex ossicles distributed. Many large plates were found inside their skin. Ossification degeneration phenomena had also been found in some other deepsea holothurians (Lee Hufford, 1968; Martinez et al., 2019), and ossicle degeneration has been reported in hadal snailfish (Wang et al., 2019). In Paelopatides sp., eight PSGs (ACP5, ACP2, COL2A, COL5AS, DLG1, FBN2, PSEN1, and RPL38) were enriched in the biological process of skeletal system development (Table 2, Supplementary Table S6), and previous investigations had shown that lacking MITF (Lu et al., 2010, 2014) and ACP5 (Hayman et al., 1996) disrupt the ossification. In addition, nine skeleton related PS Gs (MITF, ACP5, ACP2, COL2A, COL5AS, DLG1, FBN2, PSEN1 and RPL38) were also consistently expressed at a lower level in the hadal Paelopatides sp. (Fig.3), which suggests that these PSGs may be the molecular reminders for ossification degeneration of hadal Paelopatides sp.
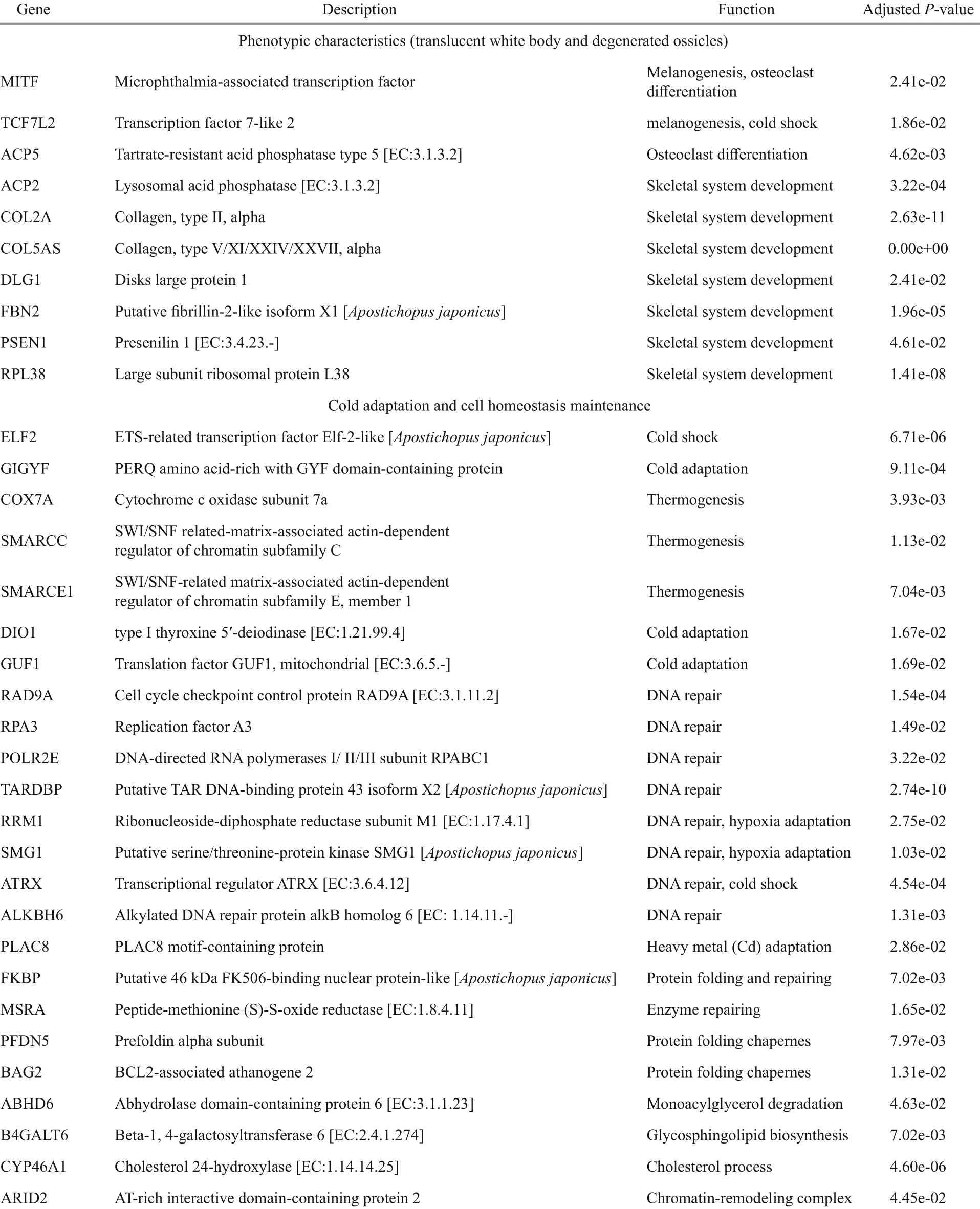
Table 2 Positively selected genes ( PSGs) from Paelopatides sp. related to its distinct phenotypic characteristics (translucent white body and degenerated ossicles) and some deep-sea adaptation (cold adaptation and cell homeostasis maintenance)
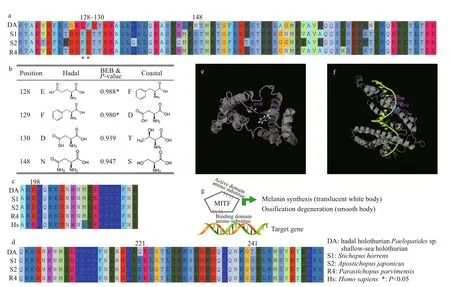
Fig.2 Positively selected amino acid sites in Paelopatides sp. mainly distributed in the transactivation region of MITF
4.3 Cold adaptation
Temperature is an important environmental driver for species distribution (Danovaro et al., 2004). The temperature of the habitat where the Paelopatides sp. was collected was about 1.68 °C (record from HOV Jiaolong). Sea cucumbers are poikilothermal benthos, which cannot regulate their body temperature by themselves (Yang et al., 2006). That means the biochemical systems in the hadal holothurians have to operate in such frozen temperature. In the ice-cold temperature, some enzymes lose their activity, nucleic acids tend to adopt unfavorable structure, and some proteins are out of their normal function when involved in genetic information expression (Feller and Gerday, 2003; Lan et al., 2017, 2018). Moreover, chemical reaction rate have been proofed to be decreased in the low temperature (Carney, 2005; Jamieson, 2015). Even living in such frozen temperature, Paelopatides sp. still adapt and thrive in hadal trenches (observation from Jiaolong; Jamieson, 2015; Martinez et al., 2019). Therefore, how Paelopatides sp. adapt to their ice-cold surroundings is a fantastic point to be further explored.
It is reported that some cold shock domain containing proteins can be involved in cold adaptation by unwinding the unfavorable secondary structures and by facilitating the genetic processes (Thieringer et al., 1998; Lim et al., 2000; Lan et al., 2018). Cold shock proteins are synthesized to overcome the deleterious effects of cold temperatures (Phadtare et al., 1999; Kang et al., 2015; Lan et al., 2017). In this study, ETS domain (TCF7L2, ELF2) and helicase conserved C-terminal domain (ATRX) are positively selected in hadal Paelopatides sp., which are important cold shock domains (Gualerzi et al., 2003; Fry et al., 2018). The homologs of TCF7L2 and ELF2, TCFIIA and ELF1, have been reported to have experienced significant expansion in the hadal amphipod Hirondellea gigas (Lan et al., 2017) and the Antarctic amphipod Gondogeneia antarctica (Kang et al., 2015), and they are suggested to be involved in cold adaptation (Tang et al., 2015; Nie et al., 2019). The Helicase conserved C-terminal domain containing proteins are commonly positively selected across hadal Hirondellea gigas (EIF4G; Lan et al., 2017), bathyal Aldrovandia aき nis (EIF4B; Lan et al., 2018), hadal Pseudoliparis swirei (IFIH1; Wang et al., 2019) and the hadal Paelopatides sp. (ATRX). Meanwhile, the gene GIGYF can regulate mRNA surveillance for cold adaptation by selective interaction with the helicase conserved C-terminal domain containing protein EIF4F (Peter et al., 2017), which was positively selected in Paelopatides sp.
In addition, three thermogenesis related genes (COX7A, SMARCC, and SMARCE1) are positively selected in the hadal Paelopatides sp. (Table 2, Supplementary Table S6). COX7A is an adipocyte marker gene, while adipocytes play important roles in adaptive thermogenesis (Fisher et al., 2012; Maurer et al., 2015). SMARCC and SMARCE1 are molecular determinants in response to temperature (Jin, 2018; Leal et al., 2018). The core thermogenesis PSGs (COX7A) had a consistent higher expression in the hadal Paelopatides sp. than its shallow-water relatives (Fig.3). Moreover, the gene DIO1 and mitochondrial translation factor GUF1 are also positively selected in Paelopatides sp. DIO1 is a deiodinase for thyroid hormone that plays a critical role in cold adaptation in invertebrate (Heyland and Moroz, 2005; Bianco and Kim, 2006; Gereben et al., 2008; Panicker et al., 2008). Mutation of GUF1 would be more sensitive to temperature change and diminished rates of protein synthesis in low temperatures (Bauerschmitt et al., 2008). Therefore, positively selected GUF1 may promote mitochondrial protein synthesis under hadal cold adversity.
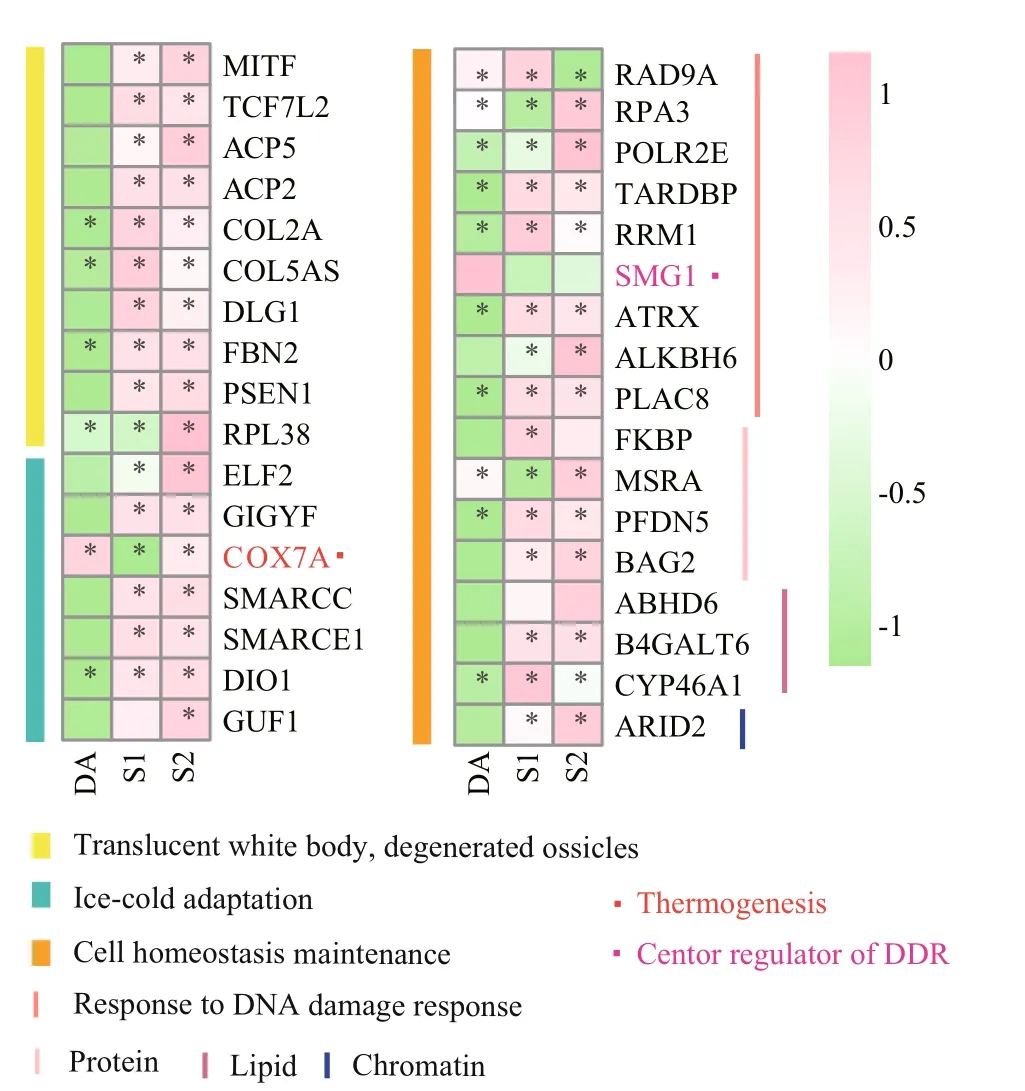
Fig.3 Expression of the PSGs mentioned in discussion part in the hadal Paelopatides sp. and two shallow-water relatives
4.4 Cell homeostasis maintenance
DNA is one of the most important biomacromolecules carrying genetic information for most organisms. Deep-sea animals are susceptible to DNA damage from high hydrostatic pressure (Dixon et al., 2004; Lan et al., 2017) and ice-cold temperature (Anderson and Foote, 1975; Gualerzi et al., 2003). To ensure genetic fidelity, organisms in the deep sea have evolved a variety of mechanisms to eき ciently repair DNA lesions (Dixon et al., 2004). At least eight out of all the PSGs in the hadal Paelopatides sp. are involved in DNA repair including RAD9A, RPA3, POLR2E, TARDBP, RRM1, SMG1, ATRX, and ALKBH6 (Table 2). RPA3 is involved in repairing genetic materials in the replication process (Zou et al., 2006). POL2RE (Bhagavan and Ha, 2015), RAD9A (Lieberman et al., 2017) and ATRX (De La Fuente et al., 2011; Han et al., 2018) are involved in the transcription-coupled repair. TARDBP assembles DNA repair endonucleases to seal DNA breaks (Mitra et al., 2019). RRM1 is necessary for DNA repair especially for the uracil-DNA repair (Ladner, 2001). SMG1 is one of the stress responsive PIKK family members, which serves as the central regulator of DNA damage responses ( DDR; Lloyd et al., 2018). In this study, SMG1 had a consistent higher expression in the hadal Paelopatides sp. than its shallow-water relatives (Fig.3). In addition, ALKBH6 may mediate alkylation damage repair by similarity (Fedeles et al., 2015). These PSGs related to DNA repair may play an important role in maintaining the fidelity of genetic materials in deep-sea surroundings.
In addition to high hydrostatic pressure and low temperature, high concentrations of heavy metals may also cause DNA damages (Lin et al., 2007; Abbà et al., 2011). The upper Mariana Trench (6 500- 7 626 m) is reported to be exposed to the higher concentrations of cadmium (Welty et al., 2018). Cadmium is genotoxic and can induce DNA damage (Lin et al., 2007), while a previous experiment proved that PLAC8 motif-containing protein (OmFCR) and cell cycle checkpoint control protein ( RAD9) are involved in the pathway for the response to cadmium exposure (Abbà et al., 2011). In the hadal Paelopatides sp., PLAC8 and RAD9A are positively selected, which might be involved in the adaptation to the high concentration of heavy-metal surroundings in the upper Mariana Trench.
Moreover, many of the rest of the PSGs (Supplementary Table S6) in Paelopatides sp. are enriched in protein folding, lipid metabolism and chromosome regulated, including FKBP (Kuzuhara and Horikoshi, 2004), MSRA (Stadtman et al., 2005), PFDN5, and BAG2 (Supplementary Table S6), which are dedicated to protein folding. ABHD6, B4GALT6, and CYP46A1 are involved in lipid metabolism (Supplementary Table S6). H3 (Liu et al., 2012) and ARID2 (Zhao et al., 2011) are involved in the chromatin-remodeling complex. High hydrostatic pressure may also result in cellular and macromolecular structural changes including causing protein denaturation (Balny et al., 1997; Somero, 2003; Simonato et al., 2006), reducing the fluidity of cell membranes (Yano et al., 1998; Simonato et al., 2006), and changing the characters of chromosome (Pease, 1946). These structural related PSGs might contribute to the adaptation to the hadal environment with extrahigh hydrostatic pressure.
5 CONCLUSION
In this study, the deep-sea adaptation of a hadal holothurian Paelopatides sp. was investigated. A high-quality transcript and a phylogenetic tree based on omics data were obtained. Based on the positive selection analysis, 113 PSGs were identified in Paelopatides sp. Some PSGs (such as MITF) may contribute to its translucent white body and degenerated ossicles. Some are thermogenesis related genes (COX7A, SMARCC, SMARCE1, DIO1, and GUF1) or cold shock genes (TCF7L2, ELF2, ATRX, and GIGYF) that may be dedicated to cold adaptation. Many of the other PSGs are mainly involved in cell homeostasis maintenance: at least nine PSGs (RAD9A, RPA3, POLR2E, TARDBP, RRM1, SMG1, ATRX, ALKBH6, and PLAC8) may repair the DNA damage induced by the high hydrostatic pressure and ice-cold temperature. In addition to the DNA repair, PLAC8 may facilitate the adaptation to the high concentration of cadmium in the upper Mariana Trench. The valuable in-situ fixed sample from the Mariana Trench, the high-quality assembly, and the positive selection analysis on the hadal holothurian in this study shed light on deep-sea adaptation research.
6 DATA AVAILABILITY STATEMENT
All transcriptome data generated from this study were deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive database (PRJNA613016, SRA Accession Nos.: SRR11535194-SRR11535196).
7 ACKNOWLEDGMENT
We would like to thank Huaining GAO, Zhiqiang WANG, Yanying YE, Yang YANG, Baosheng LI for their helps in in-situ sampler preparation. The authors also want to thank the captains and crews of the R/V Xiangyanghong 09 and the pilots of HOV Jiaolong for their technical support.
8 ABBREVIATION
PSG( s) abbreviate for positively selected gene(s), and full description of abbreviations for each PSG can be found in Table 2 and Supplementary Table S5.
References
Abbà S, Vallino M, Daghino S, Di Vietro L, Borriello R, Perotto S. 2011. A PLAC8-containing protein from an endomycorrhizal fungus confers cadmium resistance to yeast cells by interacting with Mlh3p. Nucleic Acids Research, 39(17): 7 548-7 563, https://doi.org/10.1093/nar/gkr336.
Altschul S F, Madden T L, Schäffer A A, Zhang J H, Zhang Z, Miller W, Lipman D J. 1997. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Research, 25(17): 3 389-3 402, https://doi.org/10.1093/nar/25.17.3389.
Anderson G B, Foote R H. 1975. Effects of low temperature upon subsequent nucleic acid and protein synthesis of rabbit embryos. Experimental Cell Research, 90(1): 73-78, https://doi.org/10.1016/0014-4827(75)90358-4.
Anderson T R, Rice T. 2006. Deserts on the sea floor: Edward Forbes and his azoic hypothesis for a lifeless deep ocean. Endeavour, 30(4): 131-137, https://doi.org/10.1016/j.endeavour.2006.10.003.
Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Balny C, Mozhaev V V, Lange R. 1997. Hydrostatic pressure and proteins: basic concepts and new data. Comparative Biochemistry and Physiology Part A: Physiology, 116(4): 299-304, https://doi.org/10.1016/S0300-9629(96)00355-6.
Bauerschmitt H, Funes S, Herrmann J M. 2008. The membranebound GTPase Guf1 promotes mitochondrial protein synthesis under suboptimal conditions. Journal of Biological Chemistry, 283(25): 17 139-17 146, https://doi.org/10.1074/jbc.M710037200.
Beliaev G M, Brueggeman P L. 1989. Deep Sea Ocean Trenches and Their Fauna. UC San Diego Scripps Institution of Oceanography Technical Report (Translated from Russian. The original book was published in Russian by Nauka Publishing House, Moscow, 1989), 255p. ISBN5-02-00, https://escholarship.org/uc/item/46n6148x.
Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B ( Methodological), 57(1): 289-300, https://doi.org/10.1111/ j.2517-6161.1995.tb02031.x.
Bhagavan N V, Ha C E. 2015. DNA replication, repair, and mutagenesis. In: Bhagavan N V, Ha C E eds. Essentials of Medical Biochemistry. 2ndedn. Academic Press, Amsterdam. p.401-417, https://doi.org/10.1016/b978-0-12-416687-5.00022-1.
Bianco A C, Kim B W. 2006. Deiodinases: implications of the local control of thyroid hormone action. Journal of Clinical Investigation, 116(10): 2 571-2 579, https://doi.org/10.1172/JCI29812.
Buchfink B, Xie C, Huson D H. 2015. Fast and sensitive protein alignment using DIAMOND. Nature Methods, 12(1): 59-60, https://doi.org/10.1038/nmeth.3176.
Cannon J T, Kocot K M, Waits D S, Weese DA, Swalla BJ, Santos S R, Halanych K M. 2014. Phylogenomic resolution of the hemichordate and echinoderm clade. Current Biology, 24(23): 2 827-2 832, https://doi.org/10. 1016/j.cub.2014.10.016.
Carney R S. 2005. Zonation of deep biota on continental margins. In: Gibson R N, Atkinson R J A, Gordon J D M eds. Oceanography and Marine Biology-An Annual Review. Taylor & Francis. New York.
Chen S F, Zhou Y Q, Chen Y R, Gu J. 2018. FASTP: an ultrafast all-in-one FASTQ preprocessor. Bioinformatics, 34(17): i884-i890, https://doi.org/10.1093/bioinformatics/bty560.
Cheng J, Hui M, Sha Z L. 2019. Transcriptomic analysis reveals insights into deep-sea adaptations of the dominant species, Shinkaia crosnieri (Crustacea: Decapoda: Anomura), inhabiting both hydrothermal vents and cold seeps. BMC Genomics, 20(1): 388, https://doi.org/10. 1186/s12864-019-5753-7.
Chieu H D, Suwansa-ard S, Abramov T, Elizur A, Cummins S F. 2018. In vitro oocyte maturation by radial nerve extract and early development of the black sea cucumber ( Holothuria leucospilota). Aquaculture, 495: 247-254, https://doi.org/10.1016/j.aquaculture.2018.05.032.
Danovaro R, Dell’Anno A, Pusceddu A. 2004. Biodiversity response to climate change in a warm deep sea. Ecology Letters, 7(9): 821-828, https://doi.org/10.1111/j.1461- 0248.2004.00634.x.
Darriba D, Taboada G L, Doallo R, Posada D. 2011. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics, 27(8): 1 164-1 165, https://doi.org/10. 1093/bioinformatics/btr088.
De La Fuente R, Baumann C, Viveiros M M. 2011. Role of ATRX in chromatin structure and function: implications for chromosome instability and human disease. Reproduction, 142(2): 221-234, https://doi.org/10.1530/REP-10-0380.
Dehcheshmeh I S, Karimi M M, Jafarisani M. 2018. Increased expression of CCAT2 LncRNA in non-melanoma skin cancer. International Journal of Health Studies, 4(1): 1-4, https://doi.org/10.22100/ijhs.v4i1.349.
Dixon D R, Pruski A M, Dixon L R J. 2004. The effects of hydrostatic pressure change on DNA integrity in the hydrothermal-vent mussel Bathymodiolus azoricus: implications for future deep-sea mutagenicity studies. Mutation Research/ Fundamental and Molecular Mechanisms of Mutagenesis, 552(1-2): 235-246, https://doi.org/10.1016/j.mrfmmm.2004.06.026.
Downey R V, Fuchs M, Janussen D. 2018. Unusually diverse, abundant and endemic deep-sea sponge fauna revealed in the Sea of Okhotsk (NW Pacific Ocean). Deep Sea Research Part II: Topical Studies in Oceanography, 154: 47-58, https://doi.org/10.1016/j.dsr2.2018.02.005.
Fedeles B I, Singh V, Delaney J C, Li D Y, Essigmann J M. 2015. The AlkB Family of Fe(II)/α-Ketoglutarate-dependent Dioxygenases: repairing nucleic acid alkylation damage and beyond. Journal of Biological Chemistry, 290(34): 20 734-20 742, https://doi.org/10.1074/jbc.R115.656462.
Feller G, Gerday C. 2003. Psychrophilic enzymes: hot topics in cold adaptation. Nature Reviews Microbiology, 1(3): 200-208, https://doi.org/10.1038/nrmicro773.
Finn R D, Clements J, Eddy S R. 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Research, 39(Web Server issue): W29-W37, https://doi.org/10.1093/nar/gkr367.
Fisher F M, Kleiner S, Douris N, Fox E C, Mepani R J, Verdeguer F, Wu J, Kharitonenkov A, Flier J S, Maratos-Flier E, Spiegelman B M. 2012. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes & Development, 26(3): 271-281, https://doi.org/10.1101/gad.177857.111.
Foote A D, Liu Y, Thomas G W C, Vinař T, Alföldi J, Deng J X, Dugan S, van Elk C E, Hunter M E, Joshi V, Khan Z, Kovar C, Lee S L, Lindblad-Toh K, Mancia A, Nielsen R, Qin X, Qu J X, Raney B J, Vijay N, Wolf J B W, Hahn M W, Muzny D M, Worley K C, Gilbert M T P, Gibbs R A. 2015. Convergent evolution of the genomes of marine mammals. Nature Genetics, 47(3): 272-275, https://doi.org/10.1038/ng.3198.
Fry E A, Mallakin A, Inoue K. 2018. Translocations involving ETS family proteins in human cancer. Integr ative Cancer Sci ence and Ther apeutics, 5(4): 1-12, https://doi.org/10.15761/ICST.1000281.
Gereben B, Zavacki A M, Ribich S, Kim B W, Huang S A, Simonides W S, Zeold A, Bianco A C. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocrine Reviews, 29(7): 898-938, https://doi.org/10.1210/er.2008-0019.
Gualerzi C O, Giuliodori A M, Pon C L. 2003. Transcriptional and post-transcriptional control of cold-shock genes. Journal of Molecular Biology, 331(3): 527-539, https://doi.org/10.1016/s0022-2836(03)00732-0.
Haas B J, Papanicolaou A, Yassour M, Grabherr M, Blood P D, Bowden J, Couger M B, Eccles D, Li B, Lieber M, MacManes M D, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey C N, Henschel R, LeDuc R D, Friedman N, Regev A. 2013. De novo transcript sequence reconstruction from RNA-Seq using the Trinity platform for reference generation and analysis. Nature Protocols, 8(8): 1 494-1 512, https://doi.org/10. 1038/nprot.2013.084.
Han B, Cai J Q, Gao W D, Meng X Q, Gao F, Wu P F, Duan C B, Wang R J, Dinislam M, Lin L, Kang C S, Jiang C L. 2018. Loss of ATRX suppresses ATM dependent DNA damage repair by modulating H3K9me3 to enhance temozolomide sensitivity in glioma. Cancer Letters, 419: 280-290, https://doi.org/10.1016/j.canlet.2018.01.056.
Hauswirth R, Haase B, Blatter M, Brooks S A, Burger D, Drogemuller C, Gerber V, Henke D, Janda J, Jude R, Magdesian K G, Matthews J M, Poncet P A, Svansson V, Tozaki T, Wilkinson-White L, Penedo M C T, Rieder S, Leeb T. 2012. Mutations in MITF and PAX3 cause "splashed white" and other white spotting phenotypes in horses. PLoS Genetics, 8(4): e1002653, https://doi.org/10.1371/journal.pgen.1002653.
Hayman A R, Jones S J, Boyde A, Foster D, Colledge W H, Carlton M B, Evans M J, Cox T M. 1996. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development, 122(10): 3 151-3 162.
Heyland A, Moroz L L. 2005. Cross-kingdom hormonal signaling: an insight from thyroid hormone functions in marine larvae. Journal of Experimental Biology, 208: 4 355-4 361, https://doi.org/10.1242/jeb.01877.
Hui M, Song C W, Liu Y, Li C L, Cui Z X. 2017. Exploring the molecular basis of adaptive evolution in hydrothermal vent crab Austinograea alayseae by transcriptome analysis. PLoS One, 12(5): e0178417, https://doi.org/10.1371/journal.pone.0178417.
Jamieson A. 2015. The Hadal Zone: Life in the Deepest Oceans. Cambridge University Press, Cambridge.
Jamieson A J, Gebruk A, Fujii T, Solan M. 2011. Functional effects of the hadal sea cucumber Elpidia atakama (Echinodermata: Holothuroidea, Elasipodida) reflect small-scale patterns of resource availability. Marine Biology, 158(12): 2 695-2 703, https://doi.org/10.1007/s00227-011-1767-7.
Janies D A, Witter Z, Linchangco G V, Foltz D W, Miller A K, Kerr A M, Jay J, Reid R W, Wray G A. 2016. EchinoDB, an application for comparative transcriptomics of deeplysampled clades of echinoderms. BMC Bioinformatics, 17: 48, https://doi.org/10.1186/s12859-016-0883-2.
Jin D X. 2018. Molecular Determinants of Mammary Differentiation and Breast Cancer Progression. PhD diss., Massachusetts Institute of Technology, Cambridge, Massachusetts, United States. http://hdl.handle.net/ 1721.1/117874.
Jones P, Binns D, Chang H Y, Fraser M, Li W Z, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn A F, Sangrador-Vegas, Scheremetjew M, Yong S Y, Lopez R, Hunter S. 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics, 30(9): 1 236-1 240, https://doi.org/10.1093/bioinformatics/btu031.
Kang S, Kim S, Park H. 2015. Transcriptome of the antarctic amphipod Gondogeneia antarctica and its response to pollutant exposure. Marine Genomics, 24: 253-254, https://doi.org/10.1016/j.margen.2015.07.012.
Katoh K, Standley D M. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30(4): 772-780, https://doi.org/10.1093/molbev/mst010.
Kuzuhara T, Horikoshi M. 2004. A nuclear FK506-binding protein is a histone chaperone regulating rDNA silencing. Nature Structural & Molecular Biology, 11(3): 275-283, https://doi.org/10.1038/nsmb733.
Ladner R D. 2001. The role of dUTPase and uracil-DNA repair in cancer chemotherapy. Current Protein & Peptide Science, 2(4): 361-370, https://doi.org/10.2174/1389203013380991.
Lan Y, Sun J, Tian R M, Bartlett D H, Li R S, Wong Y H, Zhang W P, Qiu J W, Xu T, He L S, Tabata H G, Qian P Y. 2017. Molecular adaptation in the world’s deepest-living animal: insights from transcriptome sequencing of the hadal amphipod Hirondellea gigas. Molecular Ecology, 26(14): 3 732-3 743, https://doi.org/10.1111/mec.14149.
Lan Y, Sun J, Xu T, Chen C, Tian R M, Qiu J W, Qian P Y. 2018. De novo transcriptome assembly and positive selection analysis of an individual deep-sea fish. BMC Genomics, 19: 394, https://doi.org/10.1186/s12864-018-4720-z.
Lan Y, Sun J, Zhang W P, Xu T, Zhang Y, Chen C, Feng D, Wang H B, Tao J, Qiu J W, Qian P Y. 2019. Host-symbiont interactions in deep-sea chemosymbiotic vesicomyid clams: insights from transcriptome sequencing. Frontiers in Marine Science, 6: 680, https://doi.org/10.3389/fmars.2019.00680.
Leal L N, Romao J M, Hooiveld G J, Soberon F, Berends H, Boekshoten M V, Van Amburgh M E, Martín-Tereso J, Steele M A. 2018. Nutrient supply alters transcriptome regulation in adipose tissue of pre-weaning Holstein calves. PLoS One, 13(8): e0201929, https://doi.org/10. 1371/journal.pone.0201929.
Lee Hufford G. 1968. Some Aspects of the Biology of the Deep Sea Holothuroid Paelopatides sp.. Oregon State University, Corvallis.
Li B, Dewey C N. 2011. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12: 323, https://doi.org/10.1186/1471-2105-12-323.
Li B, Ruotti V, Stewart R M, Thomson J A, Dewey C N. 2010. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics, 26(4): 493-500, https://doi.org/10.1093/bioinformatics/btp692.
Li L, Stoeckert C J Jr, Roos D S. 2003. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Research, 13(9): 2 178-2 189, https://doi.org/10. 1101/gr.1224503.
Li W Z, Godzik A. 2006. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics, 22(13): 1 658-1 659, https://doi.org/10.1093/bioinformatics/btl158.
Liang L Y, Chen J W, Li Y N, Zhang H B. 2020. Insights into high-pressure acclimation: comparative transcriptome analysis of sea cucumber Apostichopus japonicus at different hydrostatic pressure exposures. BMC Genomics, 21: 68, https://doi.org/10.1186/s12864-020-6480-9.
Liao Y L. 1997. Fauna Sinica: Phylum Echinodermata, Class Holothuroidea. Science Press, Beijing. (in Chinese)
Lieberman H B, Panigrahi S K, Hopkins K M, Wang L, Broustas C G. 2017. p53 and RAD9, the DNA damage response, and regulation of transcription networks. Radiation Research, 187(4): 424-432, https://doi.org/10. 1667/RR003CC.1.
Lim J, Thomas T, Cavicchioli R. 2000. Low temperature regulated DEAD-box RNA helicase from the antarctic archaeon, Methanococcoides burtonii. Journal of Molecular Biology, 297(3): 553-567, https://doi.org/10. 1006/jmbi.2000.3585.
Lin A J, Zhang X H, Chen M M, Cao Q. 2007. Oxidative stress and DNA damages induced by cadmium accumulation. Journal of Environmental Sciences, 19(5): 596-602, https://doi.org/10.1016/S1001-0742(07)60099-0.
Liu B H, Yip R K H, Zhou Z J. 2012. Chromatin remodeling, DNA damage repair and aging. Current Genomics, 13(7): 533-547, https://doi.org/10.2174/138920212803251373.
Liu R Y, Wang K, Liu J, Xu W J, Zhou Y, Zhu C L, Wu B S, Li Y X, Wang W, He S P, Feng C G, Zhang H B. 2020. De novo genome assembly of limpet Bathyacmaea lactea (Gastropoda: Pectinodontidae): the first reference genome of a deep-sea gastropod endemic to cold seeps. Genome Biology and Evolution, 12(6): 905-910, https://doi.org/10.1093/gbe/evaa100.
Lloyd J P B, Lang D, Zimmer A D, Causier B, Reski R, Davies B. 2018. The loss of SMG1 causes defects in quality control pathways in Physcomitrella patens. Nucleic Acids Research, 46(11): 5 822-5 836, https://doi.org/10.1093/nar/gky225.
Long K A, Nossa C W, Sewell M A, Putnam N H, Ryan J F. 2016. Low coverage sequencing of three echinoderm genomes: the brittle star Ophionereis fasciata, the sea star Patiriella regularis, and the sea cucumber Australostichopus mollis. Gigascience, 5(1): 20, https://doi.org/10.1186/s13742-016-0125-6.
Lu S Y, Li M T, Lin Y L. 2010. Mitf induction by RANKL is critical for osteoclastogenesis. Molecular Biology of the Cell, 21(10): 1 763-1 771, https://doi.org/10.1091/mbc.e09-07-0584.
Lu S Y, Li M T, Lin Y L. 2014. Mitf regulates osteoclastogenesis by modulating NFATc1 activity. Experimental Cell Research, 328(1): 32-43, https://doi.org/10.1016/j.yexcr.2014.08.018.
Martinez M I, Solís-Marín F A, Penchaszadeh P E. 2019. First report of Paelopatides (Synallactida, Synallactidae) for the SW Atlantic, with description of a new species from the deep-sea off Argentina. Zoologischer Anzeiger, 278: 21-27, https://doi.org/10.1016/j.jcz.2018.10.010.
Maurer S F, Fromme T, Grossman L I, Hüttemann M, Klingenspor M. 2015. The brown and brite adipocyte marker Cox7a1 is not required for non-shivering thermogenesis in mice. Scientific Reports, 5: 17 704, https://doi.org/10.1038/srep17704.
McGaugh S E, Gross J B, Aken B, Blin M, Borowsky R, Chalopin D, Hinaux H, Jeffery W R, Keene A, Ma L, Minx P, Murphy D, O’Quin K E, Rétaux S, Rohner N, Searle S W J, Stahl B A, Tabin C, Volff J N, Yoshizawa M, Warren W C. 2014. The cavefish genome reveals candidate genes for eye loss. Nature Communications, 5: 5307, https://doi.org/10.1038/ncomms6307.
Mehr S, Verdes A, Desalle R, Sparks J, Pieribone V, Gruber D F. 2015. Transcriptome sequencing and annotation of the polychaete Hermodice carunculata (Annelida, Amphinomidae). BMC Genomics, 16 : 445, https://doi.org/10.1186/s12864-015-1565-6.
Miller A K, Kerr A M, Paulay G, Reich M, Wilson N G, Carvajal J I, Rouse G W. 2017. Molecular phylogeny of extant Holothuroidea (Echinodermata). Molecular Phylogenetics and Evolution, 111: 110-131, https://doi.org/10.1016/j.ympev.2017.02.014.
Mitra J, Guerrero E N, Hegde P M, Liachko N F, Wang H B, Vasquez V, Gao J L, Pandey A, Taylor J P, Kraemer B C, Wu P, Boldogh I, Garruto R M, Mitra S, Rao K S, Hegde M L. 2019. Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects. Proceedings of the National Academy of Sciences of the United States of America, 116(10): 4 696-4 705, https://doi.org/10.1073/pnas.1818415116.
Moore K J. 1995. Insight into the microphthalmia gene. Trends in Genetics, 11(11): 442-448, https://doi.org/10.1016/S0168-9525(00)89143-X.
Mozhaev V V, Heremans K, Frank J, Masson P, Balny C. 1996. High pressure effects on protein structure and function. Proteins, 24(1): 81-91, https://doi.org/10.1002/(SICI)1097-0134(199601)24:1<81::AID-PROT6>3.0.CO;2-R.
Musilova Z, Cortesi F, Matschiner M, Davies W I L, Patel J S, Stieb S M, de Busserolles F, Malmstrøm M, Tørresen O K, Brown C J, Mountford J K, Hanel R, Stenkamp D L, Jakobsen K S, Carleton K L, Jentoft S, Marshall J, Salzburger W. 2019. Vision using multiple distinct rod opsins in deep-sea fishes. Science, 364(6440): 588-592, https://doi.org/10.1126/science.aav4632.
Nguyen N T, Fisher D E. 2019. MITF and UV responses in skin: from pigmentation to addiction. Pigment Cell & Melanoma Res earch, 32(2): 224-236, https://doi.org/10. 1111/pcmr.12726.
Nie M M, Tan X G, Lu Y L, Wu Z H, Li J, Xu D D, Zhang P J, You F. 2019. Network of microRNA-transcriptional factor-mRNA in cold response of turbot Scophthalmus maximus. Fish Physiology and Biochemistry, 45(2): 583-597, https://doi.org/10.1007/s10695-019-00611-y.
Panicker V, Cluett C, Shields B, Murray A, Parnell K S, Perry J R B, Weedon M N, Singleton A, Hernandez D, Evans J, Durant C, Ferrucci L, Melzer D, Saravanan P, Visser T J, Ceresini G, Hattersley A T, Vaidya B, Dayan C M, Frayling T M. 2008. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. The Journal of Clinical Endocrinology & Metabolism, 93(8): 3 075-3 081, https://doi.org/10.1210/jc.2008-0397.
Pease D C. 1946. Hydrostatic pressure effects upon the spindle figure and chromosome movement. II. Experiments on the meiotic divisions of tradescantia pollen mother cells. The Biological Bulletin, 91(2): 145-169, https://doi.org/10.2307/1538257.
Peter D, Weber R, Sandmeir F, Wohlbold L, Helms S, Bawankar P, Valkov E, Igreja C, Izaurralde E. 2017. GIGYF1/2 proteins use auxiliary sequences to selectively bind to 4EHP and repress target mRNA expression. Genes & Development, 31(11): 1 147-1 161, https://doi.org/10. 1101/gad.299420.117.
Phadtare S, Alsina J, Inouye M. 1999. Cold-shock response and cold-shock proteins. Current Opinion in Microbiology, 2(2): 175-180, https://doi.org/10.1016/S1369-5274(99)80031-9.
Purcell S W, Samyn Y, Conand C. 2012. Commercially Important Sea Cucumbers of the World. FAO, Roma.
Reich A, Dunn C, Akasaka K, Wessel G. 2015. Phylogenomic analyses of echinodermata support the sister groups of asterozoa and echinozoa. PLoS One, 10(3): e0119627, https://doi.org/10.1371/journal.pone.0119627.
Sanderson M J. 2003. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics, 19(2): 301-302, https://doi.org/10.1093/bioinformatics/19.2.301.
Schmutz S M, Berryere T G, Dreger D L. 2009. MITF and white spotting in dogs: a population study. Journal of Heredity, 100(S1): S66-S74, https://doi.org/10.1093/jhered/esp029.
Simonato F, Campanaro S, Lauro F M, Vezzi A, D'Angelo M, Vitulo N, Valle G, Bartlett D H. 2006. Piezophilic adaptation: a genomic point of view. Journal of Biotechnology, 126(1): 11-25, https://doi.org/10.1016/j.jbiotec.2006.03.038.
Smirnov A V, Gebruk A V, Galkin S V, Shank T. 2000. New species of holothurian (Echinodermata: Holothuroidea) from hydrothermal vent habitats. Journal of the Marine Biological Association of the United Kingdom, 80(2): 321-328, https://doi.org/10.1017/S0025315499001897.
Somero G N. 2003. Protein adaptations to temperature and pressure: complementary roles of adaptive changes in amino acid sequence and internal milieu. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 136(4): 577-591, https://doi.org/10. 1016/s1096-4959(03)00215-x.
Stadtman E R, Van Remmen H, Richardson A, Wehr N B, Levine R L. 2005. Methionine oxidation and aging. Biochimica et Biophysica Acta ( BBA)- Proteins and Proteomics, 1703(2): 135-140, https://doi.org/10.1016/j.bbapap.2004.08.010.
Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30(9): 1 312-1 313, https://doi.org/10. 1093/bioinformatics/btu033.
Sun J, Chen C, Miyamoto N, Li R S, Sigwart J D, Xu T, Sun Y N, Wong W C, Ip J C H, Zhang W P, Lan Y, Bissessur D, Watsuji T O, Watanabe H K, Takaki Y, Ikeo K, Fujii N, Yoshitake K, Qiu J W, Takai K, Qian P Y. 2020. The Scaly-foot Snail genome and implications for the origins of biomineralised armour. Nature Communications, 11(1): 1657, https://doi.org/10.1038/s41467-020-15522-3.
Sun J, Zhang Y, Xu T, Zhang Y, Mu H W, Zhang Y J, Lan Y, Fields C J, Hui J H L, Zhang W P, Li R S, Nong W Y, Cheung F K M, Qiu J W, Qian P Y. 2017. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes. Nature Ecology & Evolution, 1(5): 0121, https://doi.org/10.1038/s41559-017-0121.
Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56(4): 564-577, https://doi.org/10.1080/10635150701472164.
Tang C, Wang Y W, Lan D L, Feng X H, Zhu X, Nie P T, Yue H. 2015. Analysis of gene expression profiles reveals the regulatory network of cold-inducible RNA-binding protein mediating the growth of BHK-21 cells. Cell Biology International, 39(6): 678-689, https://doi.org/10.1002/cbin.10438.
The R Core Team. 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
Thieringer H A, Jones P G, Inouye M. 1998. Cold shock and adaptation. Bioessays, 20(1): 49-57, https://doi.org/10. 1002/(SICI)1521-1878(199801)20:1<49::AID-BIES8>3.0. CO;2-N.
Wang K, Shen Y J, Yang Y Z, Gan X N, Liu G C, Hu K, Li Y X, Gao Z M, Zhu L, Yan G Y, He L S, Shan X J, Yang L D, Lu S X, Zeng H H, Pan X Y, Liu C, Yuan Y, Feng C G, Xu W J, Zhu C L, Xiao W H, Dong Y, Wang W, Qiu Q, He S P. 2019. Morphology and genome of a snailfish from the Mariana Trench provide insights into deep-sea adaptation. Nature Ecology & Evolution, 3(5): 823-833, https://doi.org/10.1038/s41559-019-0864-8.
Waterhouse R M, Seppey M, Simao F A, Manni M, Ioannidis P, Klioutchnikov G, Kriventseva E V, Zdobnov E M. 2018. BUSCO applications from quality assessments to gene prediction and phylogenomics. Molecular Biology and Evolution, 35(3): 543-548, https://doi.org/10.1093/molbev/msx319.
Welty C J, Sousa M L, Dunnivant F M, Yancey P H. 2018. High-density element concentrations in fish from subtidal to hadal zones of the Pacific Ocean. Heliyon, 4(10): e00840, https://doi.org/10.1016/j.heliyon.2018.e00840.
Wilton D J, Ghosh M, Chary K V A, Akasaka K, Williamson M P. 2008. Structural change in a B-DNA helix with hydrostatic pressure. Nucleic Acids Research, 36(12): 4 032-4 037, https://doi.org/10.1093/nar/gkn350.
Xing L L, Sun L N, Liu S L, Wan Z X, Li X N, Miao T, Zhang L B, Bai Y C, Yang H S. 2018. Growth, histology, ultrastructure and expression of MITF and astacin in the pigmentation stages of green, white and purple morphs of the sea cucumber, Apostichopus japonicus. Aquaculture Research, 49(1): 177-187, https://doi.org/10.1111/are.13446.
Yang H S, Zhou Y, Zhang T, Yuan X T, Li X X, Liu Y, Zhang F S. 2006. Metabolic characteristics of sea cucumber Apostichopus japonicus (Selenka) during aestivation. Journal of Experimental Marine Biology and Ecology, 330(2): 505-510, https://doi.org/10.1016/j.jembe.2005. 09.010.
Yang L D, Wang Y, Zhang Z L, He S P. 2015. Comprehensive transcriptome analysis reveals accelerated genic evolution in a Tibet Fish, Gymnodiptychus pachycheilus. Genome Biology and Evolution, 7(1): 251-261, https://doi.org/10.1093/gbe/evu279.
Yang Z H, dos Reis M. 2011. Statistical properties of the branch-site test of positive selection. Molecular Biology and Evolution, 28(3): 1 217-1 228, https://doi.org/10.1093/molbev/msq303.
Yang Z H. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution, 24(8): 1 586-1 591, https://doi.org/10.1093/molbev/msm088.
Yano Y, Nakayama A, Ishihara K, Saito H. 1998. Adaptive changes in membrane lipids of barophilic bacteria in response to changes in growth pressure. Applied and Environmental Microbiology, 64(2): 479-485, https://doi.org/10.1128/AEM.64.2.479-485.1998.
Zhang J Z, Nielsen R, Yang Z H. 2005. Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Molecular Biology and Evolution, 22(12): 2 472-2 479, https://doi.org/10.1093/molbev/msi237.
Zhang J, Sun Q L, Luan Z D, Lian C, Sun L. 2017a. Comparative transcriptome analysis of Rimicaris sp. reveals novel molecular features associated with survival in deep-sea hydrothermal vent. Scientific Reports, 7(1): 2 000, https://doi.org/10.1038/s41598-017-02073-9.
Zhang Y J, Sun J, Chen C, Watanabe H K, Feng D, Zhang Y, Chiu J M Y, Qian P Y, Qiu J W. 2017b. Adaptation and evolution of deep-sea scale worms (Annelida: Polynoidae): insights from transcriptome comparison with a shallow-water species. Scientific Reports, 7(1): 46205, https://doi.org/10.1038/srep46205.
Zhang Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics, 9: 40, https://doi.org/10.1186/1471-2105-9-40.
Zhang Z, Xiao J F, Wu J Y, Zhang H Y, Liu G M, Wang X M, Dai L. 2012. ParaAT: A parallel tool for constructing multiple protein-coding DNA alignments. Biochemical and Biophysical Research Communications, 419(4): 779-781, https://doi.org/10.1016/j.bbrc.2012.02.101.
Zhao H, Wang J, Han Y Q, Huang Z, Ying J M, Bi X Y, Zhao J J, Fang Y, Zhou H T, Zhou J G, Li Z Y, Zhang Y F, Yang X, Yan T, Wang L F, Torbenson M S, Cai J Q. 2011. ARID2: a new tumor suppressor gene in hepatocellular carcinoma. Oncotarget, 2(11): 886-891, https://doi.org/10.18632/oncotarget.355.
Zheng P, Wang M X, Li C L, Sun X Q, Wang X C, Sun Y, Sun S. 2017. Insights into deep-sea adaptations and hostsymbiont interactions: a comparative transcriptome study on Bathymodiolus mussels and their coastal relatives. Molecular Ecology, 26(19): 5 133-5 148, https://doi.org/10.1111/mec.14160.
Zou Y, Liu Y Y, Wu X M, Shell S M. 2006. Functions of human replication protein A (RPA): from DNA replication to DNA damage and stress responses. Journal of Cellular Physiology, 208(2): 267-273, https://doi.org/10.1002/jcp.20622.
 Journal of Oceanology and Limnology2021年1期
Journal of Oceanology and Limnology2021年1期
- Journal of Oceanology and Limnology的其它文章
- Influence of sequential tropical cyclones on phytoplankton blooms in the northwestern South China Sea*
- Simulated perturbation in the sea-to-air flux of dimethylsulfide and the impact on polar climate
- Performance of ecological restoration in an impaired coral reef in the Wuzhizhou Island, Sanya, China*
- Investigating factors driving phytoplankton growth and grazing loss rates in waters around Peninsular Malaysia
- Effects of oxytetracycline dihydrate and sulfamethoxazole on Microcystis aeruginosa and Chlamydomonas microsphaera*
- Reproductive cycle of Ophiopholis mirabilis (Echinodermata: Ophiuroidea) in Zhangzi Island area, northern Yellow Sea*
