Efficacy and safety of intraoperative radiotherapy in rectal cancer:A systematic review and meta-analysis
Bin Liu,Long Ge,Jing Wang,Ya-Qiong Chen,Shi-Xun Ma,Pei-Lan Ma,Yun-Qiang Zhang,Ke-Hu Yang,Hui Cai
Bin Liu,Ya-Qiong Chen,Shi-Xun Ma,Pei-Lan Ma,Yun-Qiang Zhang,Gansu Provincial Hospital,General Surgery Clinical Medical Center,Lanzhou 730000,Gansu Province,China
Long Ge,Evidence Based Social Science Research Center,School of Public Health,Lanzhou University,Lanzhou 730000,Gansu Province,China
Jing Wang,Gansu University of Chinese Medicine,Gansu University of Chinese Medicine,Lanzhou 730000,Gansu Province,China
Ke-Hu Yang,Evidence Based Medicine Center,School of Basic Medical Sciences,Lanzhou University,Lanzhou 730000,Gansu Province,China
Hui Cai,General Surgery Clinical Medical Center,Gansu Provincial Hospital,Lanzhou 730000,Gansu Province,China
Abstract BACKGROUND In recent years,intraoperative radiotherapy (IORT) has been increasingly used for the treatment of rectal cancer.However,the efficacy and safety of IORT for the treatment of rectal cancer are still controversial.AIM To evaluate the value of IORT for patients with rectal cancer.METHODS We searched PubMed,Embase,Cochrane Library,Web of Science databases,and conference abstracts and included randomized controlled trials and observational studies on IORT vs non-IORT for rectal cancer.Dichotomous variables were evaluated by odds ratio (OR) and 95% confidence interval (CI),hazard ratio (HR)and 95%CI was used as a summary statistic of survival outcomes.Statistical analyses were performed using Stata V.15.0 and Review Manager 5.3 software.RESULTS In this study,3 randomized controlled studies and 12 observational studies were included with a total of 1460 patients,who are mainly residents of Europe,the United States,and Asia.Our results did not show significant differences in 5-year overall survival (HR=0.80,95%CI=0.60-1.06;P=0.126);5-year disease-free survival (HR=0.94,95%CI=0.73-1.22;P=0.650);abscess (OR=1.10,95%CI=0.67-1.80;P=0.713),fistulae (OR=0.79,95%CI=0.33-1.89;P=0.600);wound complication (OR=1.21,95%CI=0.62-2.36;P=0.575);anastomotic leakage (OR=1.09,95%CI=0.59-2.02;P=0.775);and neurogenic bladder dysfunction (OR=0.69,95%CI=0.31-1.55;P=0.369).However,the meta-analysis of 5-year local control was significantly different (OR=3.07,95%CI=1.66-5.66;P=0.000).CONCLUSION The advantage of IORT is mainly reflected in 5-year local control,but it is not statistically significant for 5-year overall survival,5-year disease-free survival,and complications.
Key Words:Intraoperative radiotherapy;Rectal cancer;Systematic review;External beam radiation therapy;Randomized controlled trials;Meta-analysis
INTRODUCTION
Rectal cancer is a common malignant tumor of the digestive tract[1].Because of its characteristics of being difficult to locate,high mortality,and poor prognosis,it is a killer,thereby threatening human health[2].Surgical resection is one of the main clinical treatment methods,and tumor tissue can be removed as much as possible to achieve good clinical treatment results[3].Currently,laparoscopic surgery is commonly used in the clinical treatment of rectal cancer[4].For advanced or recurrent rectal cancer,the combination of surgery and radiotherapy can prolong the survival rate of patients,but external beam radiation therapy (EBRT) alone has a poor response to treatment and a high recurrence rate[5,6].
Intraoperative radiotherapy (IORT) involves the precise delivery of large doses of ionizing radiation to a tumor or tumor bed during surgery[7,8].Direct visualization of the tumor bed and the ability to separate healthy tissue from the tumor bed maximize the radiation dose to the tumor,while minimizing the dose to healthy tissue,thereby leading to an increased treatment rate for IORT[9,10].Although IORT was introduced in the 1960s[5],its popularity increased with the introduction of self-shielding mobile linear accelerators and low-voltage IORT devices[11].In May 2019,the American Society of Brachytherapy reached a consensus on IORT:IORT can be considered at the time of surgical resection of locally advanced or recurrent colorectal cancer in cases with concern for a positive margin,particularly when pelvic EBRT has already been delivered[12].The National Comprehensive Cancer Network guidelines for the treatment of rectal cancer (Version 4.2020) described the following:IORT,if available,may be considered for very close or positive margins after resection,as an additional boost,especially for patients with T4 or recurrent cancers[13].At present,the number of studies that focus on IORT is increasing and includes breast cancer,colorectal cancer,pancreatic cancer,gastric cancer,head and neck cancer,glioma,and gynecological tumors[14-16].
In the past 10 years,cases of rectal cancer patients receiving IORT have gradually increased[17].In previous studies[18],it was demonstrated that adding IORT to traditional treatment of rectal cancer not only reduces the local recurrence rate of advanced rectal cancer but also influences the local control (LC) rate of locally recurrent rectal cancer.However,a recent randomized controlled trial (RCT) showed that IORT cannot be recommended as a standard therapy to compensate less radical resection for advanced lower rectal cancer[19].Although several research reports on IORT for the treatment of rectal cancer have been published,due to the small sample size,there is still a lack of reliable evidence regarding the efficacy and safety of IORT.
Therefore,to draw more reliable conclusions,we conducted a systematic review and meta-analysis to evaluate the effectiveness and safety of IORTvsnon-IORT in the treatment of rectal cancer.
MATERIALS AND METHODS
This systematic review and meta-analysis are reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis statement[20].
Search strategy
Up to November 2020,PubMed,Embase,Cochrane Library,Web of Science,letters to the editor and abstracts of conferences were searched to compare the efficacy and safety of IORT and non-IORT for the treatment of rectal cancer.The following medical subject heading terms and keywords were used:“intraoperative radiotherapy”,“IORT”,“intra-operative radiation therapy”,“intraoperative radiation therapy”,“rectal neoplasms”,and “rectal cancer”.The search strategy for PubMed is revealed in the supplementary material (Item 1).
Selection criteria
Studies were included if the RCT and observational study published compared IORT and non-IORT treatment for rectal cancer,and at least 20 patients were included in the study.Studies were excluded if the study was a review,expert opinion,or metaanalysis,lack of original data,no control group,duplicate studies,and animal studies.Titles and abstracts of retrieved studies were screened by two independent reviewers,and any conflicts were resolved by discussion.Any potentially eligible study was retrieved for further reviewer.
Data extraction and quality assessment
Two reviewers (BL and LG) independently assessed the eligibility of each trail and extracted the data (first author name,publication date,country/region,study type,number of patients per group,age,tumor site,stage,pre-operative radiotherapy;chemotherapy;post-operative radiotherapy and IORT dose from each study.The main results were 5-year overall survival (OS),5-year disease-free survival (DFS),5-year LC,and complications (abscess,fistulae,wound complications,anastomotic leakage,and neurogenic bladder dysfunction).
The Cochrane risk of bias tool[21]was used to evaluate the quality of RCTs including selection bias,performance bias,detection bias,attrition bias,reporting bias,and other bias through high-risk,low-risk,and unknown risk.The quality of the study was assessed by the Newcastle-Ottawa Scale[22]for observational studies.We analyzed the representativeness of the exposed observational,selection of the non-exposed observational,ascertainment of exposure,demonstration that outcome of interest was not present at the start of the study,comparability of cohorts based on the design and analysis,assessment of outcome,whether follow-up time was long enough for outcomes to occur,and the adequacy of follow-up of the cohorts.A score of 0-9 was assigned to each study.In general,studies were considered of high quality if a score of 6 was reached.Disagreements were resolved by discussion and consultation with the senior investigator.
Statistical analysis
Dichotomous variables were evaluated by odds ratio (OR) and 95% confidence interval (CI),including LC and complication results.In addition,hazard ratio (HR)was used as a summary statistic of survival outcomes (5-year OS and 5-year DFS).Heterogeneity was evaluated using the HigginsI2value,and values <25,25 to 50,and>50 were defined as corresponding to low,moderate,and high heterogeneity,respectively.The OR and HR values are reported with the 95%CIs.P<0.05 was considered statistically significant.All statistical analyses were performed with Review Manager 5.3 software (Cochrane Collaboration’s Information Management System) and Stata version 15.0 software (STATA,College Station,TX,United States).
Subgroup analysis was performed on the basis of study type,and sensitivity analysis was performed on the outcome indicators of more than 10 studies to explore their potential sources and assess the robustness of these results.The Begg’s test and Egger’s test were used to test publication bias.
RESULTS
Search results
Initially,645 studies were included in the study through electronic retrieval.A total of 169 duplicate studies were removed and 448 articles were excluded after reading the title and abstract;thus,a total of 28 studies were obtained.After reading the full text,another 15 studies were excluded.Finally,15[19,23-36]studies were included,involving 1460 patients (687 in the IORT group,773 in the non-IORT group).The studies included 3 RCTs[19,30,32]and 12 observational studies[23-29,31,33-36]comparing IORT with non-IORT for rectal cancer.Tables 1 and 2 summarize the baseline characteristics of the included studies.Basic characteristics of the included studies were as follows.(1)There was a large sample size gap between the studies,with the largest being 163 cases[34]and the smallest being 43 cases[27];(2) The publication year of the literature varied greatly,and the time span ranged from 1991 to 2020;(3) The literature was mainly obtained from European,American,and Asian countries;and (4) The literature mostly consisted of observational studies and a few RCTs.
The quality of RCTs showed that attrition bias was at high risk and the quality of all observational studies showed that two studies received nine stars,four received eight stars,and four received seven stars (Table 1).Figure 1 presents the screening flow chart of the included studies.Figure 2 shows the quality assessment of the three RCTs,which indicates that the overall quality of the three RCTs was sufficient.
Meta-analysis results
The results of the meta-analysis were arbitrated by the study type subgroup (RCTs and observational studies) (Table 3).
Five-year OS and five-year DFS
A total of 9[19,25,26,28,30-32,35,36]of the 15 studies included the 5-year OS results reported in their results (Figure 3A).We did not observe statistically significant differences in the meta-analysis (HR=0.80,95%CI=0.60-1.06;P=0.189).The meta-analysis of RCTs(HR=0.68,95%CI=0.29-1.63;P=0.390) and observational studies (HR=0.81,95%CI=0.60-1.11;P=0.189) also showed similar results.Furthermore,the results showed no heterogeneity in the subgroup of observational studies (χ2=2.21,I2=0.0%;P=0.819).
In 6[23,28,30,32,35,36]of the 13 studies,a 5-year DFS period was reported (Figure 3B).No significant differences were observed in the data:Totality (HR=0.94,95%CI=0.73-1.22;P=0.650).The meta-analysis of RCTs (HR=1.61,95%CI=0.74-3.53;P=0.231)and observational studies (HR=0.89,95%CI=0.68-1.16;P=0.378) showed similar results.The results showed no heterogeneity in the subgroup of observational studies (χ2=1.72,I2=0.0%;P=0.633).
Five-year local control
In 14[19,23-33,35,36]cases,the meta-analysis of 5-year LC revealed statistically significant differences (OR=3.07,95%CI=1.66-5.66;P=0.000) (Figure 4).However,the metaanalysis of RCTs (OR=1.37,95%CI=0.35-5.35;P=0.655) and observational studies(OR=3.45,95%CI=1.54-7.73;P=0.000) showed different results.High heterogeneity was found in the subgroup of observational studies (χ2=41.31,I2=73.4%;P=0.000).
Abscess
In 6[19,24,26,30,33,34]of the 13 studies,abscess results reported were included in the study(Figure 5).No statistical significance was observed (OR=1.10,95%CI=0.67-1.80;P=0.833).The meta-analysis of RCTs (OR=1.83,95%CI=0.65-5.11;P=0.252) and observational studies (OR=0.94,95%CI=0.53-1.66;P=0.833) also showed similar results.The results showed no heterogeneity in the subgroup of observational studies (χ2=1.99,I2=0.0%;P=0.575).
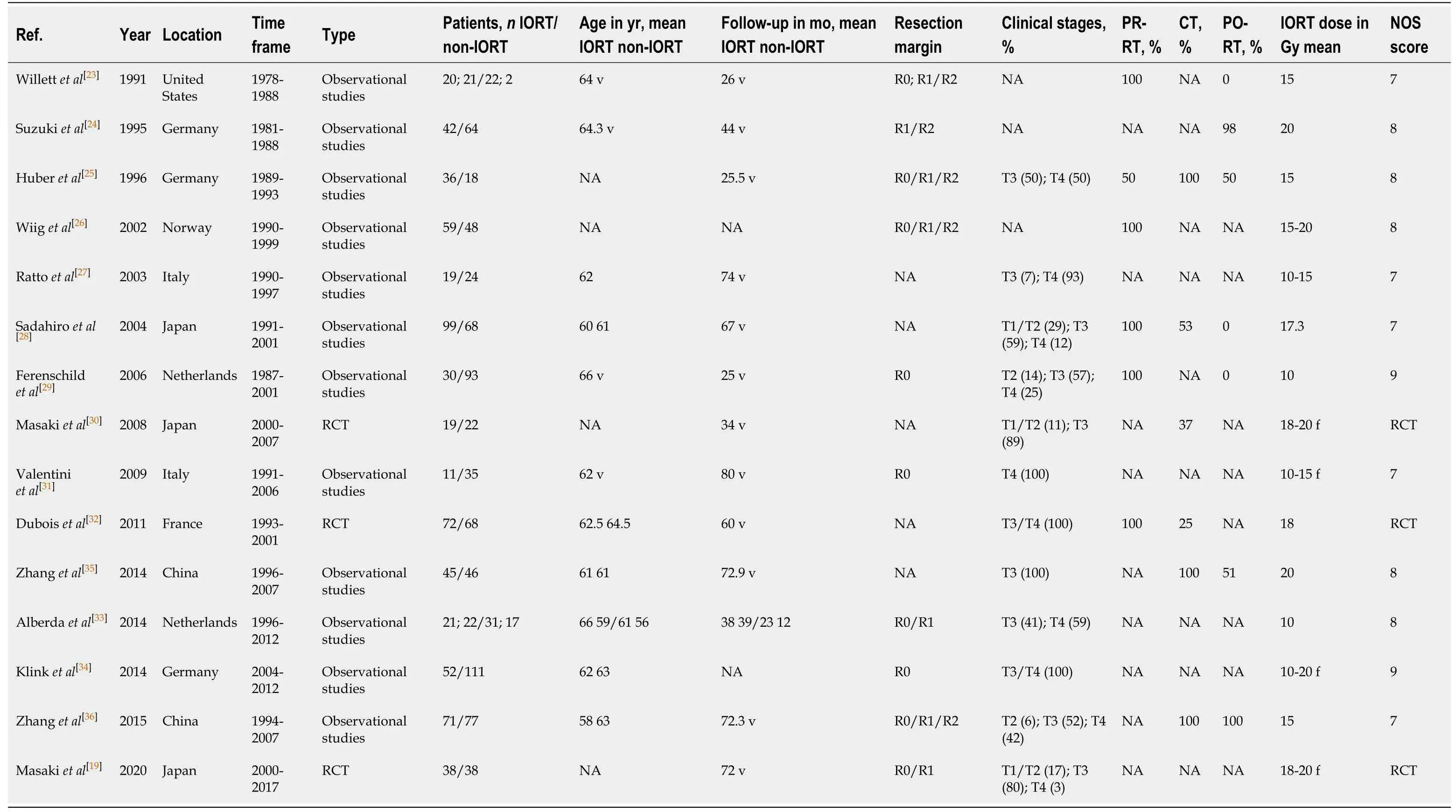
Table 1 Baseline characteristics of the included studies
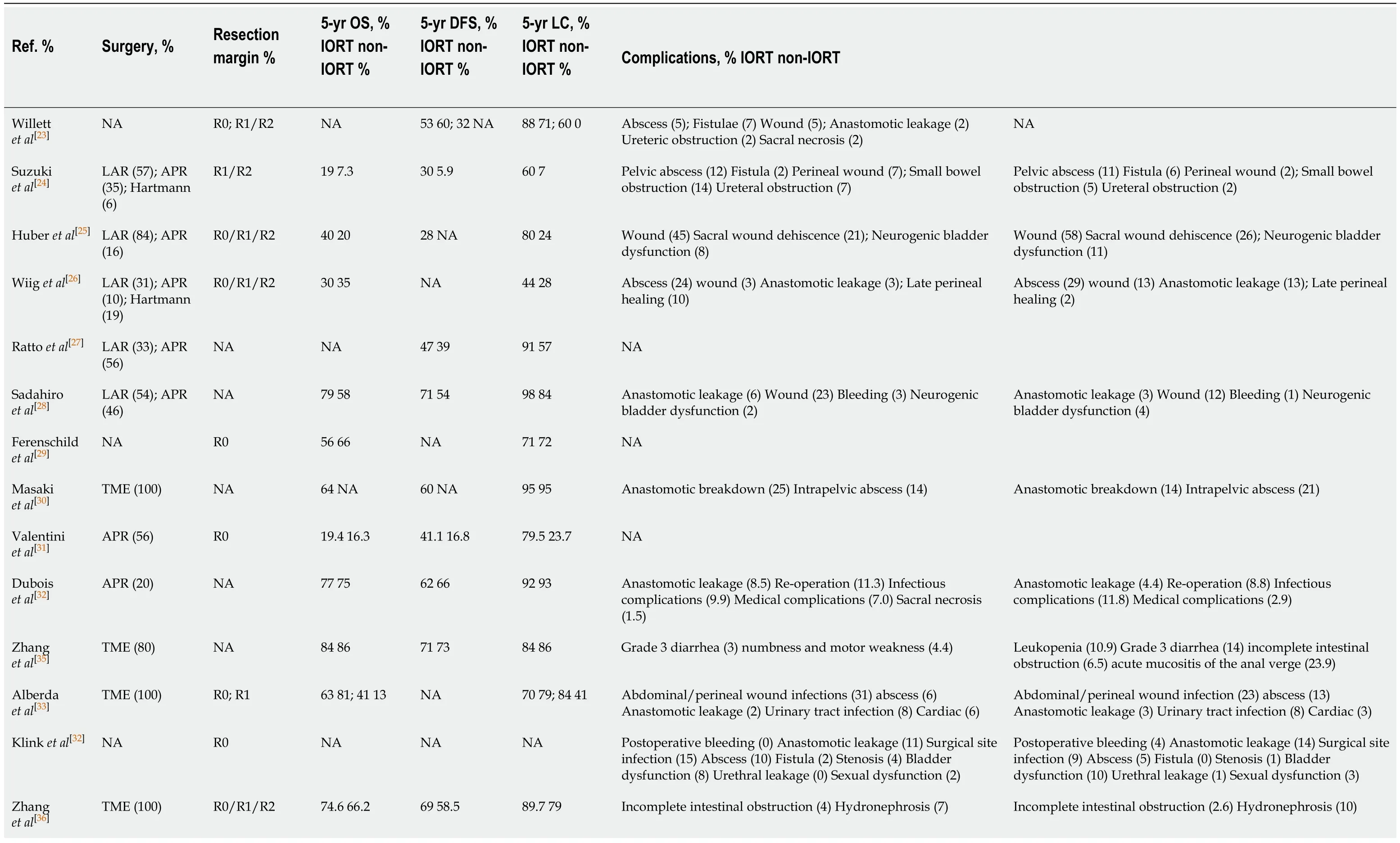
Table 2 Outcome indicators of the included studies

APR:Abdominoperineal resection;DFS:Disease-free survival;LAR:Low anterior resection;LC:Local control;NA:Not reported;non-IORT:Non-Intraoperative radiotherapy;OS:Overall survival;TME:Total mesorectal excision.
Fistulae
In 3[24,32,34]of the 13 studies,the fistulae results were included in the study (Figure 6).The results were not statistically significant (OR=0.79,95%CI=0.33-1.89;P=0.600).The meta-analysis of RCTs (OR=0.75,95%CI=0.30-1.88;P=0.542) and observational studies (OR=1.22,95%CI=0.08-18.77;P=0.888) showed similar results.High heterogeneity was found in the subgroup of observational studies (χ2=2.02,I2=50.4%;P=0.156).
Wound complications
In 8[24-26,28,32-35]of the 13 cases,wound complications results were included in the study(Figure 7) and were not statistically significant (OR=1.02,95%CI=0.52-2.02;P=0.948).The meta-analysis of RCTs (OR=0.75,95%CI=0.30-1.88;P=0.542) and observational studies (OR=1.06,95%CI=0.47-2.37;P=0.893) also showed similar results.High heterogeneity was found in the subgroup of observational studies (χ2=16.09,I2=62.7%;P=0.013).
Anastomotic leakage
In 7[19,26,28,30,32-34]of the 13 cases,the anastomotic leakage results were not statistically significant (OR=1.09,95%CI=0.59-2.02;P=0.775) (Figure 8).RCTs (OR=2.18,95%CI=0.89-5.33;P=0.087) and observational studies (OR=0.66,95%CI=0.31-1.41;P=0.283) The results showed no heterogeneity in the subgroup of observational studies(χ2=2.46,I2=0.0%;P=0.482).
Neurogenic bladder dysfunction
In 3[25,28,34]of the 13 cases,the neurogenic bladder dysfunction results were included in the study (Figure 9).No statistically significant differences were observed (OR=0.69,95%CI=0.31-1.55;P=0.369).The results showed no heterogeneity in the subgroup of observational studies (χ2=0.25,I2=0.0%;P=0.874).
Publication bias and sensitivity analysis
Our data showed that Begg’s tests (P=0.855) and Egger’s tests (P=0.483) did not have publication bias (Figure 10).Sensitivity analysis on the primary outcomes was performed with high and moderate heterogeneity (5-year LC,fistulae,and wound complications) to explore their potential source and assess the robustness of these outcomes.After ignoring each included study in turn for each outcome,the results of 5-year LC,fistulae,and wound complications were stable after testing.
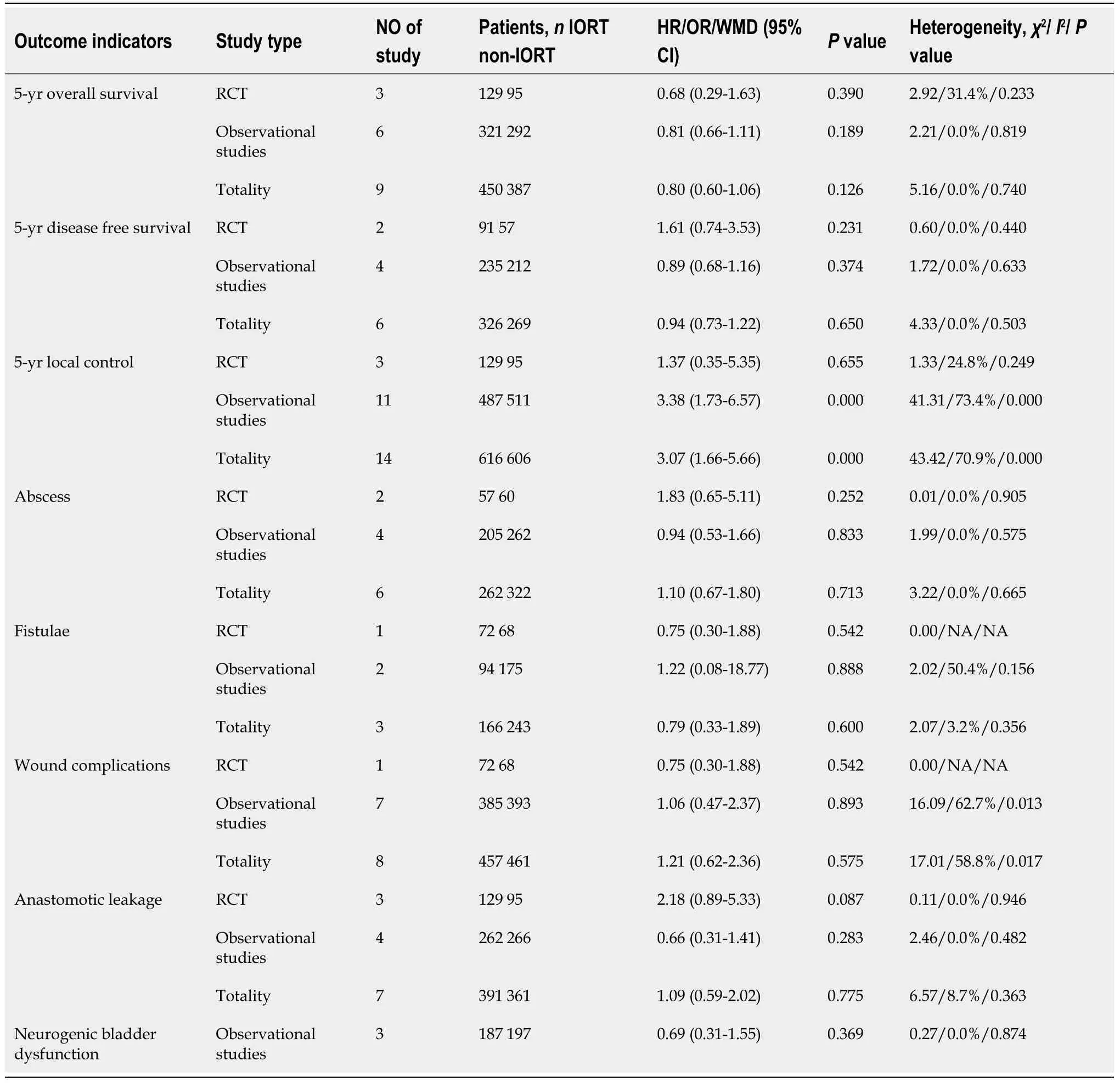
Table 3 Results of meta-analysis and subgroup analysis by intraoperative radiotherapy compared with non-intraoperative radiotherapy for rectal cancer
DISCUSSION
For the treatment of rectal cancer,total mesorectal excision is a treatment method that clearly improves the condition;however,recurrence is a major challenge for the prognosis of patients[37].Multidisciplinary treatment methods including surgery,chemotherapy and radiotherapy significantly improve the prognosis of patients[38].The total dose of radiotherapy may be an important determinant of LC of advanced and recurrent tumors;however,treatment with EBRT alone has not achieved sufficient results[39].Therefore,IORT allows for the direct administration of high-dose radiation and the area that is at the greatest risk after resection[40].Although research reports on IORT for rectal cancer have been published,the sample sizes were small,and therefore,there is still a lack of reliable evidence regarding treatment efficacy and safety.The systematic review and meta-analysis of this study show that IORT is associated with improved LC after resection.
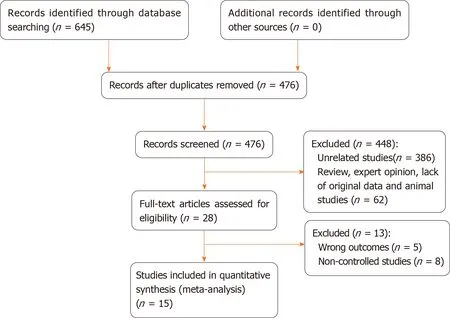
Figure 1 Study identification and selection flow chart.
When subgroup analysis was conducted by study type (RCTs or observational studies),the 5-year survival rate,fistulae,and wound complications showed moderate heterogeneity,which were likely to be different from the original research design,racial various,and inconsistent measurement methods[41].Concerning the 5-year survival rate,whether or not to undergo preoperative radiotherapy,postoperative radiotherapy,and chemotherapy regimen may be influencing factors in all studies included.In addition,differences in the dose of IORT will likely lead to a shift in survival rates in each study[42].The difference in complication results may be due to the longer IORT time compared with simple surgery and more blood loss[43].The studies included primary rectal cancer and recurrent rectal cancer.Due to the destruction of the anatomical plane in cases with recurrent rectal cancer[44]as well as the limitation of the pelvic area,achieving an R0 resection is more complicated.In addition,compared with primary rectal cancer,a more systematic radiotherapy and chemotherapy regimen was received before surgery,thereby leading to differences in both outcomes and bias[45].
The benefit of IORT after R0 resection is a potential confounding factor between studies[46].Many reports have confirmed that the 5-year DFS rate of the IORT group and non-IORT group after R0 resection is equivalent[31,36].In addition,in a recent RCT[19],IORT and non-IORT treatment were compared and the 5-year overall survival rates were 71.5% and 81.8%,respectively,and support the view that IORT may not be beneficial after complete tumor resection.When compared with patients who did not receive adjuvant therapy (preoperative radiotherapy,postoperative radiotherapy,chemotherapy),patients who received adjuvant therapy clearly showed beneficial effects of the treatment[28,32,36],which indicates the importance of adjuvant therapy for IORT.
In this study,we present the first pooled analysis of the impact of IORT on longterm oncology outcomes after rectal cancer resection.In a previous study,the safety and effectiveness of IORT in the treatment of colorectal cancer was systematically evaluated in 2011[47],and in one study,the benefits of IORT treatment for colorectal cancer were reported in 2013[48].However,due to the differences in anatomical location and biological function between colon cancer and rectal cancer,we analyzed rectal cancer separately.By contrast to previous studies,our research incorporated more original studies and detailed subgroup analysis and sensitivity analysis were performed.Despite the inherent limitations of meta-analysis using observational studies,our findings suggested that the use of IORT during rectal cancer surgery may improve LC and has a more moderate impact on disease prognosis and survival.The application of IORT in the treatment of various types of tumors has significant benefits.Indeed,early breast cancer patients who received IORT during breastconserving surgery had a better survival period[49],and in patients with brain metastases,using IORT can deliver auxiliary radiation to the resection cavity with a high LC rate and low incidence of radiation necrosis[50].
This research also had limitations.At first,the randomization in the original research was limited.There were few controlled experiments and the sample size was irregular.Second,although most patients were treated in large tertiary cancer centers,the inclusion criteria for patients were different.Moreover,during treatment,the assessment methods of the outcome index was related to the proficiency of the surgeon.In addition,there were differences in the surgical procedures in this research,which may be a confounding factor for the results.Finally,our research is a secondary study and differences in the original data cannot be controlled for,including experimental design,inclusion criteria,and the original study included,which may affect the reliability of the results.
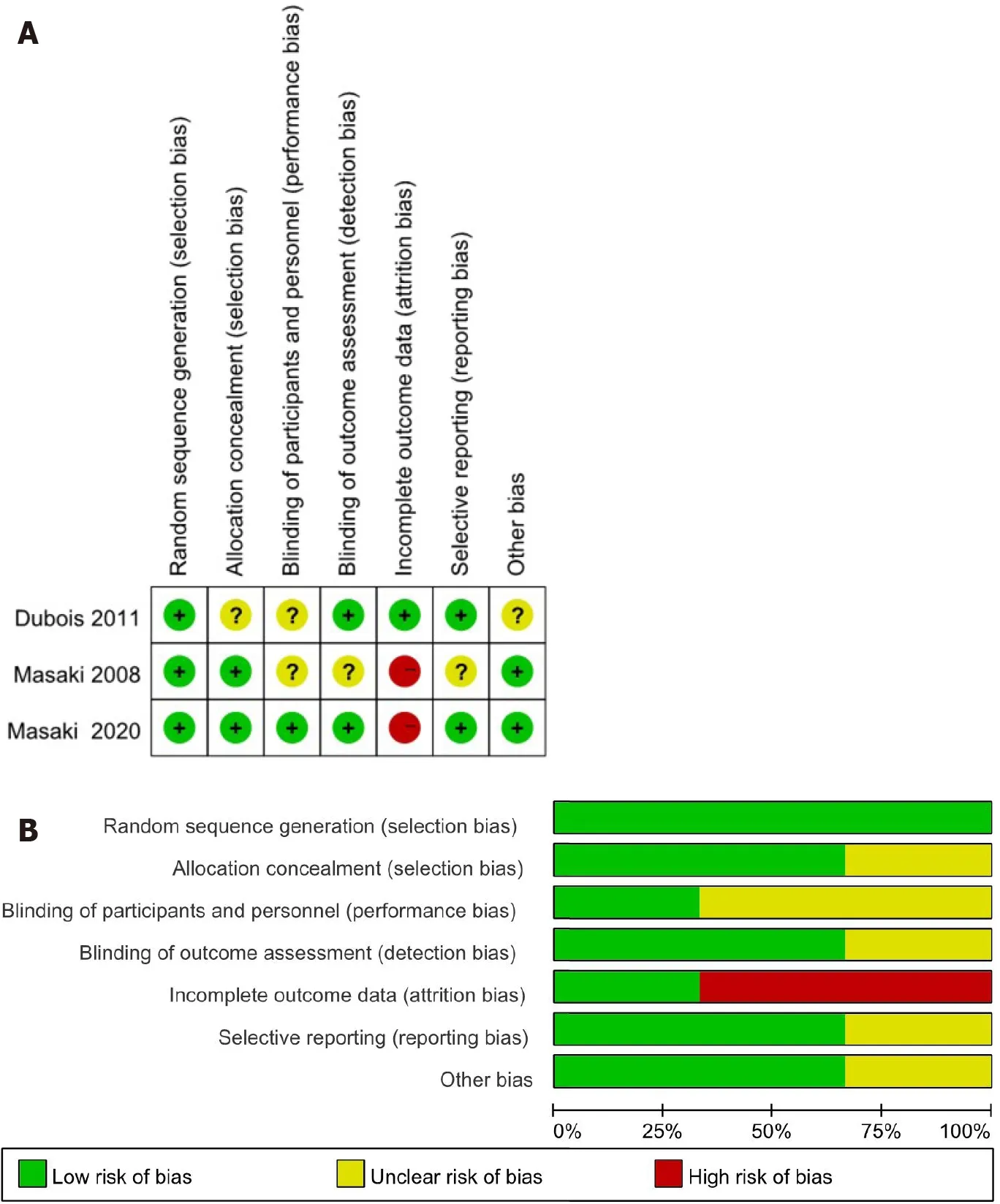
Figure 2 Quality assessment of three randomized controlled trials.
CONCLUSION
Our findings demonstrate that in patients with rectal cancer,adding IORT to traditional multimodal treatment strategies can improve LC but does not significantly improve the survival rate and complications of patients.In the future,well-designed prospective RCTs are warranted to better define the treatment effects using IORT.
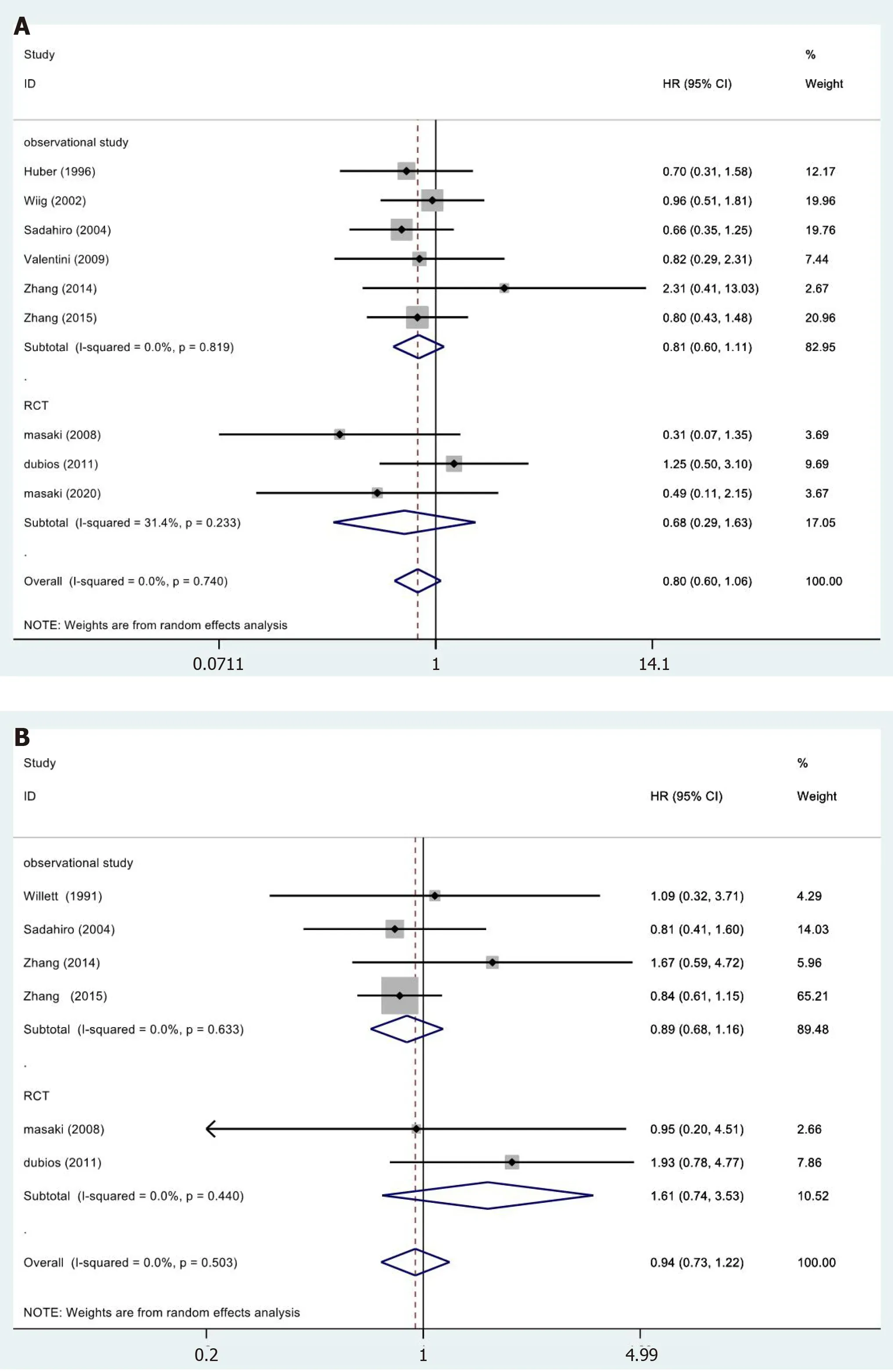
Figure 3 Results of meta-analysis.
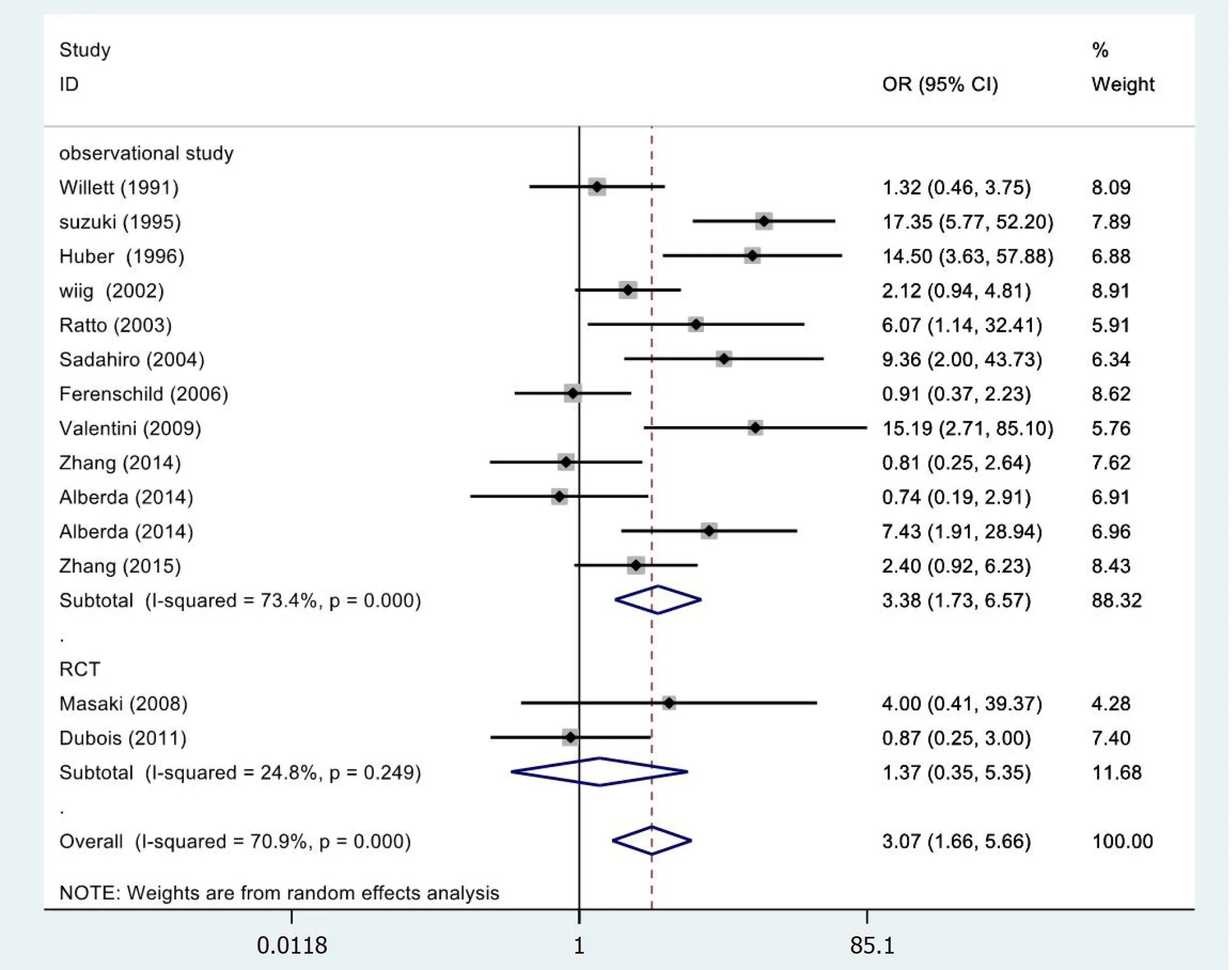
Figure 4 Results of meta-analysis:5-year local control.
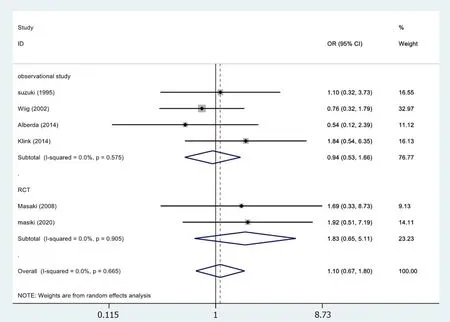
Figure 5 Results of meta-analysis:Abscess.
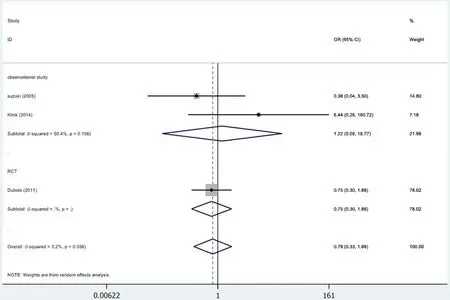
Figure 6 Results of meta-analysis:Fistulae.

Figure 7 Results of meta-analysis:Wound complications.
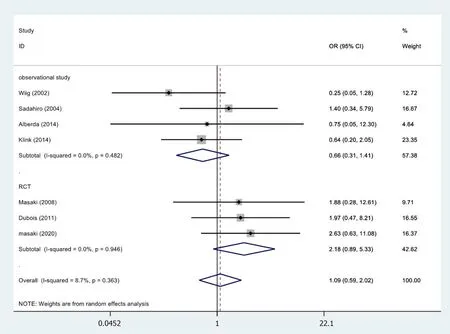
Figure 8 Results of meta-analysis:Fistulae anastomotic leakage.
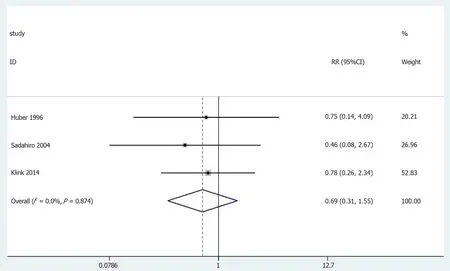
Figure 9 Results of meta-analysis:Neurogenic bladder dysfunction.
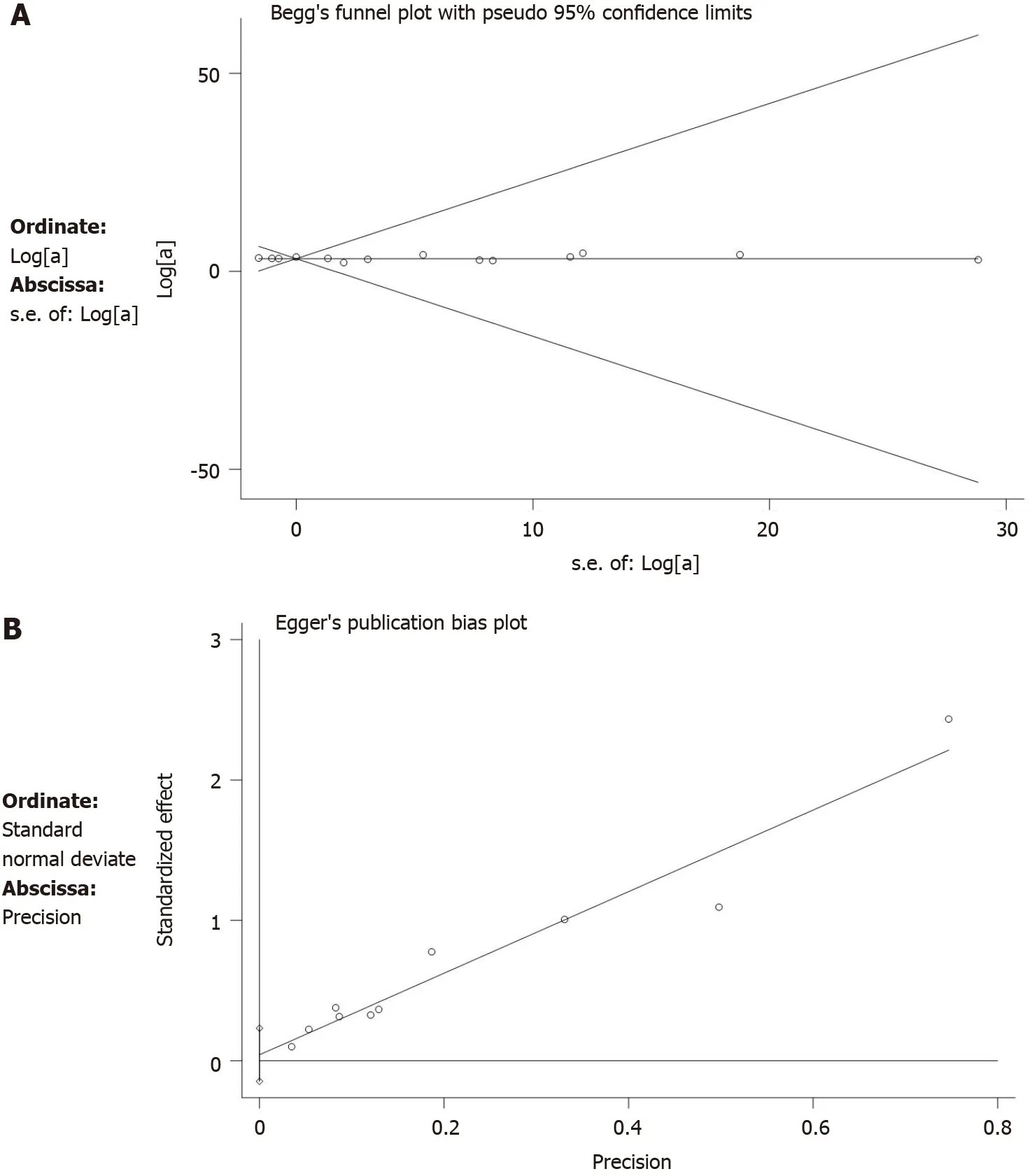
Figure 10 Publication bias.
ARTICLE HIGHLIGHTS
Research background
The prognosis of patients with rectal cancer is poor and the mortality rate is high.The effectiveness and safety of intraoperative radiotherapy (IORT) for rectal cancer still controversial.
Research motivation
Previous studies have demonstrated that adding IORT to traditional treatment of rectal cancer not only reduces the local recurrence rate of advanced rectal cancer but also influences the local control rate of locally recurrent rectal cancer.However,a recent randomized controlled trial (RCT) showed that IORT cannot be recommended as a standard therapy to compensate less radical resection for advanced lower rectal cancer.It is necessary to perform a meta-analysis to systematically and comprehensively investigate the effectiveness and safety of IORT in the treatment of rectal cancer.
Research objectives
A systematic review and meta-analysis to evaluate the value of IORT for patients with rectal cancer.
Research methods
We searched PubMed,Embase,Cochrane Library,Web of Science databases and conference abstracts and included RCTs and observational studies on IORT vs non IORT for rectal cancer.Dichotomous variables were evaluated by odds ratio (OR) and 95% confidence interval (CI),hazard ratio (HR) and 95%CI was used as a summary statistic of survival outcomes.Statistical analyses were performed using Stata V.15.0 and Review Manager 5.3 software.
Research results
In this study,3 RCTs and 12 observational studies were included with a total of 1460 patients,who were mainly residents of Europe,the United States,and Asia.Our results did not show significant differences in 5-year overall survival (HR=0.80,95%CI=0.60-1.06;P=0.126),5-year disease-free survival (HR=0.94,95%CI=0.73-1.22;P=0.650);abscess:(OR=1.10,95%CI=0.67-1.80;P=0.713);fistulae (OR=0.79,95%CI=0.33-1.89;P=0.600);wound complication (OR=1.21,95%CI=0.62-2.36;P=0.575);anastomotic leakage (OR=1.09,95%CI=0.59-2.02;P=0.775);and neurogenic bladder dysfunction (OR=0.69,95%CI=0.31-1.55;P=0.369).However,the metaanalysis of 5-year local control was significantly different (OR=3.07,95%CI=1.66-5.66;P=0.000).
Research conclusions
The advantage of IORT is mainly reflected in 5-year local control but it is not statistically significant for 5-year overall survival,5-year disease-free survival,and complications.
Research perspectives
Several limitations in this analysis should be carefully addressed.First,the randomization in the original research was limited.There were few controlled experiments and the sample size was irregular.Second,although most patients were treated in large tertiary cancer centers,the inclusion criteria for patients were different.Moreover,during treatment,the assessment methods of the outcome index was related to the proficiency of the surgeon.In addition,there were differences in the surgical procedures in this research,which may be a confounding factor for the results.Finally,our research is a secondary study and differences in the original data cannot be controlled for,including experimental design,inclusion criteria,and the original study included,which may affect the reliability of the results.
ACKNOWLEDGEMENTS
The authors thank Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province and the DaVinci Surgery System Database(DSSD,www.davincisurgerydatabase.com) and for their help and support in the methodology and meta-analysis process.
 World Journal of Gastrointestinal Oncology2021年1期
World Journal of Gastrointestinal Oncology2021年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Internal hemorrhoid harboring adenocarcinoma:A case report
- Diagnostic performance of narrow-band imaging international colorectal endoscopic and Japanese narrow-band imaging expert team classification systems for colorectal cancer and precancerous lesions
- Mining The Cancer Genome Atlas database for tumor mutation burden and its clinical implications in gastric cancer
- Survival outcomes and prognostic indicators for gastric cancer patients with positive peritoneal wash cytology but no peritoneal metastasis after radical gastrectomy
- Laparoscopy-assisted transanal total mesorectal excision for lower rectal cancer:A feasible and innovative technique
- Myeloid-derived suppressor cells in gastrointestinal cancers:A systemic review
