Reproductive cycle of Ophiopholis mirabilis (Echinodermata: Ophiuroidea) in Zhangzi Island area, northern Yellow Sea*
Nan YU , Song SUN , , Guangtao ZHANG Fang ZHANG
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
5 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Ophiopholis mirabilis is a common species with a high population density on the western coasts of the northern Pacific Ocean. The number of O. mirabilis has been increasing recently in the scallop aquaculture zone (the Zhangzi Island area, northern Yellow Sea) in China. To explore the mechanism of its population variation, the reproductive cycle of O. mirabilis was investigated in this area (39°04′N; 122°51′E) from February 2017 through January 2018 and determined by the monthly gonad index (GI), histological examinations of the gonads and the oocyte size-frequency distribution. O. mirabilis had a clear annual reproductive cycle that was synchronous between males and females. Sea temperature and food availability played important roles in O. mirabilis reproduction. The GI value was less reliable for determining reproductive activity in O. mirabilis because the nutritive tissues within the gonads may be utilized to synthesize gametes, leading to a decrease in GI during maturation. The histological results also show that abundant nutritive phagocytes were present in the gonads of O. mirabilis, which, together with the germ cells, affected the weight of the gonads. In addition, the mature oocytes of O. mirabilis were relatively small (75-150 μm), indicating that the larval development was planktotrophic. This study provided insights into the reproductive patterns and biology of O. mirabilis and is an essential basis for the quantity control of this species in aquaculture areas.
Keyword: reproductive biology; Ophiuroidea; the northern Pacific Ocean; Ophiopholis mirabilis
1 INTRODUCTION
Ophiuroidea is the largest group in the phylum Echinodermata, with 2 064 extant species found in nearly all oceans, latitudes and habitats, from the intertidal zone to the deep sea (Stöhr et al., 2012). The characteristics of aggregated distribution and dominant abundance make them an important group in marine benthic communities (Fedra et al., 1976; Warner, 1979; Aronson, 1989; Fujita and Ohta, 1989; Gage and Tyler, 1991; Metaxas and Giき n, 2004). However, there is relatively scarce information on the reproductive biology and larval development of ophiuroids, with only 4% of all species having been studied (Hendler, 1991; Hendler and Tran, 2001).
The northern Pacific Ocean is a region rich in ophiuroid species, as approximately 20% of these species are distributed in this region (Stöhr et al., 2012). Ophiopholis mirabilis is a common species on the western coasts of the northern Pacific Ocean. They aggregated in large numbers at the bottom of the southern Sea of Okhotsk (Djakonov, 1954) and near the coasts of Japan (Matsumoto, 1941), Korea (Shin and Rho, 1996), and the Yellow Sea (Liu, 2008; Liao and Xiao, 2011; Li et al., 2016) at depths of 30-50 m and are important contributors to biomass in benthic ecosystem. Despite the abundance and distribution of O. mirabilis, little is known about its biology, especially its reproductive biology. Recently, the number of O. mirabilis has been increasing in the Zhangzi Island area, northern Yellow Sea, which is the largest aquaculture zone for the Japanese scallop Patinopecten yessoensis in China (Zhang et al., 2008). Therefore, the investigation of the reproduction of O. mirabilis is essential in exploring its life history and developing control strategies for its population in aquaculture areas.
Reproductive activity in ophiuroids may occur throughout the year with an aperiodic pattern, or there may be a distinct reproductive cycle or periodicity ranging from annual to monthly (Hendler, 1991; Mercier and Hamel, 2009). In temperate areas, where the sea temperature varies seasonally, most ophiuroids show annual reproductive cycles, which are influenced by water temperature, photoperiod, food availability and other external factors (Rumrill and Pearse, 1985; Hendler and Tyler, 1986; Hendler, 1991; Mercier and Hamel, 2009). Giese and Pearse (1974) suggested that environmental change is not restricted to only one factor that interacts with reproductive activities but may consist of several factors. In ophiuroids, temperature is considered to be the most likely proximate factor affecting reproduction, and food availability for either adults or larvae is an important ultimate factor explaining the adaptive significance of reproductive periodicity (reviewed by Mercier and Hamel, 2009). O. aculeata is the only species in the genus Ophiopholis whose reproduction has been described, and its spawning may be initiated by changes in temperature. Blake (1978) reported that O. aculeata spawned during a decreasing temperature period in Marine and Newfoundland, whereas Himmelman et al. (2008) observed that the spawning activity in northern Gulf of St. Lawrence coincided with a major increase in temperature. Furthermore, laboratory experiments indicated that phytoplankton was probably the major environmental factor regulating the annual reproduction of O. aculeata (Doyle, 2011).
In this study, we analyzed the reproductive cycle of O. mirabilis based on samples collected monthly in the Zhangzi Island area of the northern Yellow Sea. The oogenesis and spermatogenesis of O. mirabilis were described by histological examination. The reproductive cycle of this species was documented by the gonad index, the patterns of gametogenesis and the oocyte size-frequency distribution. We also analyzed the main external factors affecting the growth and development of gonads to elucidate the reproductive biology of O. mirabilis in the northern Yellow Sea.
2 MATERIAL AND METHOD
The specimens of Ophiopholis mirabilis were collected by trawls from a site in the Zhangzi Island area, northern Yellow Sea (39°04′N; 122°51′E) from February 2017 to January 2018, at a depth ranging from 24 to 30 m. The specimens were then preserved in 4% formaldehyde in sea water. In each month, 10 males and 10 females (disc diameter >7 mm) were randomly selected for the following analysis.
The arms of each individual were removed from the base using dissecting scissors. The disc diameter was photographed and measured using a Zeiss SteREO Discovery Stereomicroscope with a precision of 0.1 mm, and the wet weight of the whole disc without arms was recorded with a Mettler Toledo precision balance (0.000 1 g). The gonads of each individual were then dissected out carefully and weighed. Therefore, the gonad index (GI) was calculated using the following formula:

Two lobes of the gonads were used for the histological analysis. The lobes were dehydrated in 20% sucrose solution for 24 h and then embedded in OCT compound (Sakura) and quick-frozen. Sections were cut at 8-10 μm thickness using a freezing microtome (Microm HM505E) and stained with hematoxylin and eosin. The sections were examined under a Leica DMIL LED microscope equipped with a digital camera.
Five maturity stages of oogenesis and spermatogenesis were defined: early growing, growing, mature, spawning and spent, based on the development of gametes observed in the sections and other studies of ophiuroid reproduction (Hendler, 1991; Doyle et al., 2012). The reproductive cycles of males and females were documented by analyzing the proportions of the different maturity stages in each month.
Oocyte size frequencies were determined by image analysis of histological sections of the ovaries. Two sections for each female were observed under a 100× microscope. All complete oocytes sectioned through the nucleus in the microscopic field were counted and measured at the longest axis (the maximum Feret diameter) using the software Leica Application Suite V4.9 (Doyle, 2011; Doyle et al., 2012). The data from females for each month were pooled to construct oocyte size-frequency histograms.
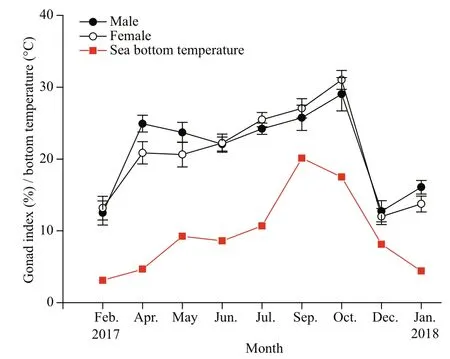
Fig.1 Mean monthly values of gonad index (%±SE) for males and females of O. mirabilis and sea bottom temperature (°C) from February 2017 to January 2018 in the Zhangzi Island area
The sea bottom temperature in each month at the sampling site was monitored by a CTD (AAQ1183-1F, Alec Electronics Co., Ltd.).
The significance of the differences in GI values among months for males and females was tested with one-way ANOVA, and the differences between GI values in males and females were also tested with one-way ANOVA. Correlations between the GI values and the sea bottom temperature and between the GI values of males and females were assessed using Pearson’s correlation coeき cient. Statistical analyses were performed with IBM SPSS Statistics (V. 23.0) software.
3 RESULT
Ophiopholis mirabilis is a gonochoric species without evident morphological differences between males and females. It is hard to sex individuals based on the appearance of the gonads except by using histological sections. Whether from males or females, the gonads are composed of yellowish lobes. Each individual generally has ten lobes of the same size. No hermaphroditic individuals were found.
3.1 Gonad index
There were significant differences in the gonad indices for O. mirabilis among the months (Male, F8,89=16.746, P <0.01; Female, F8,89=23.846, P <0.01), and the indices for males and females followed a similar temporal pattern. The value of the gonad index was low (<20%) in February 2017 and increased sharply in April 2017, after which the values increased gradually until October 2017, then dropped below 20% in December 2017 and January 2018 (Fig.1). This pattern was correlated with the sea bottom temperature (Male, R=0.676, n=9, P <0.05; Female, R=0.805, n=9, P <0.05). There was no significant difference in gonad index values between males and females ( F1,179=0.241, P >0.05), but the close correlation between them ( R=0.947, n=9, P <0.001) indicated that the development of testes and ovaries was synchronous in time.
3.2 Histology
3.2.1 Female
The disc diameter of females was between 7.7 and 12.4 mm (mean±SD: 9.7±1.0 mm). The oogenic cycle was divided into five stages based on the development and staining properties of the oocytes in the ovaries (Fig.2).
Stage I: early growing
Oocytes are very small and teardrop- or spindleshaped. They are previtellogenic oocytes stained dark blue with hematoxylin and are closely attached to the germinal epithelium (Fig.2a).
Stage II: growing
Oocytes become larger and more abundant (Fig.2b). Most are still linked with the germinal epithelium, and some of them are scattered in the ovarian cavity. In this stage, early vitellogenic oocytes begin to appear, stained light blue with hematoxylin. They are spindle-shaped with yolks and have a clear germinal vesicle and a single nucleolus. Some small previtellogenic oocytes are still present (Fig.2b).
Stage III: mature
Oocytes are irregularly round and fully grown (Fig.2c & d). They are closely arranged in the ovarian cavity with almost no tissue connection or distance between them (Fig.2c). Most of them are mature vitellogenic oocytes at their largest size, in which the cytoplasm is full of lipid droplets and yolk bodies (Fig.2d). A small number of these oocytes are early vitellogenic oocytes, and there are almost no previtellogenic oocytes. Mature oocytes are less alkalophilic than those in the growing stage and are stained lighter blue.
Stage IV: spawning
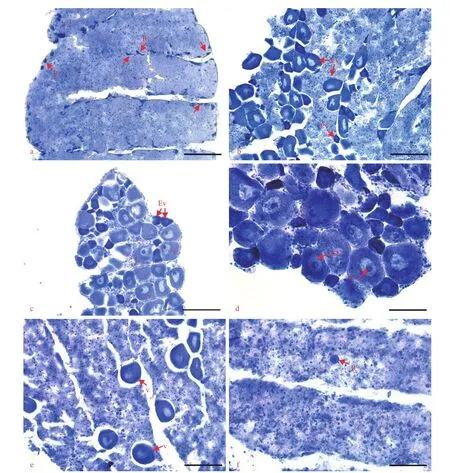
Fig.2 Histology of ovaries
The number of mature vitellogenic oocytes in the ovarian cavity has decreased due to gamete release. The remaining oocytes are loosely arranged in the ovarian cavity with a significant distance between them. Some small and round residual vitellogenic oocytes are visible that have been lysed by phagocytes and stained purple with hematoxylin (Fig.2e).
Stage V: spent
The ovary contains few mature vitellogenic oocytes but some residual vitellogenic oocytes. The ovarian cavity is filled with alkalophilic granules similar to nutritive tissues (Fig.2f). In some sections, a few previtellogenic oocytes appear on the germinal epithelium, which indicates the beginning of a new cycle of gamete development.
3.2.2 Male
The disc diameter of males was between 7.4 and 13.2 mm (mean±SD: 9.4±1.1 mm). The spermatogenic cycle was divided into five stages based on the development and arrangement of spermatozoa in the testes (Fig.3).
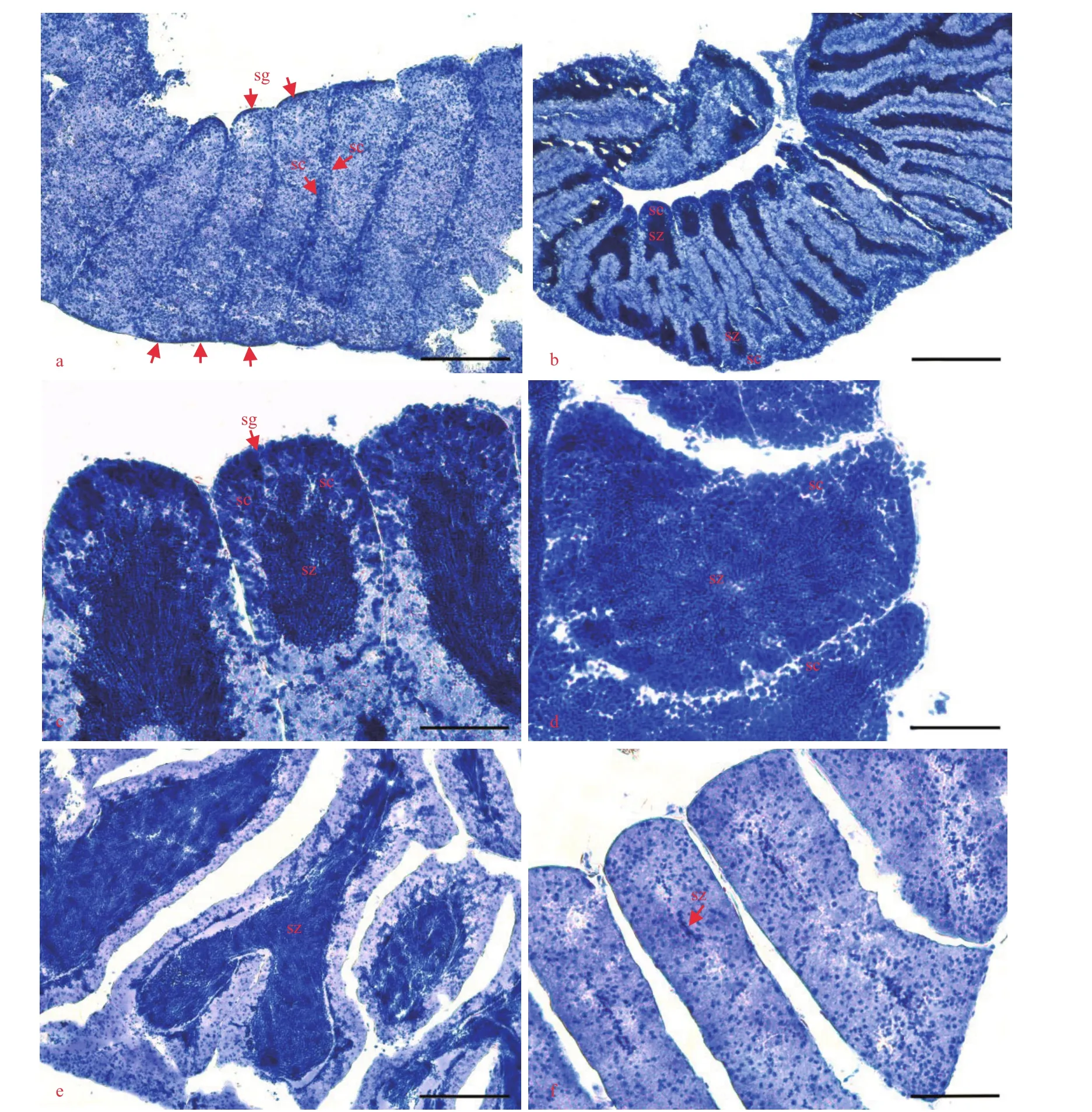
Fig.3 Histology of testes
Stage I: early growing
A large number of spermatogonia and spermatocytes appear on the germinal epithelium and are stained dark blue with hematoxylin (Fig.3a). Spermatogonia layers line the germinal epithelium. Spermatocytes are stacked in columns and are arranged under the spermatogonia. Additionally, a small amount of spermatozoa appears in the testis cavity.
Stage II: growing
The number of spermatogonia and spermatocytes continues to increase as the layers of them become thicker (Fig.3b & c). A large number of spermatozoa appear from ends of the spermatocyte columns, accumulate at both ends of the testis, and then spread to the center of the testis cavity.
Stage III: mature
There is a layer of spermatogonia and spermatocytes that are densely and evenly arranged on the germinal epithelium. The number of spermatozoa reaches its maximum, completely filling the testis cavity. Spermatozoa are more basophilic than spermatogonia and spermatocytes and are stained darker blue with hematoxylin (Fig.3d).
Stage IV: spawning
Few spermatogonia and spermatocytes appear along the germinal epithelium. With gamete release, the spermatozoa accumulate in the center of the testis cavity with an empty space between the spermatozoa and the germinal epithelium (Fig.3e). In the later part of this stage, the accumulations of spermatozoa become thinner or almost invisible, which indicates the end of spawning.
Stage V: spent
Spermatozoa are mostly released, and a small number of them are scattered in the testis cavity (Fig.3f). The assembled and orderly spermatozoa are hardly found.
3.3 Reproductive cycle
In females, the ovaries of O. mirabilis were mature in February. The main spawning activity occurred in April and lasted until May (Fig.4a), indicating a spring spawning. With the release of fully grown oocytes, most examined females were in the spent stage from May to July (Fig.4a). In July, early vitellogenic oocytes were present in the ovaries, which indicated the beginning of the renewal of gametogenesis. Females in the early growing and growing stages dominated from July to October (Fig.4a). This showed that oocytes developed in late summer and autumn. By December and January (2018), all examined individuals were mature again (Fig.4a), indicating the coming of the next spawning season.
In males, the reproductive pattern was similar to that in females. The testes were mature in February. The active spawning activity occurred from April to June (Fig.4b), indicating the same spring spawning as that in the females. Males in the spent stage first appeared in April and dominated between June and July. After the spent period, males entered a renewal of spermatogenesis in autumn, and individuals in the early growing and growing stages were prevalent in September and October (Fig.4b). In December and January (2018), all examined males were in the mature stage (Fig.4b), and the testes were full of developed spermatozoa in preparation for the next spawning season.
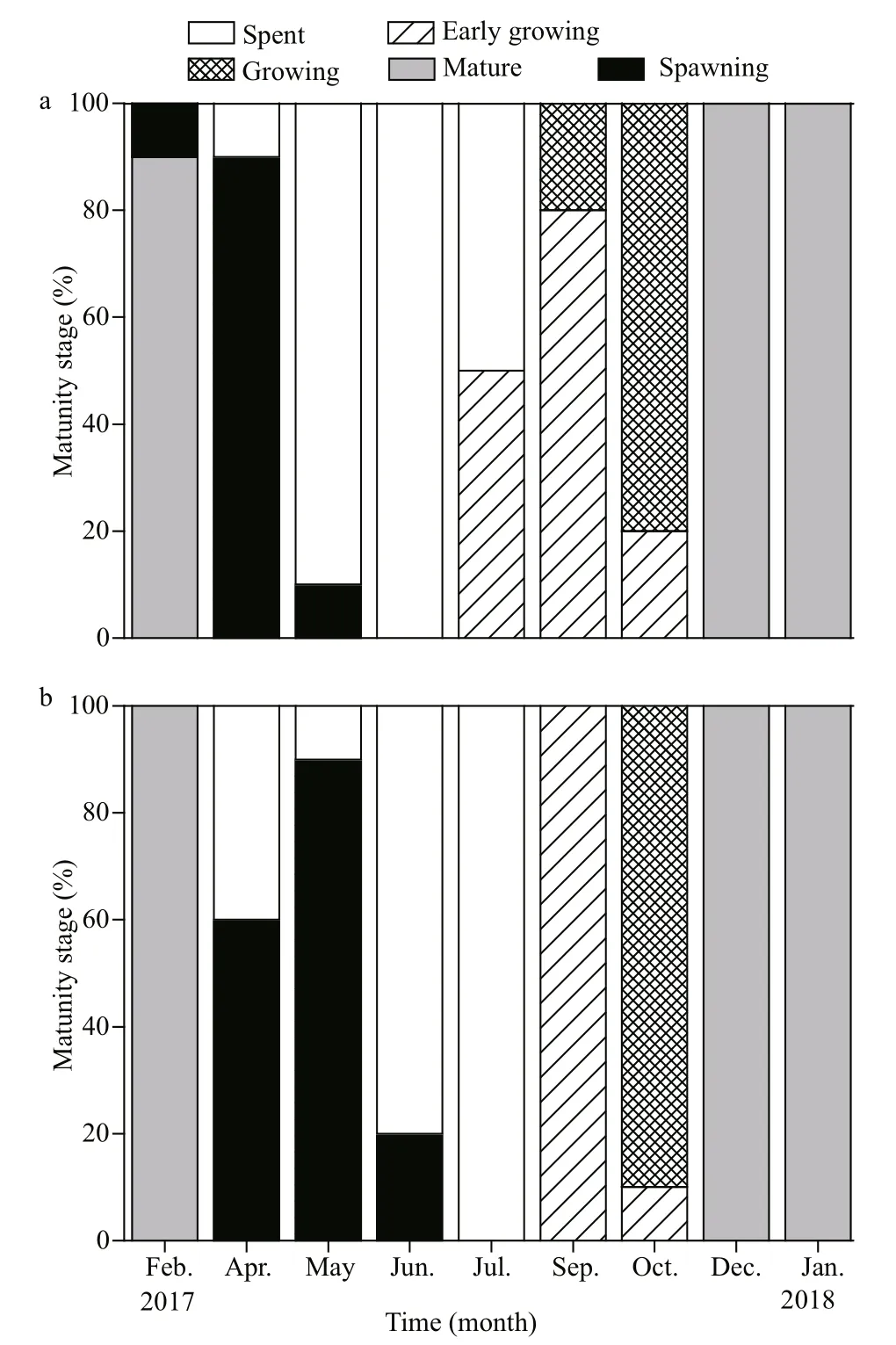
Fig.4 Gametogenic cycle of O. mirabilis in the Zhangzi Island area
Overall, for O. mirabilis, the development of ovaries and testes were synchronized and followed a similar pattern in the Zhangzi Island area. Gametogenesis occurred in late summer and autumn. The gonads with fully grown gametes were mature in winter. The spawning activity mainly occurred in spring, indicating that O. mirabilis spawns once a year. After spawning, the population of O. mirabilis entered a spent period in summer.
3.4 Oocyte size-frequency
The oocyte size-frequency distributions are shown as frequency histograms in Fig.5.
The oocyte size-frequency distributions were similar in February, December and January (2018). A large range of oocyte sizes was present, with the proportion of the large oocytes (75 to 150 μm in diameter) exceeding 50% and almost no small oocytes (0 to 25 μm in diameter) (Fig.5). The number of oocytes measured in the sections was the most abundant in these months, which indicated that the ovaries were mature and full of well-developed oocytes. Moreover, there was no obvious peak of large oocytes in the histograms of these months. This is because there were still some early- and midvitellogenic oocytes in the ovaries even when the ovaries were mature, as shown in the histological results.
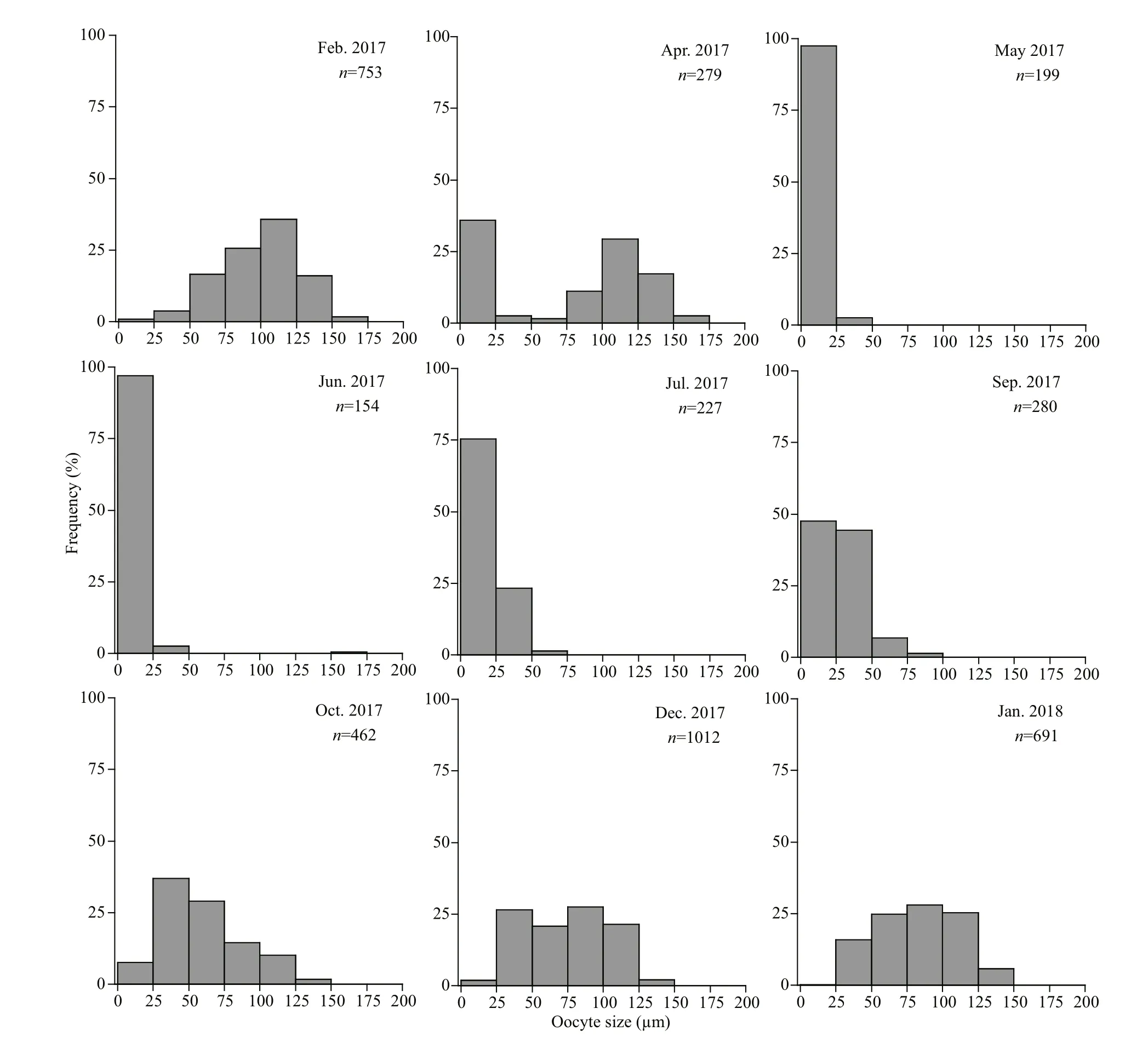
Fig.5 Monthly oocyte size-frequency distributions of O. mirabilis in the Zhangzi Island area
The distribution pattern in April was bimodal, with one peak in the size range of 0-25 μm and the other in the range of 75-150 μm (Fig.5). The lack of connection between these two peaks indicated that this was not the period of oogenesis. Therefore, the peak of small oocytes represented the residual oocytes, indicating that spawning occurred in April. Between May and June, the ovaries of O. mirabilis were dominated by small residual oocytes (0 to 25 μm in diameter) and contained few large oocytes (Fig.5), which showed that females had entered the spent period.
Then, the frequency of small oocytes (0 to 25 μm in diameter) decreased and that of larger oocytes (>25 μm in diameter), representing the early- and mid-vitellogenic oocytes, slowly increased from July to September (Fig.5), suggesting that the new generation of oocytes was developing in the ovaries. By October, the histogram showed a cohort of oocytes with diameters ranging from 25 to 125 μm (Fig.5). The ovaries were still in the stage of gametogenesis, with a large number of early- and mid-vitellogenic oocytes and a small number of fully grown oocytes.
In the histograms, it is hard to distinguish the residual oocytes from the previtellogenic oocytes based only on their sizes because they were both in the size range of 0-25 μm. However, we could summarize their different distribution characteristics in the histograms following the reproductive cycle. The residual oocytes mainly appeared alone in the spent period (May and June) or together with the mature oocytes appearing in the spawning stage (April). The previtellogenic oocytes usually appeared at the same time as all size ranges of oocytes, such as in September and October, because previtellogenic oocytes were mainly present in the growing stages, during which the ovaries contained oocytes of different developmental stages.
4 DISCUSSION
From the histological results, we documented the reproductive cycle of O. mirabilis in the Zhangzi Island area of the northern Yellow Sea. The gonadal development of O. mirabilis exhibited a strong synchrony among individuals. All samples of males and females examined in the same month were categorized as being in one or two stages. The development stages of O. mirabilis rarely overlapped. Moreover, the close correlation in GI between males and females indicated that the same synchrony also existed between sexes. In the cold waters of the Gulf of St. Lawrence, Himmelman et al. (2008) found strongly synchronous spawning in O. aculeata from field observations. Such synchronous reproduction contributes to both fertilization success and offspring survival (Mercier and Hamel, 2009), which may also be the reason for the high density of O. mirabilis in the aquaculture zone.
The gonads of male and female O. mirabilis began to develop in September and July respectively, and reached full maturity in winter when the bottom seawater temperature was at its lowest point in the year. This process of gonadal development took approximately half a year in an annual cycle, occurring in the period of decreasing seawater temperature. A similar pattern was also found in Ophiopteris papillosa. The population of O. papillosa showed maximal gonad maturity when the seawater temperature dropped in Monterey Bay (Rumrill, 1984). Other studies have also shown that gonadal development can be achieved and sometimes initiated at low temperatures (Stancyk, 1974; Yamashita and Iwata, 1983; Valentine, 1991). This is probably because the temperature difference may cause the release of the gonad-stimulating factor, which was proposed by Shirai and Walker (1988) in the study of Ophiothrix fragilis. For O. mirabilis, the timing of gonad maturation may also be related to the preparation for the subsequent spawning. Almost all males and females of O. mirabilis displayed maximal gonad maturity in winter, while waiting for the spawning season.
According to Mercier and Hamel (2009), both increasing and decreasing temperatures could be the spawning cues in ophiuroids. In the Zhangzi Island area, O. mirabilis spawned in spring when the bottom seawater temperature increased gradually. Moreover, it is proposed that the spawning is timed to correspond with optimal environmental conditions, such as optimal temperature (Fenaux, 1968; Byrne, 1991; Hendler, 1991) and adequate food supply (Skjaeveland, 1973; Rumrill, 1984), for the development and survival of larvae or juveniles. For instance, the populations of Ophiura albida and O. ophiura in Norway were reported to spawn following the period of maximum phytoplankton density (Skjaeveland, 1973), and Ophiothrix spiculata and Amphiodia occidentalis from southern Monterey Bay were reported to spawn during the late spring phytoplankton bloom (Rumrill, 1984). In the Zhangzi Island area, the chlorophyll a value and phytoplankton abundance peaked in spring (Zhang et al., 2016; Li et al., 2018), providing food support for the development of O. mirabilis larvae. In addition, O. mirabilis is a suspension feeding species, and the concentration of bottom suspended organic matter in this area increased significantly in spring (Li et al., 2018), which provided energy for their spawning activities. This is consistent with the research reviewed by Hendler (1991), which indicated that food availability might control the reproductive cycle. Therefore, we speculated that the maximum food availability for adults and larvae together with the rising temperature might synergistically trigger the spawning of O. mirabilis in this area.
From the histological sections, we found that the cavities of the gonads were always filled with somatic nutrient-like cells in addition to gametes, so that there was no evident lumen within gonads during gonadal development in O. mirabilis. This is rare in ophiuroids but common in echinoids. The germinal epithelium of gonads in sea urchins contains two types of cells: somatic cells, called nutritive phagocytes, and germ cells (Walker et al., 2005, 2007; Harrington et al., 2007). Nutritive phagocytes function as a source of nutrients for reproduction by accumulating nutritive materials before gametogenesis and transferring them to developing gametes during gametogenesis (Pearse and Giese, 1966; Meidel and Scheibling, 1998; Walker et al., 2005; Scheibling and Hatcher, 2007). Hence, gonad growth in echinoids is not only due to gamete synthesis but also to the growth and depletion of nutritive phagocytes (Walker et al., 2007). By comparing the cell morphology and arrangement, the somatic cells abundantly present in the gonads of O. mirabilis may also be such nutritive phagocytes with similar functions. In O. mirabilis, we found that GI values decreased in February, December and January (2018) when most gonads became fully mature based on histological analyses. This may be due to the utilization of the nutrients within the nutritive phagocytes for synthesizing gametes, resulting in the decline in the mass of gonads. Similar observations were also found in sea urchins (King et al., 1994; Lozano et al., 1995; Vaschenko et al., 2001). For instance, in the Sea of Japan, some individuals of the sea urchin Strongylocentrotus intermedius exhibited low GI values but high gonadal maturity (Vaschenko et al., 2001). Furthermore, histological results showed that the number of nutritive phagocytes in the gonads of O. mirabilis decreased significantly in the mature stage. In O. aculeata, Doyle et al. (2012) also mentioned that there was almost no “connective tissue” visible in the mature ovaries, but similar nutritive phagocytes could be observed clearly from the gonadal sections in other stages. The abundant nutritive phagocytes in gonads may be a feature of the genus Ophiopholis, but the evidence is insuき cient at present because only two species ( O. aculeata and O. mirabilis) of this genus have been described in reproduction. Therefore, more studies are required to focus on the reproductive biology of other species in this genus, to try to identify the components within the nutritive phagocytes using cytochemistry, and to explore the roles of such phagocytes in ophiuroids.
As mentioned above, the decrease in the GI of O. mirabilis indicated the maturation of the gonads rather than spawning. In addition to the depletion of nutritive phagocytes, the growth of gonad may be also affected by external environment. Nichol and Barker (1984) studied the relationships of the growth of the gonads, the pyloric caeca and the body wall of starfish in terms of bioenergetics and concluded that somatic maintenance and growth took priority over gonad growth when food was inadequate. This explanation has also been applied to ophiuroids (Bourgoin and Guillou, 1990). In the Zhangzi Island area, the mean value of suspended organic matter was the lowest in winter (Li et al., 2018), and the chlorophyll a concentration and phytoplankton abundance were also at low levels (Zhang et al., 2016; Li et al., 2018), which may have restricted the growth of the gonads of O. mirabilis. Moreover, we found a significant correlation between GI and bottom seawater temperature in O. mirabilis. Low temperature may also be a limiting factor for gonad growth. Thus, the decrease in GI in O. mirabilis during maturation may be due to the preferential use of energy for survival in the extreme environment, at the same time, the nutrient resources within the gonad may be utilized to synthesize gametes.
Therefore, GI was not an appropriate indicator of reproductive activity in O. mirabilis. Although the GI method is the oldest and most widely used method for estimating reproduction (Giese and Pearse, 1974), many investigators have reported that variations in GI were unrelated to reproduction (e.g., Nichols and Barker, 1984; King et al., 1994; Lozano et al., 1995; Selvakumaraswamy and Byrne, 1995; Vaschenko et al., 2001; Brogger et al., 2013). For instance, in Ophioplocus januarii from the Gulf of San José, variations in GI values were not related to gametogenesis (Brogger et al., 2013); in Ophionereis schayeri, the GI method could not detect the episodic spawning during autumn and winter (Selvakumaraswamy and Byrne, 1995). In other echinoderms, Carvalho and Ventura (2002) found that the asteroid Asterina stellifera from southeastern Brazil spawned earlier than suggested by its GI values. Lozano et al. (1995) observed that the fluctuations and peaks in GI had little to do with the gametogenic cycle of the sea urchin Paracentrotus lividus in the northwestern Mediterranean. These studies emphasized the importance and necessity of using other methods, such as histological techniques, to complement the GI method in studies of the reproductive cycle (Nichols and Barker, 1984; Vaschenko et al., 2001).
The results of the oocyte size-frequency distribution show an annual reproductive cycle of O. mirabilis in the Zhangzi Island area, which corresponded to the histological results. The mature oocytes of O. mirabilis were relatively small (75-150 μm), indicating that larval development is planktotrophic (Hendler, 1975). Its congener O. aculeata has also been reported to have mature oocytes of similar size (70-120 μm) with planktotrophic larvae (Falk-Petersen, 1982; Doyle et al., 2012). There was no distinct peak of large oocytes in February, December, and January (2018) during the mature period, but a cohort of a wide size range (25-150 μm) of oocytes was found in O. mirabilis at that time. This is because, in addition to fully grown oocytes, the mature ovaries also contain developing oocytes whose growth may be limited by nutritional supply and ovarian space. Moreover, the bimodal distribution of oocytes in April may be a sign of spawning in O. mirabilis. The peak in large oocytes represented the unreleased oocytes, and the other peak in small oocytes represented the residual oocytes resorbed by phagocytes. Such a bimodal distribution of oocytes was also present in Ophionereis schayeri, indicating gonad maturation in January and spawning in February (Selvakumaraswamy and Byrne, 1995). However, two or more peaks in the oocyte sizefrequency distribution were common in Astrobrachion constrictum, which indicated a long period of oocyte development (Stewart and Mladenov, 1995). Additionally, gonads of both males and females in O. mirabilis have a long spent period from May to July. During this period, almost no vitellogenic oocytes were found in the ovaries, suggesting that O. mirabilis released all oocytes during spawning. In other ophiuroids, such as Ophiactis resiliens (Falkner and Byrne, 2003), some pre- and early vitellogenic oocytes were conserved in the germinal epithelium for the next spawning season.
The spatial variability in the reproduction of ophiuroids has been demonstrated in many species (Hendler, 1991). Even between intertidal and subtidal zones, the timing of spawning varied in Acrocnida brachiata (Bourgoin and Guillou, 1990). O. mirabilis is a species widely distributed in the northwestern Pacific Ocean. The synchrony in gonadal development and spawning within the population indicated that the external environment acts as a strong cue for its reproduction. Thus, it is essential to conduct investigations on the reproduction of O. mirabilis among populations from different waters and latitudes to document the impacts of environmental factors. In addition, more field observations and induction experiments are required to illustrate the larval recruitment and development of O. mirabilis.
5 CONCLUSION
Ophiopholis mirabilis had a clear annual reproductive cycle in the Zhangzi Island area, northern Yellow Sea, with gametogenesis occurring during late summer and autumn, fully mature gonads being found in all winter, and a main spawning occurring in spring followed by a spent period. This could be better confirmed by successive years of investigation in the future. The gonadal development of O. mirabilis exhibited a strong synchrony among individuals and between sexes. Such synchronous reproduction contributes to both fertilization success and offspring survival, which may be the reason for the high density of O. mirabilis in the aquaculture area. Sea Temperature and food availability played an important role in O. mirabilis reproduction. Abundant nutritive phagocytes were found in the gonads of O. mirabilis, which, together with the germ cells, affected the weight of the gonads. Therefore, GI was not an appropriate indicator of reproductive activity in O. mirabilis. In addition, the mature oocytes of O. mirabilis were relatively small (75-150 μm), indicating that the larval development was planktotrophic. This study provided insights into the reproductive patterns and biology of O. mirabilis and is an essential basis for the quantity control of this species in aquaculture areas.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We would like to thank the captain and the crew of R/V Liaochangy u No. 19 for their help during the expedition to the Zhangzi Island area. Many thanks to those who helped with collections including ZHANG Yongshan, ZHAO Zengxia, WANG Shiwei, WAN Aiyong, XU Zhiqiang, LIU Mengtan, LIANG Yi, and WANG Jun from the Institute of Oceanology, Chinese Academy of Sciences (IOCAS). Thanks also to LIU Hourong from IOCAS for assistance with the histology and anonymous reviewers for comments on the manuscript.
 Journal of Oceanology and Limnology2021年1期
Journal of Oceanology and Limnology2021年1期
- Journal of Oceanology and Limnology的其它文章
- Influence of sequential tropical cyclones on phytoplankton blooms in the northwestern South China Sea*
- Simulated perturbation in the sea-to-air flux of dimethylsulfide and the impact on polar climate
- Performance of ecological restoration in an impaired coral reef in the Wuzhizhou Island, Sanya, China*
- Investigating factors driving phytoplankton growth and grazing loss rates in waters around Peninsular Malaysia
- Effects of oxytetracycline dihydrate and sulfamethoxazole on Microcystis aeruginosa and Chlamydomonas microsphaera*
- Partial function prediction of sulfate-reducing bacterial community from the rhizospheres of two typical coastal wetland plants in China*
