Selective logging enhances ecosystem multifunctionality via increase of functional diversity in a Pinus yunnanensis forest in Southwest China
Xiaobo Huang,Shuaifeng Li and Jianrong Su*
Abstract
Keywords: Biodiversity, Ecosystem multifunctionality, Functional traits, Pinus yunnanensis,Soil enzymatic activity,Structural equation modeling
Background
Global change, caused by climate change and other human activities, is threatening the health of forests(Gauthier et al.2015).Enhancing sustainable forest management has become a global trend (Siry et al. 2005).The primary criterion for assessing the success or failure of sustainable forest management is conservation of biodiversity (Lindenmayer et al. 2000; Siry et al. 2005). It has been reported that selective logging is an effective way to ensure sustainable management of forests(Montoro Girona et al. 2019). Selective logging, that is the practice of harvesting a relatively low number of commercially valuable trees while retaining mature seed trees (Ding et al. 2017), is a broadly used method in forest management that can significantly modify ecosystem functions (Smolander et al. 2015; Trentini et al. 2017).Compared to clear-cutting, selective logging is not only considered for timber production, but also for ecological restoration, reduction of fire risk and the vulnerability of seedling to pest, biodiversity conservation, and the creation and maintenance of wildlife habitat (Keyser and Zarnoch 2012; Ding et al. 2017; Cazzolla Gatti 2018;Lavoie et al. 2019). Due to the removal of some individuals, selective logging typically open small to mediumsized gaps to allow more sunlight and precipitation to enter the forest floor, which reduces competition and create a forest microclimate for species coexistence, tree regeneration and biodiversity conservation (Qi et al.2016; Montoro Girona et al. 2018; Oldén et al. 2019).Some studies indicate that selective logging could change the carbon production and allocation (Riutta et al. 2018), impact soil organic carbon and nutrient stocks (Lontsi et al. 2019), and influence decomposition(Ojanen et al. 2017;Wang et al. 2018).The simultaneous occurrence of these different ecosystem functions (EFs)is defined as ecosystem multifunctionality (‘EMF’)(Hector and Bagchi 2007). This concept was first proposed in the study of seagrass (Duffy et al. 2003) and has been widely used in grasslands (Soliveres et al. 2016;Meyer et al. 2018), aquatic (Lefcheck et al. 2015; Perkins et al. 2015) and forest ecosystems (van der Plas et al.2016; Huang et al. 2019; Luo et al. 2019).
We know little about the effect of selective logging on multiple EFs and EMF. Due to the functional uniqueness of the species, biodiversity was crucial for EMF. Studies on the relationship between biodiversity and EMF(‘BEMF’) have become a hotspot in ecology (Zavaleta et al. 2010; Gamfeldt and Roger 2017). Biodiversity consists of three components: taxonomic diversity (defined as species abundance and richness), functional diversity(defined as various resource utilization strategies and growth types), and phylogenetic diversity (defined as the presence of different evolutionary lineages) (Bagousse-Pinguet et al. 2019; Luo et al. 2019). Most studies have focused on taxonomic diversity, e.g. species richness as a metric of biodiversity, since it is a simple metric to study and could be extended to other diversity metrics (Isbell et al. 2011; Maestre et al. 2012; Gamfeldt and Roger 2017). Scientists pointed out that species richness was not a particularly good metric for biodiversity because species richness does not provide direct information about traits like functional or phylogenetic diversity does(Gamfeldt and Roger 2017; Zirbel et al. 2019). Plant functional traits are the characteristics of morphological,phenological and physiological, which could reflect how plants respond to environmental changes, and influence other trophic levels and different ecosystem processes(Pérez-Harguindeguy et al. 2013). Functional diversity is based on functional traits, which are closely related to resource utilization (Díaz and Cabido 2001). Therefore,functional diversity might be a better predictor of ecosystem function or multifunctionality than taxonomic diversity (Díaz and Cabido 2001; Gross et al. 2017).Phylogenetic diversity could capture information on unmeasured traits as they relate to ecosystem function, so it might be a key component to predict ecosystem function or multifunctionality, comparing to taxonomic or functional diversity (Flynn et al. 2011; Srivastava et al.2012;Bagousse-Pinguet et al. 2019).The effects of different biodiversity attributes on EMF might vary in magnitude (Bagousse-Pinguet et al. 2019). In addition to aboveground plant biodiversity, a growing body of studies suggests that soil microbial diversity also plays a critical role in maintaining EMF (Jing et al. 2015; Delgado-Baquerizo et al. 2016; Luo et al. 2018). Many studies have shown that the loss of soil microbial diversity, including bacterial and fungal diversity, would threaten EMF (Wagg et al. 2014; Jing et al. 2015; Delgado-Baquerizo et al. 2016, 2017). In fact, biodiversity is not the only aspect of biological systems that affects EMF,and the effects of various abiotic factors on EMF have also received much attention (Jing et al. 2015; Giling et al. 2019).
Pinus yunnanensis Franch. is the main pioneer species in forests of southwest China and is widely used in construction (Xu et al. 2016). The P. yunnanensis forests range 23°to 30°N and 96°to 108°E.They grow mainly in river valleys between 700 and 3000 m above sea level (Li et al.2020).The P.yunnanensis forests can provide a large amount of high-quality timber and industrial raw materials, which play an important role in maintaining species diversity, conserving water resources and retaining soil(Xu et al. 2016; Li et al. 2020). However, due to excessive human disturbance activities and the invasion of pests,diseases and alien species, the EFs of P. yunnanensis forests are not fully developed (Xu et al. 2016; Liu et al.2019).Therefore,how to improve the EFs of P.yunnanensis forests is an important task for forest management.
The effect of multiple plant diversity attributes, soil microbial diversity and abiotic factors on EMF under different selective logging intensities is unclear. To fill these knowledge gaps, we quantified four key ecosystem functions: nutrient cycling (including soil total nitrogen, soil hydrolysable nitrogen, soil total phosphorus and soil available phosphorus), soil carbon stocks (soil total carbon), decomposition (including βglucosidase, urease and phosphatase activity) and wood production (aboveground woody biomass). In this study we analyzed how aboveground plant biodiversity (taxonomic, functional and phylogenetic diversity), soil microbial biodiversity (bacterial and fungal diversity), and abiotic factors (soil moisture and pH) influenced EMF under five different logging intensities applied to a P. yunnanensis forest. We predicted that: (1) in the absence of management (i.e. no logging) overall lower values of diversity and EMF.Thus, selective logging could increase EMF both directly and indirectly via taxonomic, functional and/or phylogenetic diversity; (2) effects of functional and phylogenetic diversity would be greater; (3) soil microbial diversity could improve EMF. The results of this study could provide new insight for forest management and conservation.
Materials and methods
Study area
This study was conducted at the Yongren National Forest Farm (26°12′-26°20′ N; 101°27′-101°37′ E),located in the Jinsha River watershed of Yunnan Province, southwest China (Fig. 1). The site has a mean annual temperature of 17.8°C, and a mean annual precipitation of 840 mm, with an altitudinal range from 1800 to 2500 m.The soil type is classified as hilly red soil. The main forest type in this area is P. yunnanensis forest. P. yunnanensis forest is mainly divided into two layers, the upper layer is P. yunnanensis, which occupies the absolute dominant position, the main species of the lower layer is Rhododendron decorum Franch.,Lyonia ovalifolia (Wall.) Drude, Cyclobalanopsis glaucoides Schotky,Cyclobalanopsis delavayi(Franchet)Schottky,Castanopsis delavayi Franch., Quercus semicarpifolia Smith, Quercus franchetii Skan, Keteleeria evelyniana Mast., Alnus nepalensis D. Don, Coriaria nepalensis Wall.,Rhamnus davurica Pall.and Myrica rubra Siebold et Zuccarini.The main disturbance in the P. yunnanensis forests is the selective logging for valuable timber. The Yongren National Forest Farm has been producing timber since the 1950s (Li et al.2020).
Sampling and experimental design
We obtained field data from 52 plots in April 2018.These plots (20 m×30 m) were representative of the major vegetation types and management styles in the area.The plots were set as Li et al. (2020) described: selectively logged once (SL1, 10 plots, Fig. 2a), logged twice (SL2, 10 plots, Fig. 2b), logged three times (SL3, 10 plots, Fig. 2c),logged four times (SL4, 10 plots, Fig. 2d), logged five times(SL5, 9 plots, Fig. 2e), control group without selective logging (CG, 3 plots, Fig. 2f). The forest stand characteristics(stand density, basal area and height) of these treatments are presented in Additional file:Table S1.The selective logging began in the 1970s, the interval of selective logging is about 7-8 years. Selected species included P. yunnanensis and broadleaf species. On average, 10% of the number of individuals was selectively harvested at one time.
Follow the standard field protocol, within the plots,we identified species and used a diameter tape and tree altimeter to measure diameter at breast height(DBH, cm) and height (H, m) for all individuals ≥1 cm DBH (Condit 1998; Yuan et al. 2020). A total of 13 species were investigated, belonging to 7 families and 11 genera (Table S2).
To obtain plant functional traits to calculate functional diversity, following the standardized protocols of Cornelissen et al. (2003), we gathered 50 to 100 sun-exposed mature leaves from five to ten individuals of each species per plot. We labeled the leaves by species and put them into paper bags. For broadleaf species, we used a laser area meter (LI-COR 3100C Area Meter, LI-COR, USA). For conifer species, such as P. yunnanensis, we used a method described by Zhang et al. (2008) that saw the leaf of P.yunnanensis as a cylinder. The leaf area of P. yunnanensis was calculated by the equation shown below:
which LA is the leaf area of P. yunnanensis, d is the leaf diameter of P. yunnanensis and L is the leaf length of P.yunnanensis. The d and L were measured by a Vernier caliper (precision: 0.02 mm) and a ruler (precision: 1 mm),respectively.
The fresh weight of each leaf was measured immediately with an electronic balance (precision: 0.0001 g). We weighed them again after drying for 72 h at 60°C. Afterward, we used a ball mill (NM200, Retsch,Haan, Germany) to grind all leaf samples into a fine powder to prepare for the measurement of leaf N concentration and leaf P concentration.
In each plot, we collected the wooden core for woody plants (DBH>5 cm) by increment borer. Then,the volume was measured using the diameter and the length of the wooden core. For other woody plants(DBH ≤5 cm), we collected 5-10 branches and removed the bark, then measured volume using the water replacement method. The wooden cores and the branches were weighed using an electronic balance (precision: 0.0001 g) after drying for 72 h at 60°C.
To measure soil enzyme activity, belowground diversity and soil physiochemical properties, we also collected 5 soil samples (0-20 cm) from each plot and mixed them evenly. Those samples were divided into three sections and placed in plastic bags. The first portion was stored in a portable refrigerator at 4°C to maintain freshness and moistness for soil enzyme activity. The second portion was stored at −20°C to allow for DNA extractions to assess soil bacterial and fungal diversity. When we got back to the lab, the third portion was sieved by a 2-mm mesh and air-dried for 1 month for physiochemical analyses. All measurements of physiochemical data are presented in Supplementary Methods 1.
Assessing aboveground and belowground diversity
Species richness was calculated for taxonomic diversity.As a classical measure of functional diversity, Rao’s quadratic entropy (RaoQ) is a multivariate measure of functional divergence which could increase ecosystem function due to efficient use of resources (Mason et al.2005) and has been applied to the study of the relationship between biodiversity and ecosystem function(Botta-Dukát 2005; Wasof et al. 2018). RaoQ was calculated using specific leaf area (SLA=leaf area/dry weight,mm2·mg−1), leaf dry mass content (LDMC=leaf dry weight/leaf saturated fresh weight, %), wood density(WD=wood dry weight/wood volume, g·cm−3), leaf N concentration (LNC, mg·g−1) and leaf P concentration(LPC, mg·g−1). Faith’s phylogenetic diversity (PD) was calculated as a proxy of phylogenetic diversity (Faith 1992). The number of soil bacterial and fungal operational taxonomic units (OTUs) was used to assess belowground diversity. Details on the several molecular techniques employed for assessing belowground diversity are presented in Supplementary Methods 2.
Individual EFs
We used an approach described by Lucas-Borja and Delgado-Baquerizo (2019) in which EMF was assessed by nutrient cycling, soil carbon stocks, decomposition and wood production (Table 1). Soil total nitrogen, soil hydrolysable nitrogen, soil total phosphorus and soil available phosphorus were used as the proxy of nutrient cycling (Lucas-Borja and Delgado-Baquerizo 2019). The content of soil total carbon was used as the proxy of soil carbon stocks (Gamfeldt et al. 2013). The three enzyme activities (β-glucosidase, urease, phosphatase) were used as the proxy of decomposition (Lucas-Borja and Delgado-Baquerizo 2019). Finally, the aboveground woody plant biomass was used as the proxy of wood production (Mensah et al. 2020). We used the allometric equation from Li et al. (2020) to estimate the aboveground biomass of individual trees of P.yunnanensis forest. A list of the allometric regressions used in our study is included in Table S3. All variables were standardized using the Z-score transformation (Bagousse-Pinguet et al. 2019). Then, these four components of EMF were calculated as the average of the standardized values across the corresponding variables.
Assessing EMF
We used the averaging approach (Maestre et al. 2012) to obtain a multifunctionality index. Firstly, we calculated the standardized Z-score value for each of the nine variables per plot. Then the EMF index was obtained by averaging the Z-score value of each of the nine ecosystem variables across plots. We also used this approach to calculate the index of the sets of functions related tonutrient cycling, soil carbon stocks, decomposition and wood production. The averaging approach has been widely used in the study of BEMF (Valencia-Gómez et al. 2015; Berdugo et al. 2017).
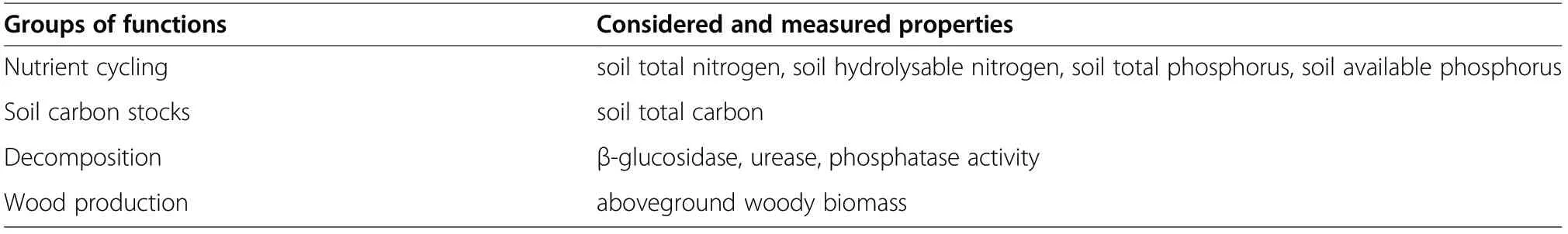
Table 1 Groups of functions along with properties considered and measured
Statistical analyses
Firstly,we used“LSD”test in one-way analysis of variance(ANOVA) to test the difference of multiple EFs and EMF among different selective logging intensities.Relationships between selective logging intensity,aboveground diversity,belowground diversity, abiotic factors and multiple EFs were assessed by calculating Kendall’tau-b correlation coefficients. Secondly, we used simple OLS regressions to evaluate the relationships between aboveground biodiversity (including taxonomic diversity, functional diversity and phylogenetic diversity), belowground biodiversity (including soil fungal OTUs and soil bacterial OTUs),abiotic factors (including soil moisture and soil pH) and multiple EFs, plus EMF. Then we identified the factors that have a significant impact on EMF. Based on our results, we conducted the structural equation model (SEM) to verify the direct and indirect effects of different selective logging intensities and biotic and abiotic factors on EMF.
All statistical analyses were conducted in R version 3.3.2. RaoQ was calculated in the “FD” package (Laliberté et al. 2014). For phylogenetic diversity, we first built the list of species found in our field survey, and then we used Phylomatic software (Webb et al. 2008) to create a phylogeny. The Phylomatic software used the Zanne et al. (2014) tree, including a dated molecular tree of >32,000 angiosperm species. Then we used the Phylocom software to calculate Faith’s PD (Webb et al.2008). The SEM was conducted in the lavaan package in R.
Results
Effect of selective logging intensity on EFs
Selective logging had different effects on different EFs(Table 2). Nutrient cycling and soil carbon stocks responded similarly to increasing selective logging intensity. It showed an increasing trend from SL1 to SL3, and a decreasing first and then increasing trend from SL3 to SL5. The values of the SL3 of nutrient cycling and soil carbon stocks were significantly greater than other selective logging intensities and CG (P<0.05), except the SL5. Decomposition responded directly to increasing selective logging intensity, with the values of the SL4 and SL5 being significantly greater than the CG (P<0.05), and the SL5 showing the highest value.For wood production,the value of the SL4 was significantly greater than other selective logging intensities and CG (P<0.05), except the SL2. The value of the SL1 was significantly lower than SL2,SL3 and SL4(P<0.05),except the CG and SL5.
We found a significant positive correlation between selective logging intensity and nutrient cycling (P<0.01), soil carbon stocks (P<0.01), decomposition(P<0.05) and wood production (P<0.01) (Table S4).With the increase of selective logging, nutrient cycling(P<0.01) (Fig. 3a), soil carbon stocks (P<0.01)(Fig. 3b), decomposition (P<0.001) (Fig. 3c) and wood production (P<0.05) (Fig. 3d) all showed an increasing trend. Selective logging intensity explained17.1% of variation of nutrient cycling, 14.7% of variation of soil carbon stocks, 33.2% of variation of decomposition and 11.4% of variation of wood production (Fig. 3).
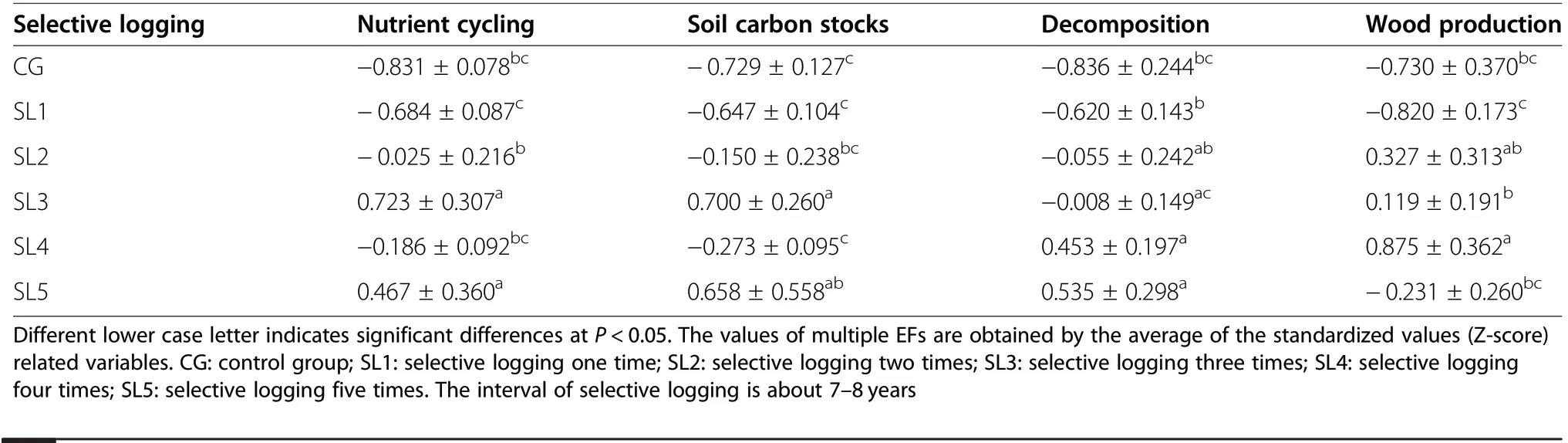
Table 2 Effect of selective logging intensity on multiple EFs (mean values ± standard error)

Table 3 Correlation coefficients between selective logging intensity and above-, belowground biodiversity and abiotic factors
EMF under different selective logging intensities
We found that except for SL1, the rest of treatments significantly improved EMF when compared to the control treatment (CG), showing a significant difference between SL1 and other logging intensities (P<0.05) (Fig. 4).Interestingly, no significant differences among SL2, SL3,SL4 and SL5 were found with regards to EMF (P>0.05)(Fig. 4).
Relationships between selective logging intensity and above-, belowground biodiversity and abiotic factors
There was a significant positive correlation between selective logging intensity and functional diversity (P<0.05,τ=0.217) (Table 3). However, there was a significant negative correlation between selective logging intensity and soil fungal OTUs (p<0.05, τ=−0.151) (Table 3). Selective logging intensity had a very significant positive correlation with soil moisture (p<0.01, τ=0.405)(Table 3).However, selective logging intensity had no significant correlation with taxonomic diversity,phylogenetic diversity, soil bacterial OTUs or soil pH (p>0.05)(Table 3).
Effect of above- and belowground biodiversity on EMF and multiple EFs
In aboveground biodiversity, functional diversity was significantly positively correlated with EMF (P<0.001)(Fig. 5b), as well as the nutrient cycling (P<0.01), soil carbon stocks (P<0.01) and decomposition (P<0.001)of multiple EFs (Figs. S1b, g, l). Neither taxonomic nor phylogenetic diversity displayed a significant linear correlation with EMF (P>0.05) (Fig. 5a, c), or with multiple EFs (P>0.05) (Figs. S1a, c, f, h, k, m, p, r). For belowground biodiversity, soil fungal OTUs were significantly negatively correlated with EMF(P<0.05)(Fig.5d),nutrient cycling (P<0.01) and carbon stocks (P<0.05) (Figs.S1d, i). There was no significant linear correlation between soil bacterial OTUs and EMF (P>0.05) (Fig. 5e)or multiple EFs(Figs.S1e,j,o,t). But functional diversity explained more of the variation in EMF (20.9%) than did soil fungal OTUs (9.4%) (Figs. 5b, d).
Effect of abiotic factors on EMF and multiple EFs
Soil moisture was significantly positively correlated with EMF (P<0.01), and explained 13.2% of the variation in EMF (Fig. 6a). There was no significant correlation between soil pH and EMF (P>0.05) (Fig. 6b). Soil moisture was significantly positively correlated with nutrient cycling(P<0.05), soil carbon stocks (P<0.05) and wood production (P<0.05) (Figs. S2a, c, g). Soil pH had no significant effect on multiple EFs(P>0.05)(Figs.S2b,d,f,h).
Effect of selective logging, biotic and abiotic factors as predictors of EMF
Selective logging might directly and indirectly through functional diversity impact EMF (P<0.05) (Fig. 7a). The variations of EMF, functional diversity, soil moisture and soil fungal OTUs explained by this model were 42.6%,13.7%, 27.5% and 5.7%, respectively (Fig. 7a). Soil fungal OTUs could also directly impact EMF (P<0.05), but there was no significant relationship between selective logging and soil fungal OTUs (P>0.05) (Fig. 7a). A strong relationship in the SEM analysis was found between logging and soil moisture, but we also found a weaker positive relationship between soil moisture and EMF (P>0.05) (Fig.7a). The standardized total effects of functional diversity, soil moisture, soil fungal OTUs and selective logging intensity derived from SEM were 0.292,0.364, −0.192 and 0.558, respectively (Fig. 7b).
Discussion
Our study explored the relationship between above- and belowground biodiversity and abiotic factors and EMF under different logging intensities. The results basically met our first prediction: selective logging could directly significantly improve EMF except logging one time also indirectly significantly improve EMF through functional diversity. Logging intensity influenced positively nutrient cycling, soil carbon stocks, decomposition and wood production. Different EFs showed different response patterns to logging intensity. Among biodiversity elements,logging intensity only influenced positively functional diversity. Similarly, logging intensity only influenced positively soil moisture between the two abiotic factors.Contrary to our second prediction, the effect of functional diversity, not phylogenetic diversity, on EMF was more significant compared to taxonomic diversity. Neither phylogenetic diversity nor taxonomic diversity had a significant effect on EMF. In addition, the relationship between belowground biodiversity and EMF was also contrary to our third prediction: only soil fungal OTUs,not soil bacterial OTUs negatively influenced EMF.
Selective logging is a widespread forest management technique that could alter forest microclimate. Studies show that air temperature,soil temperature,relative air humidity and soil moisture increased as canopy density and tree density decreased after selective logging (Masyagina et al. 2006; Oldén et al. 2019). Variations of temperature and moisture in forest microclimate directly affect some key processes in forest ecosystems (Vanwalleghem and Meentemeyer 2009). Selective logging could also alter soil conditions and nutrient contents (Smolander et al. 2013;Kim et al.2015).Soil microclimate,soil conditions and nutrient contents could affect the enzyme activities related to decomposition(Adamczyk et al.2015).In our study,an increase in logging intensity promoted multiple EFs.Consistent with previous studies(Hwang and Son 2006;Kim et al.2016), we observed that soil carbon stocks increased with logging intensity.Changes in soil carbon stock were caused by root mortality and the amount of dead woody vegetation entering the soil at different logging intensities (Kim et al.2016). After logging, changes in vegetation structure and species composition, together with the addition of necromass, increased the decomposition rate of organic carbon and thus increased the soil carbon stocks (Barros and Fearnside 2016). Wang et al. (2018) indicated that the amount of upper canopy removal would affect decomposition rates. Selective logging reduced canopy density and allowed more sunlight and precipitation to reach the ground(Simonin et al.2007).In addition,plant residues entering the soil could also increase decomposition(Adamczyk et al. 2015). The increase of soil moisture caused by logging is one of the main explanations for the increase in decomposition. In our study, logging intensity led to an increase in nutrient cycling, a relationship caused by the nutrient pool formed by logging residues entering the soil; the loss of individuals, reducing competition for nutrients; the increase of leaching loss (Carlyle 1995). Nutrient cycling implies a positive feedback between logging intensity and decomposition (Kim et al. 2016). In our study, wood production increased with logging intensities because logging promotes the radial growth of remaining trees due to the weakening of competition and the removal of water restriction (Sohn et al.2016; Cabon et al. 2018). The positive effect of logging on these four EFs leads to the direct positive effect of selective logging on EMF.
There was no significant correlation between logging intensity and taxonomic diversity. This result was consistent with the results of many previous studies (Ares et al. 2010; Nunes et al. 2014). Selective logging might change the structure of the community but not the number of species. In the field, we did not find any invasive species. Phylogenetic diversity, supposed to be the most expected predictor of EMF, was not significantly related to EMF, perhaps because the phylogenetic signal of functional traits related to each ecosystem function might not always be present (Davies et al. 2016).Zirbel et al. (2019) suggested that if the functional traits that determined an ecosystem function were not phylogenetically conserved,phylogenetic diversity might not be a good predictor of that ecosystem function. We concluded that some species, functional traits, and phylogenetic signals were lost during logging. The disappearance of a species means the loss of evolutionary history (Ribeiro et al.2016). In addition, phylogenetic diversity is a measure of biodiversity that combines species richness and evolutionary history(Cadotte 2013).The fact that taxonomic diversity was not related to EMF in our study might also lead to the failure of phylogenetic diversity prediction. Compared to taxonomic and phylogenetic diversity, functional diversity partly influenced EMF under selective logging intensity. Moreno-Gutiérrez et al. (2011) reported that the changes of soil conditions and light after logging intensity potentially influence plant functional traits.Our result that logging improves functional diversity aligns with previous studies (Ares et al. 2010; Nunes et al. 2014). It has been proven that functional diversity was the main driver of EMF in a P. yunnanensis natural secondary forest(Huang et al.2019).Increased functional diversity would aid forest ecosystems in responding to global climate change (Ares et al. 2010). In the forest ecosystem with high functional diversity,greater ecological differentiation enables coexisting species to survive through niche partitioning and complementarity of resource use among species,thus improve community resilience to maintain more EFs (Valencia et al.2015;Mensah et al.2020).Contrary to other findings(Wagg et al. 2014; Jing et al. 2015; Delgado-Baquerizo et al. 2016, 2017), this study found that soil biodiversity(mainly soil fungal OTUs) had a significant negative correlation with EMF and showed similar negative trends with multiple EFs. This finding was interesting, though the specific causality is unknown. One potential answer is that the average of multiple functions included in the EMF index might be a poor metric to accurately reflect the multiple functions performed by soil fungi and the complex interactions between soil and plant fungi (Jing et al.2015).
Our findings suggest that selectively logged twice or more is necessary if EMF is to be enhanced for forest managers. During selective logging, species with large differences in traits should be retained as much as possible. In the current study, although the expected results have been achieved, four EFs we used cannot represent all functions performed by ecosystems. The number of measured functions may influence the results of the study (Meyer et al. 2018). Gamfeldt and Roger (2017)pointed out that identifying mechanisms was the key to the success of BEMF field.Therefore,future studies need to identify underlying mechanisms behind the effects of biotic and abiotic factors on EMF.
Conclusions
Our results suggest that selective logging of P. yunnanensis forests was a significant driver of EMF when it is applied at least twice or more. However, this does not mean that more selective logging is better. For example,there was no significant difference among the EMF of selective logging two times, three times, four times and five times. Since different EFs responded to selective logging intensities differently, managers might wish to select a selective logging regime that meets their needs.For above- and belowground biodiversity, selective logging only changed the functional diversity and thus improved the EMF in the P. yunnanensis forests. Selective logging intensities did not improve the EMF through soil moisture even if the soil moisture was increased by selective logging intensities. Our study indicates that the EMF can be improved through increase of the functional diversity by selective logging. Future research should focus on the underlying mechanism explaining the relationship between soil biodiversity and multiple EFs and EMF.
Supplementary information
Supplementary informationaccompanies this paper at https://doi.org/10.1186/s40663-020-00267-8.
Additional file 1.
Abbreviations
EMF: Ecosystem multifunctionality; SEM: Structural equation model;
EFs: Ecosystem functions; SL1: Selective logging one time; SL2: Selective logging two times; SL3: Selective logging three times; SL4: Selective logging four times; SL5: Selective logging five times; CG: Control group;
OTUs: Operational taxonomic units; DBH: Diameter at breast height;
H: Height; RaoQ: Rao’s quadratic entropy; SLA: Specific leaf area; LDMC: Leaf dry mass content; WD: Wood density; LNC: Leaf N concentration; LPC: Leaf P concentration;AGB: Aboveground biomass
Acknowledgements
We would like to thank Courtney Buoncore at Princeton University for her assistance with English language and grammatical editing.
Authors’ contributions
Xiaobo Huang, Shuaifeng Li, conducted the field measurements. Jianrong Su conceived the study and revised the manuscript. Xiaobo Huang drafted the manuscript. The author(s)read and approved the final manuscript.
Funding
This manuscript was funded by the Fundamental Research Funds of CAF(CAFYBB2017ZX002) and Yunnan Basic Research Program (2019FB058).
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Received: 13 March 2020 Accepted: 6 September 2020
- Forest Ecosystems的其它文章
- Evaluating the impact of sampling schemes on leaf area index measurements from digital hemispherical photography in Larix principis-rupprechtii forest plots
- Discovering forest height changes based on spaceborne lidar data of ICESat-1 in 2005 and ICESat-2 in 2019:a case study in the Beijing-Tianjin-Hebei region of China
- Comparison of the local pivotal method and systematic sampling for national forest inventories
- Effects of firewood harvesting intensity on biodiversity and ecosystem services in shrublands of northern Patagonia
- The Siberian moth(Dendrolimus sibiricus),a pest risk assessment for Norway
- Hydrological functioning of forested catchments,Central Himalayan Region,India

