Current epidemiology of pancreatic cancer:Challenges and opportunities
Wenhao Luo ,Jinxin Tao ,Lianfang Zheng ,Taiping Zhang
1Department of General Surgery,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100730,China;2Department of Nuclear Medicine,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100730,China;3Clinical Immunology Center,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100730,China
Abstract Pancreatic cancer (PC) is an increasingly common disease worldwide.Having a better understanding of worldwide and regional epidemiologic features and risk factors of PC is essential to identify new approaches for prevention,early diagnosis,surveillance,and treatment.In this article,we review the epidemiologic features and risk factors for PC and discuss opportunities and challenges of PC future treatment.
Keywords:Pancreatic cancer;epidemiology;risk factors;novel strategies;survival
Introduction
Pancreatic cancer (PC) is one of the most aggressive and lethal malignancies, with a 5-year survival rate of approximately 10% in the USA,and it is becoming an increasingly common cause of cancer mortality (1) while remaining a devastating malignancy with limited options for effective therapy.Globally,PC is the 12th most common cancer in men,the 11th most common cancer in women,and the 7th leading cause of cancer-related deaths(2).The incidence of PC has constantly increased for the past 10 years (3).Some possible and potential reasons for constant increase in incidence rates are smoking,obesity,diabetes mellitus,physical inactivity,and consumption of high-calorie/fat diets.Improvement in patient outcomes will depend on clear knowledge of epidemiology,reasonable prevention and scientific regulation of early detection. Although current treatments have largely improved the outcomes of patients with PC, our understanding of epidemiological and risk factors for PC continues to expand and will eventually establish rational prevention approaches that will result in clinical benefit.
Epidemiology
Incidence
According to the International Agency for Research on Cancer’s GLOBOCAN 2018 estimates,458,918 new cases of PC were registered worldwide in 2018,accounting for 2.5% of all new cancer diagnoses (4).PC has ranked as the 12th most common cancer in the world,with a global agestandardized incidence rate (ASIR) of 4.8 per 100,000 persons (4).
The incidence of PC varies across geographic regions and populations.Risk is much higher in more developed regions than in less developed ones.The human development index (HDI) is a composite index measuring three dimensions:life expectancy,education period,and access to essential sources for a proper and sensible life (5).The regions and populations with remarkable progress in all HDI measurements have developed more rapidly compared with low or moderate HDI countries (6).The analyses based on cancer incidence data derived from the World Bank for Cancer in 2018 showed that the highest mean incidence of PC was related to the very high HDI(P<0.0001) (6).The inequality of PC incidence rate is more concentrated in countries with higher HDI scores (7).The ASIR (unit per 100,000 people) is approximately three to four times higher in higher HDI countries (4).The world’s highest ASIRs were recorded in Europe (7.7) and North America (7.6),followed by Oceania (6.4) and Eastern Asia(6.4),whereas the lowest incidence rates were reported in Africa (2.2) and South-Central Asia (1.1).Hungary (ASIR,
10.8 ) located in Europe and Guinea (ASIR,0.35) located in Africa represent the highest and lowest incidence rates in the world,respectively.The estimated number of new PC cases from 40 European countries in 2018 was approximately 132,600 and accounted for 3.4% of all cancer sites,with an estimated ASIR of 11.5 per 100,000(8).In terms of North America,the United States is expected to have nearly 57,600 estimated new cancer cases of pancreas in 2020,representing 3.2% of all expected numbers of new cancer cases (9).Additionally,the projected estimates of new cases account for 2.7% of all cancer sites,and the projected estimates of ASIR for PC are up to 13 per 100,000 in Canada in 2020 (10).It was estimated that in 2020,there would be an ASIR of 13 cases per 100,000 persons (14 for males and 11 for females) in Australia (11).When we look at Eastern Asia,the ASIR(males,10.6;females,6.7) associated with PC is higher in Japan than in other Asian countries such as India and China(12,13).There were 36,239 PC cases (4.1% of all sites)diagnosed in Japan in 2014,which had the 7th-highest incidence rate among all cancers (12).According to the Report of Cancer Epidemiology in China,2015,released by the National Cancer Center in 2019,new cases and incidence rates of PC have reached 950,000 (2.4% of all sites) and 6.92 (males,7.67) per 100,000,respectively,remaining in the 10th among all cancers (14).On the basis of the latest release of cancer statistical data,the comparison of estimated percentages of PC in all new cancer cases and all deaths from cancer as well as the 5-year relative survival rates for PC among the representative continents or countries is summarized inTable 1.
HDI inequality occurs not only internationally but also domestically and is closely associated with regional variations in PC incidence rates.PC remains an urban disease in Brazil because the highest incidence is found in the most developed regions and in large metropolitan integrated municipalities (15).A recent study on the regional variation of PC incidence in the Nile Delta region of Egypt revealed that the ASIR was 1.3-fold higher in urban areas (4.45 per 100,000) than in rural areas (3.43 per 100,000) (16).Similarly,incidence rates of PC vary considerably by urban and rural patients in China.Based on the estimated cancer incidence in China in 2015,the incidence rate ratio (IRR) between urban and rural areas was nearly 1.3 (7.79 per 100,000 in urban areasvs.5.82 per 100,000 in rural areas) (14).Improvement in diagnostic methods and notification quality,a rapidly aging population,and a great demographic dynamism in urban regions could partially explain this phenomenon (15).
Like nearly all other adult tumors,the incidence rate for PC increases with age.Advanced age (age ≥65 years old)leads to a rising burden in all socioeconomic regions of the world except in low HDI countries where PC predominantly affects populations with an age <65 years old (17).The PC incidence in Brazil from 2005 to 2012 nearly doubled from 2.4 to 4.5 per 100,000,particularly among patients older than 70 years (15).PC is seldom diagnosed before 55 years old,but it was recently reported that PC incidence increased in whites (by 57%) and blacks(by 44%) of younger age groups in the United States from 2001 to 2015 and was prominently distributed in people aged 30-39 years old (18),indicating that the age of PC onset tends to be younger over time.
Racial disparities in PC incidence have been investigated extensively.The incidence rate of PC was higher among blacks than whites (24.7vs.19.4 per 100,000;IRR,1.28)among all age groups in the United States from 2001 through 2015.Black patients had a lower incidence of local PC but a higher incidence of distant PC (IRR,1.32)compared to white patients (18).A data analysis with a special focus on racial disparities of PC in Georgia (a southeastern state in America) in 2000-2011 revealed that African Americans displayed an overall significantly greater ASIR compared to Caucasians (14.6vs.10.8 per 100,000).Moreover,a disproportionate burden of PC incidence was exhibited among African Americans in Georgia.An excess and disproportionate spatial distribution of PC incidence among African Americans may be related to the physical environment (P=0.16,P=0.04),such as water quality or industry (19).Additionally,cloud-adjusted solar ultraviolet-B (UVB) irradiance inversely correlates with PC incidence(P=0.0182 for males and P=0.0029 for females afteradjustment for traditional risk factors),which is consistent with an inverse association between overall vitamin D deficiency in lower UVB-irradiated countries and PC incidence (20).Black Americans have much lower mean 25(OH)D concentrations than white Americans during this period,which may help explain racial disparities in the United States (21).Apart from racial disparities in PC incidence between blacks and whites,disparity in other ethnic populations has also been observed.Subgroups of Asian Americans exhibit significant heterogeneity in PC incidence,with the ASIRs ranging from the highest of 8.1 in Japanese and 7.5 in Koreans to the lowest of 4.4 in South Asians (22).A multiethnic cohort study with an average 16.9-year follow-up showed a higher incidence of PC among African Americans (ASIR,52.7,P<0.01),Native Americans (ASIR,73.4,P<0.001),and Japanese Americans(ASIR,56.8,P<0.0001) but no difference for Latino Americans (ASIR,42,P=0.87) compared to European Americans (ASIR,41.3) (23).Only 20% of racial disparities in PC incidence could be attributed to the interethnic differences in the distribution and effects of predominant environmental risk factors,including smoking,adiposity,and red meat intake.The residual racial disparities may be explained by other genetic and biological factors.Moreover,compared to higher-stage PC cases,the proportion of blacks was smaller (10.2%vs.12.5%),while the proportion of other non-Caucasians was higher (11.9%vs.8.4%) among stage IA cases (24).Nevertheless,the trends of incidence are significantly rising across almost all racial/ethnic groups over time (22),and disparities between black and white patients were observed to decrease over 5-year time periods from 2001 through 2015 (18).
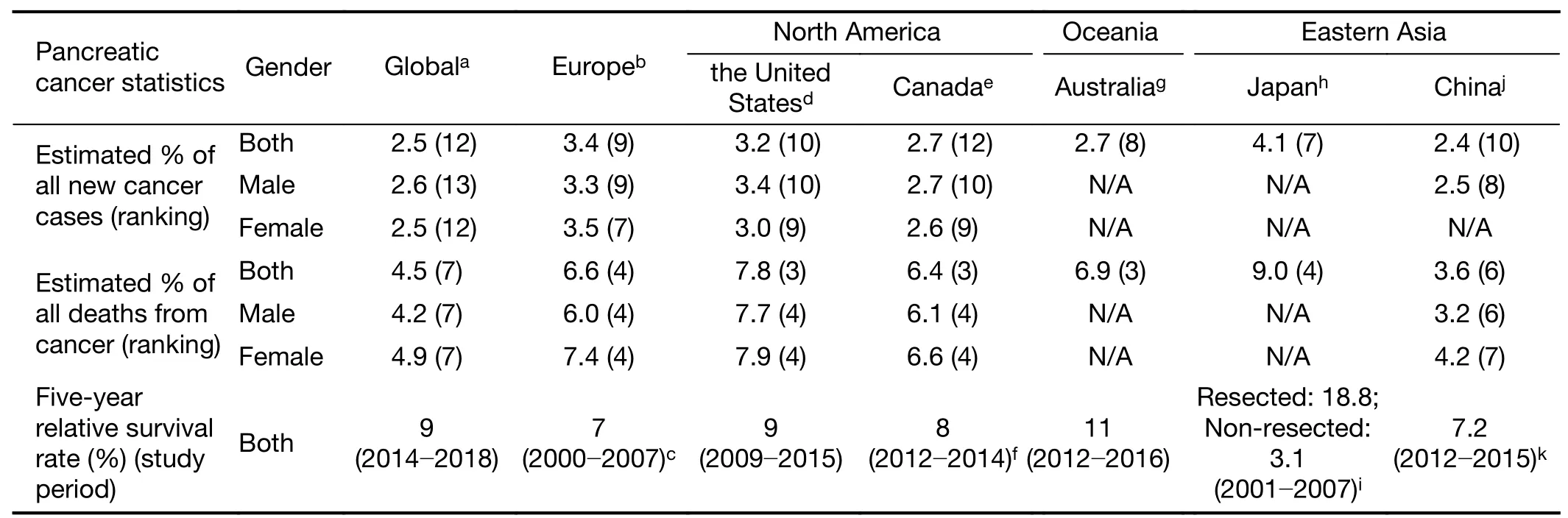
Table 1 Comparison on cancer statistical data among representative continents or countries
There is a slight difference in PC incidence among the sexes.The highest ASIR for men is distributed in central and eastern Europe (9.9),while the region with the highest ASIR for women is Western Europe (7.2) (4).The ASIRs for both sexes in eastern Asia are 7.0 and 4.8,respectively,ranking 7th in the world.Incidence is higher in men than in women across almost all regions worldwide,with a global ASIR per 100,000 of 5.5 for men and 4.0 for women(4).Differences in sex-steroid hormones and a higher prevalence of risk factors for health behaviors such as smoking could be responsible for the slight sex disparities(25).Potential sex differences in the association between smoking and PC incidence are indicated in a Japanese population-based cohort study (26).Although PC risk for current smoking is increased in both sexes compared to never smokers,the risk for former smoking and the small cumulative dose of ≤20 pack-years is significantly elevated only among females.More surprisingly,after 5 years of smoking cessation,PC risk became comparable to those who had never smoked in males,whereas no risk attenuation was observed in females.In addition to smoking,higher parity may account for a decreased risk of PC in females (27).Therefore,sex disparities should be taken into account when risk factors for PC incidence are assessed.
The incidence of PC has been increasing in both sexes in most countries over recent decades and presumably will continue to rise worldwide (4,9,25).There are some exceptions;for example,PC incidence in Iceland did not show major changes during the past two decades (28).In contrast to the favorable effect of decline in smoking prevalence,at least in males,changing prevalence of other lifestyle risk factors,including increase in population with obesity and diabetes,increase in the intake of red or processed meat with an inadequate intake of fruits and vegetables,and increase in physical inactivity,as well as improved diagnostic methods are likely to parallel the temporal trend of PC incidence worldwide (25,29).
Mortality
On the basis of GLOBOCAN mortality estimates in 2018,PC is the 7th leading cause of cancer-related death worldwide,corresponding to 432,242 deaths from PC and 4.5% of all cancer deaths.Mortality rates for PC are closely parallel to the incidence rates across geographic regions (4),indicating that they may share similar epidemiological characteristics.The global age-standardized mortality rate(ASMR) was 4.4 per 100,000.The highest ASMRs were seen in Europe (Western Europe,7.6;central and eastern Europe,7.3;northern Europe,6.5;southern Europe,6.4),followed by North America (6.5),Oceania (5.9),and eastern Asia (5.4),whereas the lowest were observed in South-Central Asia (1.0) (4).The latest cancer statistical data on the ASMR in Canada,Australia,and China are 12,10,and 6.16 per 100,000 people in both sexes,respectively(10,11,14).Countries with the highest and lowest ASMRs were registered in Uruguay (South America) and Guinea(9.9vs.0.32),respectively.A total of 52.3% of PC deaths were distributed in the most developed countries,and more than 90% of PC deaths were recorded in high HDI or very high HDI countries (4).In China,ASMR was 1.5-fold higher in urban areas (7.15) than in rural areas (4.90) in 2015 (14).Therefore,socioeconomic development is associated with global mortality variations in PC (6,7).Similar to incidence,racial disparities also exist in PC mortality across the United States (19,21),and they share similar temporal patterns among ethnic populations (18).
Mortality rates rose over the past decade for PC among males in the United States (9).The observed trends in PC mortality rates in Europe and Canada are rather stable or slightly increasing relative to the declining rates of breast cancer,and thus,it has been projected that the number of deaths caused by PC will surpass breast cancer as the 3rd leading cause of cancer death in the near future (4,8,10),as has already been done in the United States (9).In 2018,PC was the 4th most common cause of death from cancer in Australia,and it is expected to become the 3rd most common cause of cancer death in 2020 (11).
The mortality-to-incidence ratio (MIR) is a parameter measuring the relationship between mortality and incidence,whose value can serve as a proxy for the 1-year survival rate (30).The global MIR reached 94% in 2018(4).Adequate cancer screening,early diagnosis,and effective treatment provided by the healthcare system help achieve low MIR values.By obtaining and analyzing the incidence and mortality rates,the World Health Organization (WHO) rankings,and total expenditures on health/gross domestic product (e/GDP) from public databases,it was observed that the MIR variations for PC are not associated with healthcare disparities among countries,reflecting the highly lethal nature of PC despite decades of continuous efforts (30).The mortality rate should mirror its incidence (31);however,Bouvieret al.identified an unusual PC incidence and mortality pattern in which the incidence continuously increased over the last 30 years in France,whereas mortality remained flat in men and moderately increased in women at only a quarter of the rate at which the incidence rose (32).The lack of parallel temporal trends in incidence and mortality and the MIR of more than 100% from the beginning of the study period until 2005 primarily set the French PC statistics apart.On the one hand,improvements over time in the quality of PC notifications to registries and in the diagnosis of PC can contribute to an artifactual increase in incidence (33).On the other hand,the fact that incident cases notified by death certificates only are not recorded by registries leads to the unusual MIR.Underreporting of incident cases of PC commonly also occurs in other cancer registries,such as Denmark (34) and the Netherlands (35).
Surgical resection remains the only potentially curative approach for PC,but it still carries risks for postoperative mortality.Quantitation of risk factors for postoperative mortality following surgical resection for PC is beneficial for judicious patient selection and shared decision-making between surgeons and patients (36).The risk of mortality increased with age,eclipsing 10% in some cases (36).Data presented from the current retrospective analysis show that the 30-day in-hospital mortality rate after pancreaticoduodenectomy for patients >76 years old was 4.11% and for patients <76 years old was 2.77% (37).More extensive surgery is also an independent predictor of postoperative mortality.The 30-/90-day mortality rates peak at 7.08%and 13.27%,respectively,in the oldest group of patients treated with a total pancreatectomy with partial gastrectomy (36).Compared to nonteaching hospitals,patients undergoing surgery at high-volume teaching centers [odds ratio (OR),0.61;P=0.008] had a lower risk of mortality (37),indicating the positive correlation of case volume in postoperative outcomes.A significant impact of higher surgeon volume (≥4 resections/year) on lower perioperative mortality was also found in the states of Queensland and New South Wales in Australia (38).Preoperative endoscopic retrograde cholangiopancreatography(ERCP) is considered a safe procedure because patients who receive ERCP have no increased risk of death compared to those who proceed to surgery directly (39).Venous resection is widely used during PC radical surgery,and it was found that 30-day postoperative mortality and perioperative mortality were equal among patients with and without vascular resection (40).A systematic analysis of prospective data has revealed that chemoradiotherapy(CRT) for (borderline) resectable patients and neoadjuvant chemotherapy exhibit decreased risks of postoperative mortality and complications compared with surgery alone(41).The 1-year/6-month mortality following pancreatectomy for elderly patients with PC treated with early adjuvant therapy (either neoadjuvant or adjuvant chemotherapy or radiation therapy along with surgery within 12 weeks of surgery) is significantly lower than surgery alone,and the late adjuvant therapy (after 12 weeks) group has the lowest odds of 1-year/6-month mortality (42).Rural and older patients had significantly higher odds of 1-year mortality than their urban counterparts,but rural/urban differences in early (surgical or 90-day) postoperative outcomes were not significant,suggesting that regional disparities exist in the long-term postoperative mortality of patients with PC (43).The influence of service-related and sociodemographic factors on the disparities in the use of chemotherapy may in part explain regional disparities in postoperative mortality rate.
Survival
The 5-year survival rate indicates what percent of people live at least 5 years after the cancer is diagnosed.Globally,the 5-year survival rate for PC improved from 6% in 2014 to 9% in 2018 (4).PC had the lowest 5-year relative survival rate of 9% among all cancer types in the United States during 2009 through 2015 (9).In Canada,PC continues to have an exceptionally low 5-year net survival of 8% (10). The EUROCARE-5 cooperative study analyzed data of patients with PC diagnosed from 2000 to 2007 in 107 cancer registries of (29) European countries and showed that the mean age-standardized 5-year relative survival for adult patients with PC is the lowest (7%)among 46 cancers (44).Among 29 European countries involved in cancer survival statistics,Croatia had the highest 5-year relative survival rate of 10.9%,while Malta had the lowest survival rate of 0%,followed by Northern Ireland (3.02%) (44).In 2012-2016,individuals diagnosed with PC had an 11% chance of surviving for 5 years compared to their counterparts in the general Australian population (11).In China,the age-standardized 5-year relative survival for PC was 7.2% in 2012-2015,remaining the lowest for both male and female patients,and no improvements in relative survival during 2003-2015 were observed for PC (45).The 5-year relative survival for PC improved from 3.2% to 11% between 1987-1991 and 2012-2016 in Australia (11).According to a recent population-based cohort study in Australia,PC had the lowest proportion cured (6.1% for males and 7.0% for females) and the lowest median survival time (0.38 years for males and 0.40 years for females).In Japan,the 5-year overall survival (OS) rate from 2001 to 2007 was 18.8% for patients with resected PC and was extremely low at 3.1%for nonresection cases (46),and the 10-year relative survival rate was 4.9% (12).
Unlike incidence and mortality,survival rates of PC vary little by race (9).However,it seems controversial whether socioeconomic development or HDI affects survival rates(4).Burmeister and colleagues found that remoteness correlates with significantly reduced survival (P<0.01) (47).Martinet al.also noted a significantly poorer survival(P<0.02) for patients with PC living in rural regions compared to metropolitan areas (48).Such geographical variations in survival rates were likely to rely on disparities in quality of care and supportive care needs.However,it was also reported that survival rates remain relatively constant between very high/high-HDI and low-HDI regions all over the world (4).Survival rates and individual outcomes vary significantly by specific stage of PC when it is diagnosed.Based on PC statistics approved by the American Cancer Society (05/2020),approximately 10% of people are diagnosed at an early stage (local stage) when surgical removal is possible,and the 5-year survival rate is 37% (49).For PC spreading to surrounding tissues or organs (stage III),the 5-year survival rate is 12%.Fiftythree percent of people are diagnosed with distant metastases (stage IV),for which the 5-year survival is 3%.According to a recent investigation on recent trends in the stage of newly diagnosed PC,the 5-year OS for stage IA PC increased from 44.7% to 83.7% from 2004 through 2012,and the 10-year survival improved from 36.7% in 2004 to 49.0% in 2007 (24).Moreover,the average age of diagnosis for stage IA and IB cases declined by 3.5 years and 5.5 years,respectively (24).These trends may be attributed to advancements in early diagnosis and early detection.
PC is associated with the highest incidence of venous thromboembolism (VTE) compared to any other cancer types.Frereet al.analyzed data from a prospective,observational study performed at multiple centers in France from 2014 through 2018 and found that 20.79% of patients developed VTE,with a median time of 4.49 months from PC diagnosis to the onset of VTE.Those who developed VTE during follow-up had shorter times of progressionfree survival (PFS) [hazard ratio (HR),1.74;P=0.004] and OS (HR,2.02;P<0.001) (50).The incidence of VTEs among Korean patients with advanced PC is 18.6%,which is comparable to that of Caucasian patients.Patients diagnosed with classic concurrent VTEs [pulmonary embolism (PE) and/or deep vein thrombosis (DVT) of the lower extremities] had a significantly poorer OS than those who developed VTEs later (median OS,2.1 monthsvs.10.7 months;P<0.001) (51).However,VTE incidence was found to be lower (8.0%) in the Taiwanese population with newly diagnosed locally advanced or metastatic PC,and patients with VTE did not show significantly poorer survival outcomes than those without (52).Furthermore,the Taiwan study revealed that early onset of VTE is associated with significant decreases in OS,which is consistent with that conducted by Frereet al.(50,52).
Epidemiologic risk factors for PC
To avoid hazards of late diagnosis of PC and to focus on early detection efforts on individuals with higher risks,we need to fully discuss the elevated risk factors to seek out potential high-risk screening groups and establish appropriate supervision.Important modifiable PC risk factors include tobacco smoking,obesity,and diet type.Several prospective studies have shown a positive association between red meat and animal fat and the risk of PC and an inverse association between fruits,vegetables,and folate and the risk of PC (53).Other established risk factors for PC include inherited or acquired cancer predisposing genetic mutations/familial syndromes,mucinous pancreatic cysts,diabetes mellitus and chronic pancreatitis.These factors will be fully discussed below.
Tobacco smoking
Multiple reports from meta-analyses and pooled analyses concluded that smoking is associated with an increased risk of PC (54,55).Cigarette smoking has been reported to be the most important environmental factor,with a population attributable risk estimated at 25% (56).In the early 1970s,Charles Set al.analyzed data from two large cohorts,which had 2,116,229 person-years of follow-up.They found that the relative risk (RR) of PC is approximately 2.5-fold among current smokers and that 25% of PCs were attributable to past or present cigarette smoking (57).Moreover,they demonstrated that smoking cessation could eliminate 25% of 27,000 deaths from PC development every single year in the United States.SC Larssonet al.analyzed data during 560,666 person-years and found that PC risk was associated with cigarette smoking with an RR of 3.06 [95% confidence interval (95% CI),1.99-4.72],and they found that current smokers of ≥40 pack-years had a 5-fold elevated risk compared with never smokers (58).Although a number of studies have proven the close association with PC and smoking,detailed examination of the association of smoking intensity,smoking duration,and cumulative smoking dose with PC is rare.Shannon M.Lynchet al.analyzed pooled data from 1,481 cases and 1,539 controls and found that when compared with never smokers,current smokers had a significantly elevated risk[OR,1.77;95% CI,1.38-2.26].In this analysis,risk increased significantly with greater intensity (30 cigarettes/day:OR,1.75;95% CI,1.27-2.42),duration (50 years:OR,2.13;95% CI,1.25-3.62),and cumulative smoking dose (40 pack-years: OR, 1.78;95% CI,1.35-2.34).Furthermore,the risk after more than 15 years of smoking cessation was similar to that for never smokers(59).Therefore,we found that smoking cessation reduces the risk of PC,with the reduction in risk observed 10-15 years after cessation.
To determine the potential mechanisms of association between PC and smoking,previous studies demonstrated that pancreatic tumors can develop in animals by administration of tobacco-specific nitrosamines or other Nnitroso compounds (60,61).The mechanism may be that tobacco-specific carcinogens can result in the activation of oncogenes and the mutation of tumor suppressor genes.Tobacco-specific carcinogens may reach the pancreas either through the blood or through refluxed bile that is in contact with the pancreatic duct.It is most likely connected with the mutagenic effect of tobacco smoke components such as heterocyclic amines and polycyclic aromatic hydrocarbons on protooncogenes in cells,which cause Kras mutations (62).Moreover,when polycyclic aromatic hydrocarbons enter the organism,they are metabolized by detoxifying enzymes into forms capable of interacting with DNA.As a result of the activity of P450 enzyme system(CYP1A and CYP1B),active epoxy compounds are formed.These are then hydrolyzed by epoxy hydrolase into diol epoxide derivatives,which can bind with DNA and lead toP53gene mutation.Hence,the activation of oncogenes and mutation of tumor suppressor genes caused by smoking create an environment prone to PC development and progression.
Obesity
The growing worldwide obesity epidemic is associated with an increased risk of PC.According to the American Cancer Society,obese patients have a higher risk of PC than people with a healthy body mass index (BMI) (18.5-24.9 kg/m2)(63).Evidence from various studies has demonstrated that obesity has been linked to metabolic abnormalities,including insulin resistance,hyperinsulinemia and glucose intolerance,which have a close relationship with PC development (64). SC Larssonet al.examined the association of BMI with the risk of PC among 83,053 individuals and found that the multivariate RR of PC for obese individuals (BMI≥30 kg/m2) was 1.81 (95% CI,1.04-3.15) compared to those with a BMI of 20-25 kg/m2(58).In this research,SC Larssonet al.found that the risk of PC was nearly double for obese patients compared with those with a normal weight.Moreover,Alpa V.Patelet al.(65) analyzed data among 145,627 individuals and demonstrated an increased risk of PC among obese individuals compared with those with normal BMI (RR,2.08;95% CI,1.48-2.93,P=0.0001).A meta-analysis of prospective cohorts proved that an overall 5 kg/m2increase in BMI is associated with a 12% increased risk of PC,which suggested that obese individuals can be a valid target for early detection (66). Mechanically, a metabolic consequence of obesity is the development of insulin resistance,which leads to an elevation in the secretion of insulin from pancreas.Hyperinsulinemia can culminate in an increase in local blood flow and cell division in the pancreas.High concentrations of insulin are able to activate the insulin-like growth factor 1 (IGF-1) receptor.IGF-1 is a peptide hormone with structural similarity to insulin.Binding of insulin or IGF-1 to the insulin receptor or to IGF-1 receptor stimulates lipogenesis,inhibits lipolysis and increases protein synthesis.Furthermore,increased insulin can also downregulate insulin-like growth factor binding protein-I,leaving more bioavailable IGF-1 to promote cell proliferation (67).A recent study proved that IGF-1 promotes growth and proliferation of cancer cells by activating PI3K-mTOR and MAPK signaling pathways (68).
An alternative mechanism for the association between obesity and PC may be related to DNA adduct formation.Positive correlations were found between obesity and lipid peroxidation-related DNA adducts in patients with cancer.Thus,an increase in DNA damage to the pancreas caused by increased lipid peroxidation in individuals may be a mechanism for the association of PC with BMI.Recent research found that adipose cells around the pancreas can produce an obesity-associated inflammatory environment(69).Cancer-associated adipocytes as well as infiltrating inflammatory and immune cells in the peripancreatic adipose tissue microenvironment can secrete higher than normal levels of adipokines,proinflammatory cytokines,chemokines,and growth factors,which may accelerate PC progression.Leptin and adiponectin are important adipokines.In obesity,increasing leptin levels and decreasing adiponectin levels are associated with a more aggressive malignant phenotype.A case-control study found that high plasma levels of leptin are associated with an elevated risk of PC (70).The mechanism may involve the activity of the leptin-Notch axis,which promotes the invasiveness of PC cells.Moreover,increased lipid metabolism is an important sign of cancer invasiveness that increases the use of lipids in the hypoxic TME (71).In a hypoxic microenvironment,adipocytes undergo lipolysis to produce more fatty acids that provide energy for cancer cells (72).Hence,systemic circulation of adipokines and the adipocyte-mediated inflammatory and immunosuppressive microenvironment create fertile soil for the development and progression of PC.Above all,obesity underlies chronic systemic inflammation and metabolic syndrome,and epidemiological evidence confirms the positive association between the risk of PC and obesity (Figure 1).
Diet type
A study proved that nutritional factors,such as folate,fruit,red meat,cereals,and fat,can affect the risk of PC (73).A study analyzed data on PC morbidity in 1960-1989,which showed positive correlations between PC incidence rates and cholesterol (0.87 and 0.80),the consumption of animal fats (0.90 and 0.82),sugar (0.88 and 0.87),and alcohol (0.86 and 0.82) and a negative association between PC and folate(-0.45 and -0.49) intake,cereals (-0.93 and -0.91),and fiber (-0.84 and -0.89). From 1990 to 2008, the correlations between PC and diet were as follows:red meat(0.67 and 0.48),fruit (-0.62 and -0.50) and poultry (-0.88 and -0.57) (74).Therefore,we suggest lower red meat consumption and more fruit consumption to reduce the morbidity of PC.Mechanistically,red meat is a source of heme iron,and free iron can increase free radicals.Fruits can protect the pancreas against cancer because they contain vitamin C and other antioxidants and have the ability to trap free radicals and reactive oxygen molecules,protecting against oxidative damage (75). Moreover,flavonoids in fruit inhibit metabolic activation of carcinogens by cytochrome P450 enzymes or by detoxifying and cellular defensive enzymes.Some studies supported that whole-grain or high-fiber foods may reduce the risk of PC,which could be explained through the association with insulin resistance, triglycerides, and elevated lipoprotein levels (76).
Genetic mutations/familial syndromes
The genetic basis of the inherited susceptibility to PC remains unexplained,but family history is still important in risk stratification.Familial PC is defined as PC occurring in two or more first-degree relatives who do not meet the criteria for other hereditary cancer syndromes.PC risk is affected by the number of relatives.There is an 8%-12%lifetime risk with two first-degree relatives with PC and a 40% lifetime risk with three or more first-degree relatives(77).Family history of PC is also a vital risk factor because 5%-10% of patients with PC have a close relative with PC(78).Recognition of members of high-risk families is important for early detection of PC.
An estimated 5%-10% of PC occurs as part of a familial cancer syndrome associated with a known genetic mutation(79).Jennifer Permuth-Weyet al.conducted a systematic review of studies about familial risks of PC and found that individuals with a family history of PC have nearly a 2-fold increased risk of developing PC (RR,1.80;95% CI,1.48-2.12) (80).Wanget al.established a risk prediction model for PC and successfully validated that gene carrier status contributes to the development of PC and highlights that family history improves risk prediction (81).Another study collected over 200,000 person-years of follow-up from 8,564 first-degree relatives of probands and 1,007 spouse controls and found that individuals with a family history of PC are more likely to experience cancer-related mortality (82).Gene mutations may explain the increased risk of PC with a familial cancer history.In summary,our study suggested that patients who have a family history of PC have a higher risk of PC development,and more specific cancer screening methods should pay more attention to these individuals.
Mucinous pancreatic cysts
Mucinous lesions can undergo transformation from adenoma to carcinoma.Mucinous lesions have much more potential for malignant transformation than serous lesions.Up to 15% of PCs have been proven to arise from mucinous cysts such as mucinous cystic neoplasms (MCNs)and intraductal papillary mucinous neoplasms (IPMNs)(83).Studies have demonstrated a direct relationship between IPMN size and the risk of PC.Anandet al.performed a meta-analysis and found that risks of PC associated with individual cyst features were as follows:cyst size greater than 3 cm (OR,62.4;95% CI,30.8-126.3),a mural nodule (OR,9.3;95% CI,5.3-16.1) and dilatation of the main pancreatic duct (OR,7.27;95% CI,3.0-17.4)(84).In this meta-analysis,cyst size was associated most strongly with malignant IPMN,and cyst size greater than 3 cm might be considered a high-risk feature based on the significantly increased risk of PC.In addition,according to all guidelines,the presence of a mural nodule is another predictor of malignant disease.Mural nodes are present in 36%-70% of IPMN-associated cancers (85).Furthermore,a study proved that a thickened cyst wall can indicate 65%of cases with PC (86).Hence,we should pay more attention to individuals who have mucinous pancreatic cysts detected but with high-risk features.
Diabetes mellitus
Diabetes mellitus (DM) is one of the major public health challenges in the world.The prevalence of DM in PC ranges from 4% to 65% (87).Therefore,individuals with DM have a significantly increased risk of PC.To determine whether DM has associations with PC, R Huxley conducted a meta-analysis containing 9,220 patients with PC and found that the OR was 1.82 (95% CI,1.66-1.89).Moreover,they also demonstrated that individuals with DM for ≤4 years had a 50% greater risk of PC compared with individuals who had diabetes for >5 years (OR,2.1vs.1.5;P=0.005) (88).This indicated that diabetes may be an early manifestation of PC.Furthermore,a recent study collected 35 cohort studies in a meta-analysis and found that DM was associated with an increased risk of PC (RR,1.94;95% CI,1.66-2.27) (89).Above all,individuals with diabetes have a nearly 2.0-fold increased risk of PC compared with nondiabetic individuals.Several biological mechanisms have been indicated to explain the potential relationship between DM and PC.DM is associated with insulin resistance and upregulated levels of IGF-1.IGF-1 and IGF-1R are highly expressed in PC cell lines,which can lead to increased proliferation, invasion, and angiogenesis promotion (90).In summary,DM is both a possible risk factor and an early manifestation of PC.
Chronic pancreatitis (CP)
The association between CP and PC has been discussed over the years.Increasing evidence suggests that CP can be a significant risk factor for PC.Although there is a strong link between CP and PC,<5% of patients with CP develop PC,and it is a rare cause of PC (91).D Malkaet al.collected a prospective, single-center cohort of 373 consecutive patients with proven CP and found that four cases of PC (1.1% of patients) were observed (expected number of cases 0.15;SIR,26.7;95% CI,7.3-68.3;P=0.00002) (92).They proved that patients with CP have a markedly increased risk of PC compared with the general population.The mechanism underlying the risk of PC in patients with CP may be an oncogenetic multistep sequence,such as ductal epithelial hyperplasia,metaplasia and dysplasia,andK-rasgene mutations in patients with CP (93).Further exploration needs to be conducted to determine the detailed mechanisms of the association between CP and PC.
From epidemiology to practice:opportunities and challenges
The purpose of this article is to describe the indications for screening for PC in individuals with high risks.Epidemiologic findings should be applied to identify highrisk groups and provide guidance for cancer prevention and early detection.As we discussed above,tobacco smoking and red meat consumption should be kept low.Moreover,a systematic screening project should be applied to detect individuals with high-risk factors,such as family history,CP and DM.
Usually,PC is hard to detect at an early stage because of the lack of obvious symptoms but rapid transition into higher stages.A recent study demonstrated that more early-stage patients with PC have been found.These changes may be the result of several factors,including earlier diagnosis and detection (24).With surveillance programs for individuals with risk factors and progression in the detection and management of pancreatic lesions,we suspected better early detection to improve PC survival.
Therefore,discussing the potential diagnostic methods and screening strategies for PC detection is significant to put epidemiology,surveillance and risk stratification into practice.
Imaging techniques for PC detection
Over the past two decades,a number of studies have evaluated the accuracy of endoscopic ultrasound (EUS),computerized tomography (CT),and magnetic resonance imaging (MRI) in detecting PC.MRI and EUS are currently the preferred modalities for PC screening due to their high sensitivity for the detection of pancreatic lesions.EUS is regarded as the most sensitive weapon for detecting early pancreatic disorders as small as 2-3 mm.EUS had a sensitivity and specificity of 72% and 90% for T1-2 cancers,respectively,and sensitivity and specificity of 90%and 72% for T3-4 tumors (94).Comparatively,CT has a sensitivity of 76%-92% for diagnosing PC and a specificity of 67% (95).
There are increasingly more detection methods that are emerging gradually.For instance,multi-detector CT with contrast using thin axial sections with dual-phase pancreatic protocol acquisition represents a useful method to detect pancreatic details because of its good spatial and temporal resolution.Pancreas protocol MRI with contrast is another helpful imaging method.Its advantages are independent of ionizing radiation for image acquisition,and it has better soft-tissue resolution than CT.A recent study proved that MRI is better at detecting pancreatic lesions than CT (96).
EUS and MRI have good concordance for detection of pancreas lesions and have been found to be complementary because MRI is particularly sensitive for the detection of cystic lesions and EUS for solid lesions.Precise lesion sampling can be achieved with EUS-guided fine needle aspiration.
The role of positron emission tomography (PET)imaging plays no effective role in PC detection because of its high false positive and false negative rates.Regarding the high false positives,it is difficult with PET to distinguish benign inflammation,such as pancreatitis,with PC because both of them will be detected as positive lesions.Regarding the high false negatives,there are non-F18fluorodeoxyglucose-avid tumors that could not be found in PET imaging.
Above all,to improve PC diagnosis and treatment outcomes,multiple factors should be taken into consideration.Individuals who have obvious risk factors can then be placed in longitudinal surveillance programs to identify asymptomatic diseases.Regular diagnostic imaging can be regarded as the final step in this multistep surveillance paradigm.There are substantial challenges to be overcome,but undeniably,the suggestion has its own capacity to make PC early detection a reality.
High-risk group screening
PC is frequently metastatic within a short time after it is detectable by current testing modalities;therefore,identification of these lesions along with small,localized solid tumors is the goal of dedicated screening and surveillance in high-risk populations.Several efforts have been made to define categories of individuals at high risk of developing PC to enroll in proper screening programs.A recent analysis of 354 high-risk individuals suggested a survival benefit in those with PC that was detected during surveillance.Twenty individuals diagnosed with PC or high-grade precursor neoplasm during surveillance had a median survival time of 5.3 years (interquartile range 1.2-11.1 years)vs.1.4 years (interquartile range,0.4-3.5 years) in the four individuals who did not undergo recommended surveillance (97).
Screening high-risk groups is a useful method for early detection.Many pancreas screening studies in high-risk individuals have utilized surveillance intervals of 6-12 months,with follow-up of abnormal findings within 3-6 months.Nine of ten PCs detected during surveillance were resectable with an 85% 3-year survival,vs.1/4 PC presenting symptomatically,with a 25% 3-year survival(98).Screening programs for PC in familial high-risk individuals have been widely reported,but their merits remain unclear.Screening programs for PC in familial high-risk individuals allow the community to concentrate all diagnostic efforts on a category of otherwise asymptomatic and healthy subjects at high risk of PC due to family history.Paiellaet al.performed a meta-analysis of 16 studies reporting 1,551 PC cases in familial high-risk individuals,and 30 subjects (1.82%) received a diagnosis of pancreatic neoplasm (99).Moreover,they found that the pooled proportion of screening goal achievement was 1.4%(95% CI,0.8-2.0,P<0.001,I2=0%).Therefore,individuals with familial high-risk PC should be enrolled in surveillance programs for PC to improve the efficacy of surveillance programs.
The challenges are as follows:randomized,prospective studies of the impact of screening on survival are needed.Further data are required to define groups at the highest risk for the development of PC.There is a need to further refine screening tests to achieve high sensitivity and specificity and the ability to detect high-grade precursors,including non-imaging-based biomarkers.Emerging data suggest the potential for circulating tumor DNA and other markers to be highly specific and reasonably sensitive for the detection of PC,even in a general non-high-risk population.More data are required regarding the natural history of precursor lesions stratified across high-risk groups.
Conclusions
Here we present an up-to-date summary of global and national incidence data on PC,a detailed overview of incidence and survival trends over time,and a description of potential risk factors. PC remains a devastating malignancy with limited options for effective therapy.By increasing awareness of high-risk groups, screening recommendations,and risk factors for PC,clinicians can make more powerful decisions about treatment and prevention of PC.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No.81772639,No.81972258);Natural Science Foundation of Beijing (No.7192157);National Fundamental Research Program of China (No. 2018YFE0118600);Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No.2019XK320001).
Footnote
Conflict of interest:The authors have no conflict of interest to declare.
 Chinese Journal of Cancer Research2020年6期
Chinese Journal of Cancer Research2020年6期
- Chinese Journal of Cancer Research的其它文章
- Development and validation of prognostic nomogram based on log odds of positive lymph nodes for patients with gastric signet ring cell carcinoma
- Separate lateral parametrial lymph node dissection improves detection rate of parametrial lymph node metastasis in early-stage cervical cancer:10-year clinical evaluation in a single center in China
- Prognostic factors affecting long-term outcomes in patients with brain metastasis from esophageal carcinoma
- Precision screening for esophageal squamous cell carcinoma in China
- Trends and risk factors of lung cancer in China
- Oral microbiome and risk of malignant esophageal lesions in a high-risk area of China:A nested case-control study
