SIMULTANEOUS DETECTION OF SFTSV RNA FROM SERUM, URINE AND THROAT SWAB SAMPLES FROM A PATIENT IN ANHUI PROVINCE, CHINA*
AI Le-Le HU Dan ZHU Chang-Qiang YUE Na WANG Qiu-WeiYE Fu-Qiang LYU Heng YUE Ming TAN Wei-Long **
(1.Center for Disease Control and Prevention of Eastern Theater Command,Nanjing 210002, Jiangsu, China;2.Department of Infectious Diseases, The First Affiliated Hospital of Nanjing Medical University,Nanjing 210029, Jiangsu, China)
Abstract Severe fever with Thrombocytopenia Syndrome (SFTS) is an emerging epidemic disease caused by a novel SFTS bunyavirus (SFTSV). It is widely spread in central and northeast of China, particularly in rural and hilly areas, posing a great threat to public health. Here we presented a SFTS case in Anhui Province with clinical symptoms of fever, leukopenia and thrombocytopenia which SFTSV was detected in serum, urine and throat swab samples by RT-qPCR. Two gene fragments of viral complementary DNA were obtained by RT-PCR and sequenced from the patient's body fluids. Phylogenetic analysis indicated that the sequences obtained were closely related to those from patients in other epidemic areas of China. To our knowledge, it was the first report on the simultaneous detection of SFTSV RNA from serum, throat swabs and urine in the same patient, which suggest to be alert to the possible ways of SFTSV transmission other than tick bite.
Key words SFTSV;Body fluids;Laboratory examination;Sequencing analysis
Severe fever with Thrombocytopenia Syndrome (SFTS)is an emerging infectious disease which was firstly discovered in rural areas in He′nan and Hubei provinces, China, in 2009. It is characterized by high fever, gastrointestinal symptoms, thrombocytopenia, leukopenia, elevated liver enzymes and may cause Multiple Organ Dysfunction Syndrome (MODS) in severe cases with a fatality of 12%-30%(Yuetal., 2011). The causative agent was confirmed by Chinese CDC as a novel phlebovirus in the family Phenuiviridae(Yuetal., 2011). SFTSV is a segmented phlebovirus which comprises three segments, namely small (S) segment (1 744 nucleotides), medium (M) segment (3 378 nucleotides) and large (L) segment(6 368 nucleotides) (Zhangetal., 2013). The SFTS cases have been reported in at least 16 provinces in central, eastern and northeastern regions of China since 2010(Leietal., 2015). The virus is mainly transmitted by tick bites, andHaemaphysalislongicornisare implicated as a major vector of SFTSV (Lietal., 2014). However, the specific hosts reservoir of SFTSV involved in the maintenance cycle has not been clarified. Previous studies conducted in Jiangsu province, China have revealed seropositivity of antibodies against SFTSV in domestic animals including goats, cattle, dogs, pigs as well as rodents, hedgehogs and other wild animals(Lietal., 2014). The epidemic season is demonstrated from April through November(Zhangetal., 2012)in China. Currently, the diagnosis of SFTSV infection is validated by isolation of virus, detection of viral RNA and serological methods. RT-PCR test for SFTSV infection is proved to be a rapid and reliable way with high sensitivity and specificity (Liuetal., 2014).
So far, the transmission route of the contagious virus remains elusive. Apart from tick bites by vector ticks, such asHaemaphysalislongicornis, human-to-human transmission is also reported through close contact with patients′blood or bloody secretions where high levels of SFTS viral load had been detected (Jiangetal., 2015). Body fluids and secretions of SFTS patients could also be infectious to susceptible human victims and thus, SFTSV cases have been reported clustered with high frequencies in endemic areas (Baoetal., 2011). Although SFTSV RNA has been found in serum, gastric aspirates, tracheal aspirates and urine specimens from a recent Korean case (Jeongetal., 2016), there was no solid evidence thus far to elucidate the droplets or aerosols transmission of SFTSV. Herein we reported a SFTS case with viral RNA detected in serum, urine and throat swabs simultaneously, which indicates the SFTS transmission through direct contacts with body fluids.
1 Materials and Methods
1.1 Samples and reagents
The patient's serum, throat swab and urine samples were provided by the No. 105 hospital of PLA. Premix TaqTM(TaKaRa, Dalian),RNeasy Mini Kit (QIAGEN, Germany) and novel Bunyavirus nucleic acid detection kit (ZJ Bio-Tech, Shanghai) were also purchased.
1.2 PCR Detection
The Reverse Transcription-Polymerase Chain Reaction (RT-PCR) technique was performed targeting partial gene of the SFTSV with 5 pairs of primers designed by NCBI/Primer-BLAST or Primer Premier 5 (Tab. 1). The PCR procedure was carried out in 50 μL reaction system containing 3-4 μL genomic cDNA, 2 μL of each primer (10 μmol/L) and Premix TaqTM( TaKaRa, Code No. RR901A). The reaction conditions were performed as follows: an initial step of 3 min at 94 ℃, followed by 40 cycles of 30 sec at 94 ℃, 30 sec at 52 ℃, 90 sec at 72 ℃ and a final extension step of 10 min at 72 ℃.
1.3 RT-qPCR Detection
On April 21, 2016, the blood, urine and throat swab samples of the patient were collected to extract RNA of SFTSV. Realtime quantitative Polymerase Chain Reaction (RT-qPCR) tests were performed to detect SFTSV RNA via Novel Bunyavirus nucleic acid detection kit (Shanghai Zhangjiang Bio-Tech Co., Ltd.).

Tab.1 Primer sequences used to amplify partial genome of SFTSV (Anhui, China)
1.4 Gene sequence analysis
The PCR products were sequenced by Sanger dideoxy sequencing with the same primer set. The nucleotide identity to other published SFTSV strains were calculated by multiple sequences alignments with Blastn suite. The phylogenetic analysis trees were constructed with MEGA 5.1 software using maximum likelihood method with bootstrap as 500 replicates (Shietal., 2016; Zhangetal., 2017).
2 Results
2.1 Case presentation
A 66-year-old female farmer with no notable medical history got a fever with unknown origin except that she had climbed for tea-picking several days before April 14, 2016. She visited a doctor in the local county hospital on April 16, 2016. Medical examination results showed her peak axillary temperature, 38.2℃. Blood routine test showed white blood cell (WBC) 2.1×109/L, platelet (PLT) 58×109/L on April 17 and PLT 30×109/L on April 20. The antibiotics and symptomatic treatment (details not known) was performed, but no significant improvement was observed. The patient felt anorexia and general weakness for another 2 days and was then transferred to No. 105 hospital of PLA on April 20, 2016. On hospital admission, her blood pressure was 110/80 mmHg, pulse rate was 70/min, respiratory rate was 19/min, and body temperature was 36.7℃ during physical examination. Based on clinical features and blood test, SFTS was considered as a primary clinical diagnosis. Intravenous levofloxacin lactate injection (0.2 g bid) and ribavirin (250 mg qd) were administered as anti-virus therapy. Etamsylate (2 g qd) was also administered intravenously for the prevention of possible bleeding. In addition, Qingrejiedu granules, a kind of traditional Chinese herbicides, was prescribed orally (10 g tid). On April 21, her WBC recovered (8.1×109/L), but still had marked thrombocytopenia (39×109/L) and the reticulocyte count was 7.5 ×109/L, but red blood cell (RBC) was 4.66×1012/L and hemoglobin (Hb) was 133.0 g/L. Urinalysis showed occult blood 2+, urinary protein 2+, RBC 58.40/μL. Elevated biochemical tests were demonstrated among alanine aminotransferase (ALT) 183.3 U/L, aspartate transaminase (AST) 365.4 U/L, adenosine deaminase (ADA) 37.4 U/L, mitochondrial aspartate aminotransferase (mAST) 111.9 U/L, lactate dehydrogenase (LDH) 630.96 U/L and creatinine kinase (CK) 481.7 U/L. Stool occult blood was negative. Her blood sample was delivered to Anhui Provincial Center for Disease Control and Prevention to confirm the diagnosis of SFTS. On the same day, samples of serum, urine and throat swabs were also collected for further analysis. Glucuronolactone (200 mg tid) was then administered orally to treat transaminitis. On April 25, blood routine test showed WBC 5.4×109/L, Hb 118.0 g/L, RBC 4.07×1012/L, and thrombocytopenia was relieved significantly (PLT 95×109/L). Blood biochemical test showed ALT 213.0 U/L, AST 157.6 U/L, ADA 20.0 U/L, mAST 48.5 U/L, glucose 5.25 mmol/L, LDH 305.0 U/L, CK 68.0 U/L. Ultrasonic examination showed enlargement of left cervical lymph nodes, left supraclavicular lymph nodes and right inguinal lymph nodes. Ultrasonography of liver, gallbladder, pancreas and spleen showed no abnormal. IgG or IgM antibodies in the patient′s blood was showed negative by Anhui provincial CDC. Since the patient′s physical condition were significantly improved and laboratory tests and body temperature maintained normal, she was discharged on April 28, 2016, and instructed to continue her oral glucuronolactone (200 mg tid) administration until further notice (Fig. 1).

Fig. 1 PLT detection of a severe fever with thrombocytopenia syndrome during the disease course
2.2 PCR amplification of partial S fragment from SFTS patient
As shown in 1.2% agarose gel electrophesis, results of amplification of partial S segments from three samples in the patient were all positive with primer SF2/SR2 (Fig. 2).
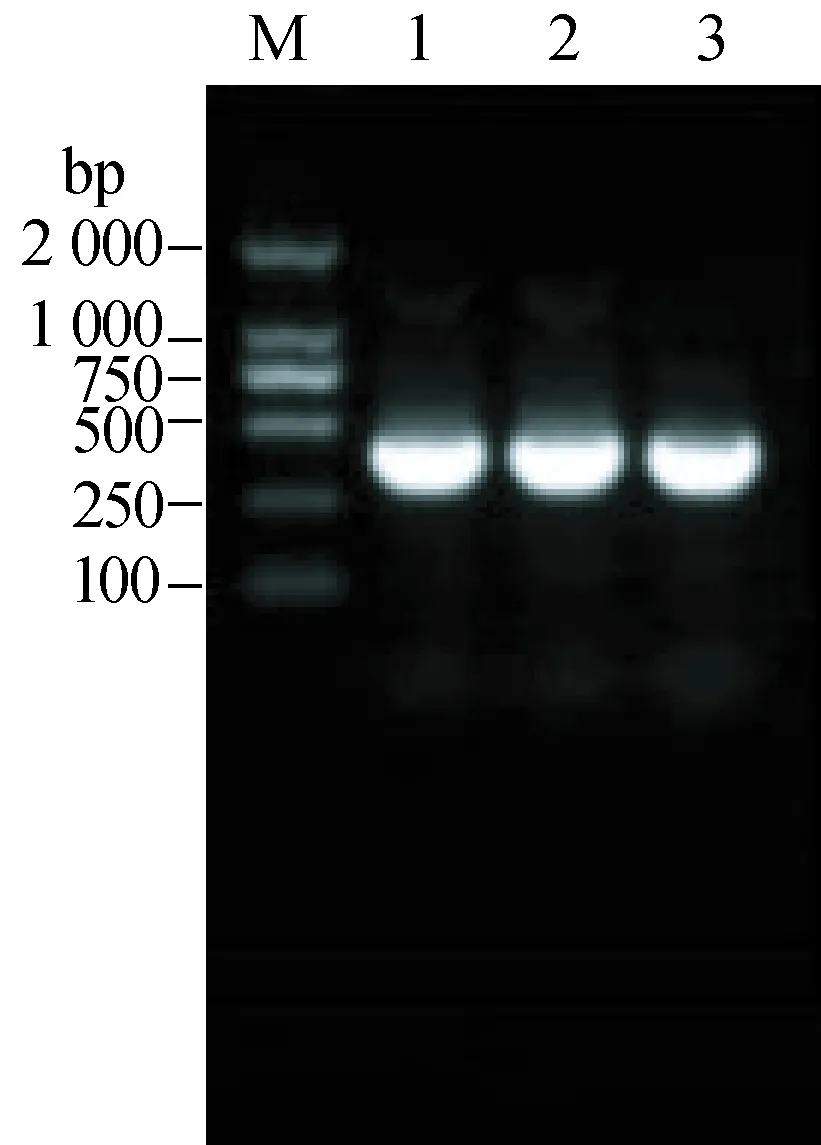
Fig. 2 PCR amplification of a partial S(using the primer pair SF2/SR2)segment sequenceM: DNA size marker; 1: Serum; 2: Throat swabs; 3: Urine.
2.3 Detecting samples with real-time quantitative polymerase chain reaction
The serum, pharyngeal swab and urine samples were also detected positive by real-time fluorescence quantitative method(Fig. 3). The Patient′s serum appeared with higher virus load (Ctvalue 17.5) than swab and urine samples (bothCtvalue 27).
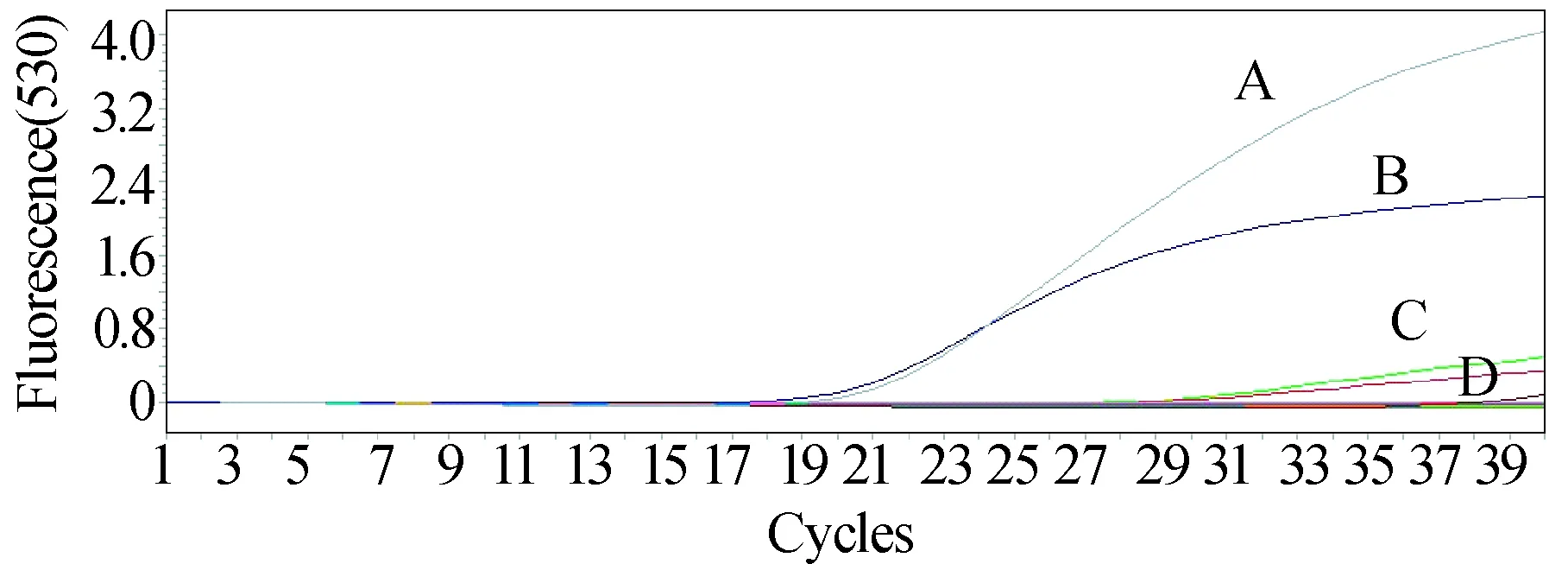
Fig. 3 The amplification curve of real-time quantitative polymerase chain reactionA: Positive control of SFTSV with Ct value 17.5; B: Patient′s serum sample with Ct value 17.5; C: Patient′s throat swab sample with Ct value 27; D: Patient′s urine sample with Ct value 27.
2.4 Phylogenetic analysis of SFTSV amplified from serum, urine and throat swabs of an Anhui patient
The PCR products were sequenced by Sanger dideoxy sequencing. After using DNAMAN v6.0 software for sequence assembly, we obtained two partial viral sequences with a length of 1 196 bp and 735 bp in S segment and L segment, respectively. Blastn suite was used for two sequence alignment to find out nucleotide identity with known SFTSV strains. We constructed phylogenetic analysis trees based on the partial sequence of SFTSV S segment (Panel A) and L segment (Panel B )in reference to previous work (Shietal., 2016; Zhangetal., 2017) (Fig. 4).
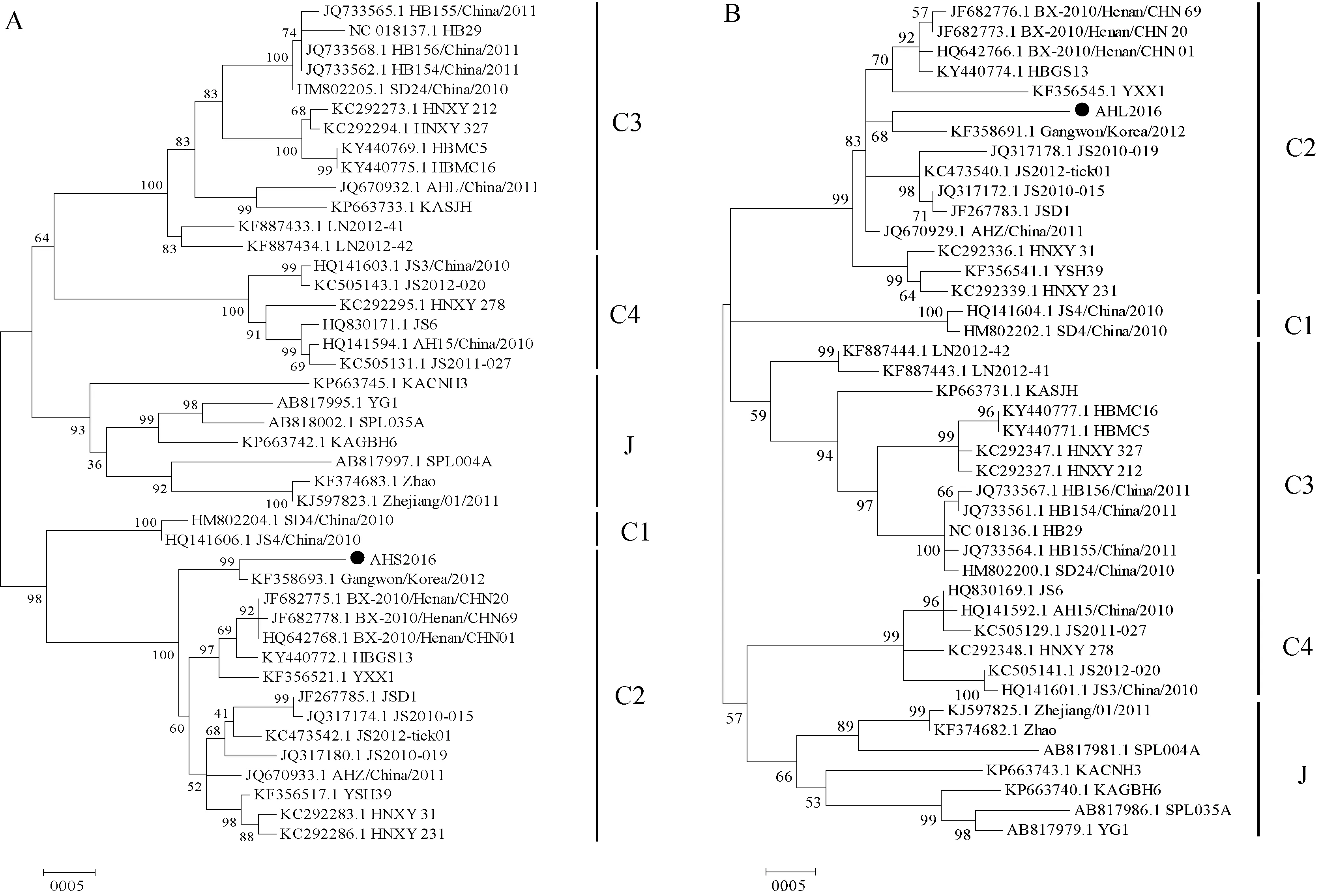
Fig. 4 Phylogenetic analysis of SFTSV amplified from serum, urine and throat swabs of an Anhui patientA: The ML tree was constructed based on the partial sequence of SFTSV S segment (from position 27 to 1 206 bp of the complete S segment); B: The ML tree was constructed based on the partial sequence of SFTSV L segment (from position 5 627 to 6 361 bp of the complete L segment). C1, C2, C3, C4, J denote 5 SFTSV genotypes currently known in mainland China, Korea and Japan. ●The partial sequence of SFTSV S/L genome segment obtained in this study.
3 Discussion
We reported a confirmed SFTS case with PCR screening from serum, urine and throat swab samples. The aged patient is a farmer living in Shucheng County, Anhui Province, where has been known as an SFTS endemic area since 2009 (Yuetal., 2011). Although no history of tick bites were recalled, gender (female), age (over the age of 60), endemic area, season and outdoor activities collectively suggest her susceptible to SFTSV which is in line with the results of a epidemiologic study of SFTS cases in Xinyang County, He′nan Province (Liuetal., 2014).
The patient had no evidence of preexisting comorbidities and received timely hospital admission, due to her relatively mild clinical symptoms. Although her peak axillary temperature was 38.2 ℃ 2 days after the onset of fever, her body temperature remained normal during the hospital stay. She had recovered from leukopenia on hospital admission,but underwent a slow recovery of thrombocytopenia. She had liver damages evidenced by elevated liver enzymes(AST, ALT, LDH) but no complaint of any gastrointestinal symptoms. Lymphadenopathy is one of the most common clinical manifestations in SFTS patients(95%), which could explain the several enlarged lymph nodes revealed by ultrasonic examination in this case (Guoetal., 2016). In the study conducted by Yuetal. (Yuetal., 2011), 84% and 59% of SFTS patients suffered from proteinuria and hematuria, respectively. In this case, although no occult blood was detected in feces, her uric occult blood, RBC and protein were all positive in the urinalysis report in April 21, 2016. However, with the remission of the disease as indicated by the recovered PLT value (95×109/L) in April 25, 2016, abnormalities in urinalysis disappeared. Thus, hematuria and proteinuria could be reversible during the clinical process of SFTS cases.
However, in the Korean cases mentioned above (Jeongetal., 2016), the condition after SFTSV infection was far worse. Nine days after onset, the patient had a fever with temperature of 39.3 ℃. Plasma exchange was then performed and oral ribavirin (4.0 g/day) was administered. The patient′s laboratory tests indicated an improvement before her condition deteriorated. The patient developed MODS and died from refractory ventricular fibrillation. The SFTS viral RNA was detected in diverse body fluids since the 12thday from symptom onset, which was similar to our case, although our case had a much better prognosis. Virus load in diverse body fluids may not be closely related to the prognosis or severity of the disease.
Laboratory diagnosis of SFTS is mainly made by virus isolation, detection of viral genomes, or detection of anti-SFTSV immunoglobulin G antibody (National Prevention guides for severe fever with thrombocytopenia syndrome of China, version 2010 ). In this case, specific antibody to IgG or IgM were failed to detect in the blood samples of our patient, which might be due to the repressed immune responses during SFTSV infection and spreading (Hirakietal., 2015; Luetal., 2015). In patients with elder age and/or underlying diseases, serologic IgG and IgM antibody detection may not be a deciduous factors to diagnosis in the acute phase of SFTS as their immune system could not produce detectable antibodies timely. However, viral RNA positivity in body fluids was achieved by qPCR and RT-PCR respectively, evidenced with partial sequences of S and L segments of SFTSV obtained. The L fragment of SFTSV has great length and variation, so it may be difficult to obtain the full length. At present, only part of the sequence has been attained. Phylogenetic analysis revealed these SFTSV sequences obtained had high identities with those of SFTS patients in other epidemic areas of China, including He′nan, Hubei, Shandong, Anhui, Jiangsu and Liaoning provinces, and the nucleotide identity was 94%-99%. We also observed high similarities between these sequences and those of SFTSV strains obtained from patients in Korea and Japan (95%-99%),Haemaphysalislongicornis, and host dog (98%-99%) in Jiangsu Province.
Phylogenetic analysis based on SFTS viral nucleotide sequences, SFTSV has been classified into 5 genotypes with C1-C4 mainly occur in China and J genotype in Korea, Japan and Zhejiang Province of China (Yoshikawaetal., 2015; Shietal., 2016). Partial nucleotide sequences of S and L segments amplified from our case should be classified into C2 genotype. Nearly half of this genotype SFTSV strains are currently known in Anhui Province (Shietal., 2016). And patients infected by SFTSV of C2 genotype were shown to develop severe neurologic and respiratory symptoms (Zhangetal., 2017), but our case did not develop such symptoms, with which the disease severity associated with the SFTS viral genotype warrants further investigation.
To our knowledge, this was the first report of simultaneous detection of SFTSV RNA in serum, urine and throat swab samples from the same patient in mainland China. The result indicates that the risk of human-to-human transmission of SFTSV should not be under estimated. Currently, sporadic infections of SFTSV are mainly observed in hilly and rural areas in China, so health education on the risk of SFTS should be accentuated among residents in these areas. Personnel engaged in outdoor activities, such as tea-picking and visiting, should be well protected from tick bites by individual measures such as long-sleeved clothes, tighten bottom of trousers, repellent (>20% diethyltoluamide) applied to the exposed skin and clothes to avoid tick-biting (Cisaketal., 2012). Moreover, direct contact with body fluids of suspicious SFTSV infection cases should be avoid during medical nursing.

