Immobilization and Properties of Polyphenol Oxidase
Zheng LI Pan WANG Yanxi SHEN Mingfei WEI Yimin HE Aoyu JI Jianfeng ZHAN








Abstract [Objectives] This study was conducted to investigate the effects of embedding conditions on activity and catalytic properties of immobilized polyphenol oxidase.
[Methods]Polyphenol oxidase was immobilized in a polymer material by the embedding method, and the optimal immobilization conditions were obtained by single factor tests: CaCl2 concentration 2.0%, sodium alginate concentration 2.0%, immobilization time 2 h and mass ratio of enzyme to carrier 10 mg/100 g, under which the immobilized enzyme activity was 93.33 U/g. Under the above conditions, the properties of polyphenol oxidase immobilized by sodium alginate (A-PPO) and free polyphenol oxidase were studied.
[Results] The thermostability of A-PPO was better than that of the free enzyme, but the pH stability of A-PPO was inhibited. The Michaelis constant Km values of free polyphenol oxidase and A-PPO were 0.37 and 0.48 mmol/L, respectively, and the maximum reaction rate Vmax values were 0.38 and 0.51 mmol/(L·g), respectively.
[Conclusions]This study provides a theoretical basis for the study of the properties of polyphenol oxidase.
Key words Polyphenol oxidase; Immobilized enzyme; Sodium alginate; Enzymatic properties; Kinetic parameter
Received: June 7, 2021 Accepted: August 8, 2021
Supported by Undergraduate Innovation and Enterpreneurship Training Program (202110514002); Huanggang Normal University High-level Cultivation Project (202108504).
Zheng LI (2000-), male, P. R. China, major: food science and engineering.
*Corresponding author. E-mail: zhanjianfeng2010@163.com.
Polyphenol oxidase (PPO, oxidoreductase; EC 1.10.3.1) is a chemically stable oxidoreductase that exists widely[1]. Free polyphenol oxidase has the disadvantages of poor stability and large loss in aqueous solution, which leads to high processing costs and limits its practical application[3]. Meanwhile, the statistical results of polyphenol oxidase produced by more than 100 species of fungi show that in most polyphenol oxidase, the optimum pH is between 3.0-5.0, while food processing generally presents high temperature, high pH, and high salt, which can inactivate and denature the active structure of polyphenol oxidase easily, and under which the enzyme not only cannot play a catalytic role, but also difficult to be separated, purified and reused[4].
An immobilized enzyme refers to an enzyme preparation in which an enzyme is immobilized or confined on a carrier by physical or chemical means within a certain range, while maintaining its catalytic activity and enabling its continuous reaction and repeated use[5]. Its main advantages are good thermal stability, and good stability and storage stability under alkaline conditions, and can be reused for multiple many times[6]. The immobilization effect of PPO on organic carriers is ideal[9], but under the conditions of severe reaction conditions or high ion concentration, the immobilized enzyme is unstable and easy to decompose. In this paper, sodium alginate was used as a carrier to study the effects of embedding conditions on enzyme activity and catalytic properties of the immobilized polyphenol oxidase prepared.
Materials and Methods
Experimental materials
Main reagents and agents
Polyphenol oxidase (PPO, derived from mushrooms, purity 25 U/UL, purchased from Shanghai Guchen Biotechnology); catechol (analytical purity, purity 99.0%, purchased from Sinopharm Chemical Reagent Co., Ltd.); sodium alginate (chemically pure, purchased from Sinopharm Chemical Reagent Co., Ltd.); glutaraldehyde 50% (analytically pure, purchased from Tianjin Damao Chemical Reagent Factory).
Main instruments
PHS-3C pH meter, Shanghai INESA Scientific Instrument Co., Ltd.; V-5600 visible spectrophotometer, Shanghai Metash Instrument Co., Ltd.; HWS-28 electric heating constant temperature water bath, Shanghai Yiheng Scientific Instrument Co., Ltd.; DF-101S collector type constant temperature heating magnetic stirrer, Gongyi Yuhua Instrument Co., Ltd.
Experimental methods
Determination method of free polyphenol oxidase activity
According to the method of Huang et al. [16] and Yuan et al. [17] for determining the activity of the free enzyme, a slight improvement was made to obtain the free polyphenol oxidase enzyme activity determination method. First, 1 ml of 0.1 mol/L catechol solution was added with 5 ml of phosphate buffer with a pH of 6.8, mixed in a constant temperature shaker at 30 ℃ for 10 min, and then immediately added with 1 ml of the enzyme solution, obtaining a system which was shaken quickly for reaction. A reaction system added with 1 ml of phosphate buffer solution instead of the enzyme solution was set as a blank control. The absorbance of the reaction systems were measured at a wavelength of 410 nm. The measurement was performed once every minute for a total of 3 min.
The activity unit of polyphenol oxidase was defined as: under the measurement conditions, each milliliter of sample caused a change in absorbance of 0.001 per minute, which was defined as 1 unit of enzyme activity [U/(g·min)][18]. For immobilized enzyme activity, the highest absorbance of each group was 100% enzyme activity, and the percentages of the remaining values to the highest value were the relative enzyme activity.
Immobilization method of polyphenol oxidase
According to the method of immobilized enzymes by Hang et al. [19] and Liu et al. [20] with some modifications, a certain amount of sodium alginate was added to an appropriate amount of phosphate buffer with a pH value of 6.8, and it was heated and melted in a 60 ℃ water bath, and then cooled. After cooling to room temperature, a 10 mg/250 ml polyphenol oxidase solution was mixed with sodium alginate in a volume ratio of 1∶2, obtaining a solution, which was added with a small amount of 4% glutaraldehyde, and shaken for 40 min at room temperature. The above mixture was dropped into a certain concentration of calcium chloride solution at a constant speed to form sodium alginate gel beads. The gel beads were added into a new calcium chloride solution and hardened for 2 h at 4 ℃, then washed with distilled water several times and cold-stored for later use.
Single factor optimization test
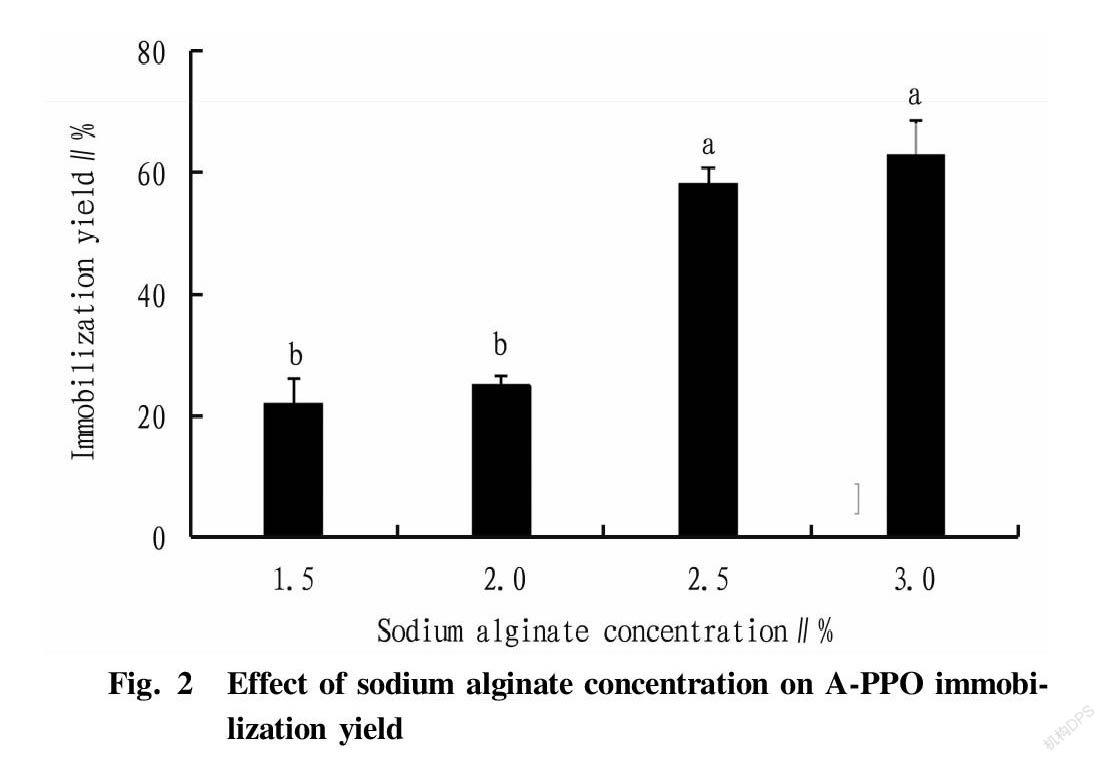
Effects of sodium alginate concentration on relative enzyme activity and immobilization yield
Sodium alginate solutions with concentrations of 1.5%, 2.0%, 2.5% and 3.0% were prepared, respectively. A-PPO was prepared using a CaCl2 solution with a concentration of 2% with an immobiliztion time of 2 h, and determined for enzyme activity. Relative enzyme activity and recovery were calculated. The calculation of the activity recovery referred to reference [21].
Activity recovery (%)=(Total activity of immobilized enzyme/Total activity of original enzyme solution)×100Total activity of immobilized enzyme (U/g)=Activity of immobilized enzyme/Mass of immobilized enzymeEffects of CaCl2 concentration on relative enzyme activity and immobilization yield of immobilized enzyme CaCl2 solutions with concentrations of 1.5%, 2.0%, 2.5% and 3.0% were prepared, respectively. A-PPO was prepared using a CaCl2 solution with a concentration of 2% with an immobiliztion time of 2 h, and determined for enzyme activity. Relative enzyme activity and recovery were then calculated.
Effects of immobiliztion time on relative enzyme activity and immobilization yield of immobilized enzyme The immobiliztion time was set to 1, 2, 3 and 4 h, respectively. A-PPO was prepared using a sodium alginate solution with a concentration of 2% and a CaCl2 solution with a concentration of 2%, and determined for enzyme activity. Relative enzyme activity and recovery were then calculated.
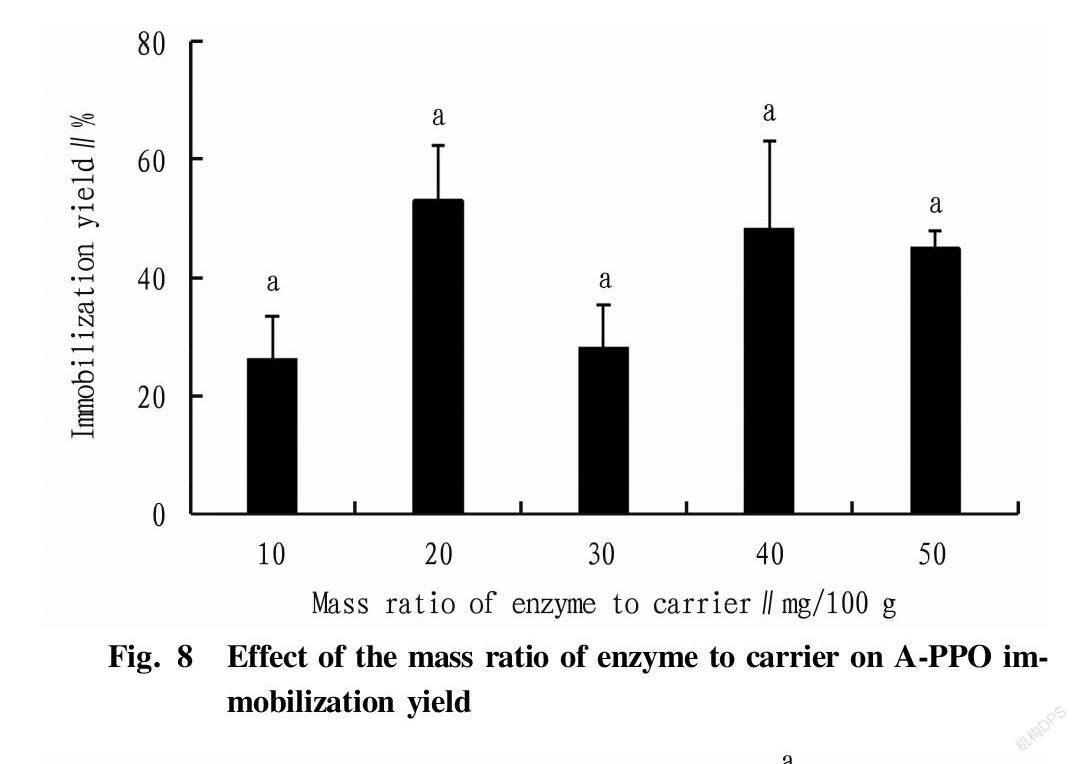
Effect of the mass ratio of enzyme to carrier on relative enzyme activity and immobilization yield The mass ratios of enzyme to carrier were set as 10, 20, 30, 40 and 50 mg/100 g, respectively. A-PPO was prepared using a CaCl2 solution with a concentration of 2% with an immobiliztion time of 2 h, and determined for enzyme activity. Relative enzyme activity and recovery were then calculated.
Research on the properties of immobilized enzyme
pH stability
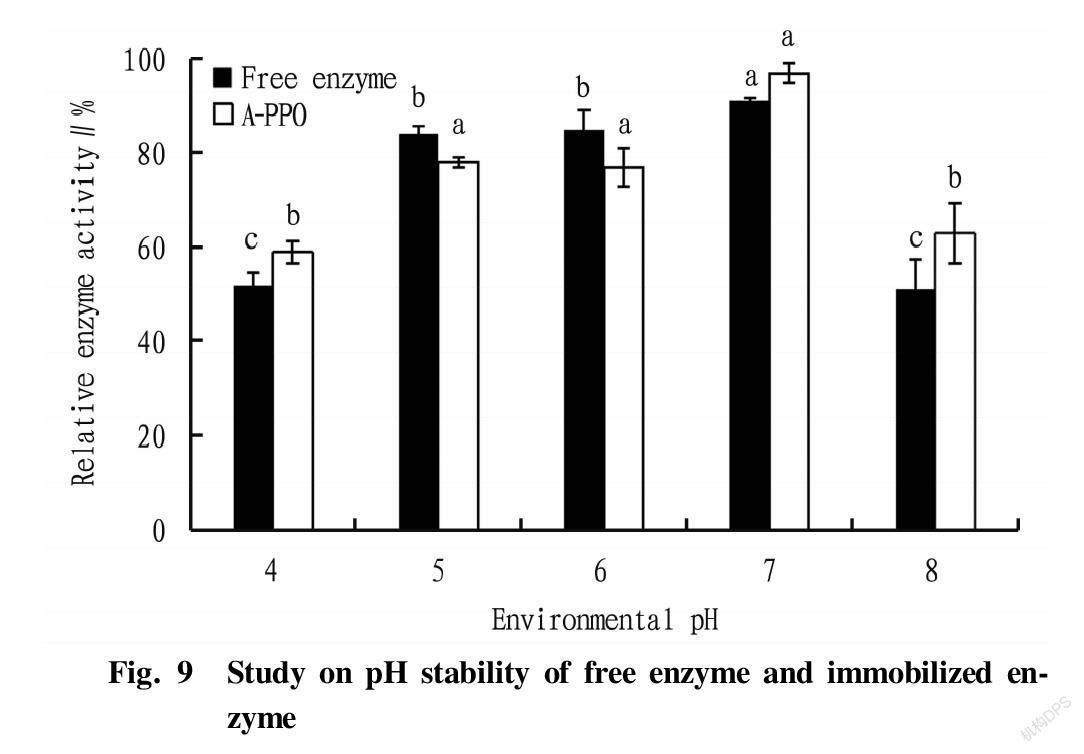
First, five parts of 0.5 g of A-PPO were added, respectively, into buffer solutions with pH of 4.0, 5.0, 6.0, 7.0 and 8.0 for 2 h, and five parts of 1 ml of free enzyme were treated the same as A-PPO. The obtained systems were then washed with phosphate buffer (pH 6.8) to neutral. A-PPO was dried at room temperature, and its enzyme activity was measured.
Thermostability
Five parts of 0.5 g of A-PPO and five parts of 1 ml of free enzyme were placed, respectively, at temperatures of 20, 30, 40 and 50 ℃ for 2 h. The enzyme activity was determined after cooling to room temperature.
Determination of Michaelis constant
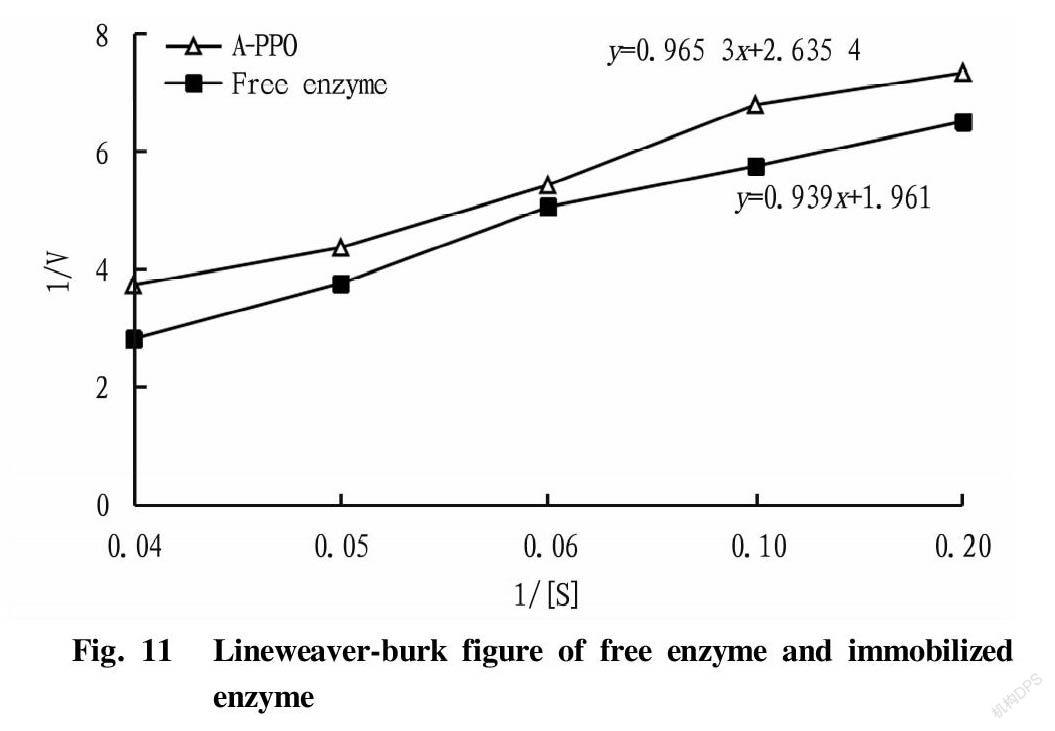
Catechol solutions with concentrations of 5, 10, 15, 20 and 25 mmol/L were prepared, respectively, and five parts of 1 g of A-PPO and five parts of 1 ml of free enzyme were taken for the determination of enzyme activity. The absorbance was measured once per minute for each group, and a total of 5 measurements were made. A scatter plot was made using the 5 measured values with [1/V] as the ordinate and [1/S] as the abscissa, and the slope of the trend line was the reaction speed. A Lineweaver-Burk equation was obtained, with a horizontal intercept of -1/Km, and a vertical intercept of 1/Vmax[22].
Results and Analysis
Analysis of single factor optimization test results
Effects of sodium alginate concentration on relative enzyme activity and immobilization yield
It can be seen from Fig. 1 and Fig. 2 that during the production process of immobilized enzyme, when the concentration of sodium alginate was 2.5%-3.0%, the viscosity of the prepared sodium alginate solution was too large, and when it was dropped into the CaCl2 solution, it was difficult for gel beads to shape, and they also stacked and agglomerated easily. When the concentration of sodium alginate was 1.5%, the viscosity of the solution was low. Although the gel beads were formed at a fast speed, the quality of the formed gel beads was unstable, the physical strength was small, and a stable structure could not be formed. When the concentration of sodium alginate was 2.0%, the speed of forming gel beads was moderate, the shape and size were uniform, and the structure was relatively stable. Moreover, the relative enzyme activity of A-PPO was relatively high. Therefore, the condition was effective for polyphenol oxidase embedding.
Effects of CaCl2 concentration on relative enzyme activity and immobilization yield of immobilized enzyme
It can be seen from Fig. 3 and Fig. 4 that although the concentration of CaCl2 had no significant effect on the relative activity of A-PPO, it was found during the production process of the immobilized enzyme that the effect of gel bead particles prepared when the concentration of CaCl2 was 2.0% was obviously better than 1.5%, 2.5% and 3.0%, and the shaping speeds were in order of 2.0%>2.5%>3.0%>1.5%. Therefore, the CaCl2 concentration of 2.0% was the best condition.
Zheng LI et al. Immobilization and Properties of Polyphenol Oxidase
Effects of immobiliztion time on relative enzyme activity and immobilization yield of immobilized enzyme
It can be seen from Fig. 5 and Fig. 6 that the relative enzyme activity of A-PPO immobilized for 2 h was higher than that of 3 and 4 h of immobilization. That is to say, the immobilization time was negatively related to the effect of gel bead shaping and hardening. A longer immobilization time might change the partial structure of the enzyme, thereby reducing the activity. Therefore, the immobilization time of 2 h was the best choice.
Effect of the mass ratio of enzyme to carrier on relative enzyme activity and immobilization yield
It can be seen from Fig. 7 and Fig. 8 that the relative enzyme activity of A-PPO was not greatly affected by the mass ratio of enzyme to carrier. The relative enzyme activity of A-PPO decreased slowly with the increase of the mass ratio of enzyme to carrier, and 10 mg/100 g was the best mass ratio.
Enzymatic properties analysis
pH stability
It can be seen from Fig. 9 that there was a difference in the pH stability between the free enzyme and the A-PPO enzyme. The free enzyme always maintained a relative enzyme activity more than 80% in the environmental pH range of 5.0-7.0, indicating that the structural properties of polyphenol oxidase itself were relatively stable, and the most suitable storage environment was neutral and weakly acidic environment. When the pH of the sodium alginate-immobilized enzyme was in the range of 5.0-7.0, the relative enzyme activity could only be maintained between 60% and 80%. It was found after experiments that A-PPO could maintain a relatively complete shape when placed in a pH 4.0-6.0 environment for 2 h, but at pH 7.0 and pH 8.0, there was an obvious dissolution phenomenon, indicating that the immobilized enzyme prepared by the sodium alginate embedding method had an inhibitory effect on the pH stability of the enzyme.
Thermostability
It can be seen from Fig. 10 that the free enzyme could maintain a relatively high relative enzyme activity when the temperature was around 30 ℃. When the temperature was lower than or higher than 30 ℃, the enzyme activity was greatly reduced. The A-PPO prepared by the sodium alginate embedding method maintained a relative enzyme activity more than 60% at 20-50 ℃. It could be seen that the immobilized enzyme could effectively improve its thermostability due to the binding between the enzyme and the carrier, which is beneficial for the enzyme to maintain its enzyme activity in a higher temperature environment.
Kinetic parameters
From Fig. 11, the intercept was calculated by the formula in the Lineweaver-burk diagram made by measuring the Michaelis constant of free and immobilized enzymes, and then the Vmax and Km values were calculated from the intercept, as shown in Table 1.
The Km value of free PPO was less than that of A-PPO, indicating that the affinity of the immobilized enzyme to the substrate is less than that of the free enzyme, which was because after the polyphenol oxidase was immobilized, the spatial structure of the enzyme molecules was restricted, and the active site could not completely contact the substrate. Meanwhile, the steric hindrance and substrate diffusion caused by the spatial structure of the carrier would also reduce the affinity between the enzyme and the substrate. The Vmax value of free PPO was lower than that of the immobilized enzyme, which was because that the untimely operation and the influence of the surrounding environment caused the measured value of the free enzyme to be too small when determining the value of the free enzyme, leading to the Vmax value of the free enzyme being lower than that of the immobilized enzyme.
Conclusions and Discussion
In the research process of polyphenol oxidase immobilization and its properties, the sodium alginate embedding method was used to immobilize polyphenol oxidase, and the optimal immobilization conditions were obtained through single factor optimization tests: sodium alginate concentration 2.0%, CaCl2 concentration 2.0%, immobilization time 2 h, mass ratio of enzyme to carrier 10 mg/100 g. Under these conditions, the enzymatic properties were determined, and the results showed that the A-PPO prepared by the sodium alginate embedding method had better thermostability than free polyphenol oxidase, but because sodium alginate selected as the immobilization carrier had weak environmental tolerance, the pH stability of the enzyme was inhibited.
References
[1] HU YY, LI LL, CHEN WX. Study on the characteristics of polyphenol oxidase and peroxidase from Capsicum chinense [J]. China Condiment, 2015, 40(7): 21-25. (in Chinese)
[2] CUI Y, LI G, MENG Y, et al. Biotransformation of catechol from phenol catalyzed by immobilized polyphenol oxidase[J]. Chemistry & Bioengineering, 2006(9): 24-26. (in Chinese)
[3] WANG Y, ZHANG D, RU J, et al. Enzymatic properties and catalytic capacity of immobilized laccase on modified chitosan for 2,4-Dichlorophenol degradation[J]. Environmental Chemistry, 2013, 32(10): 1901-1908. (in Chinese)
[4] WANG H, LI J, CHENG HL, et al. Immobilization of polyphenol oxidase and its enzymatic properties[J]. Periodical of Ocean University of China, 2015, 45(8): 90-96. (in Chinese)
[5] WU Y, MA HF, CAO YJ, et al. Advances on properties, production, purification and immobilization of fungal laccase[J]. Biotechnology Bulletin, 2019(9): 1-10. (in Chinese)
[6] LI LJ, MA GP, ZHAO LG. Research progress of immobilized enzyme carriers[J]. China Biotechnology, 2015, 35(11): 105-113. (in Chinese)
[7] TOSA T, MORI T, FUSE N, et al. Studies on continuous enzyme reactions. I. Screening of carriers for preparation of water-insoluble aminoacylasw[J]. Enzymologia, 1996, 31(4): 214-224.
[8] CHEN XC, ZHOU QZ, LIU FM, et al. Removal of nine pesticide residues from water and soil by biosorption coupled with degradation on biosorbent immobilized laccase[J]. Chemosphere, 2019(233): 49-56.
[9] YU XY, JIANG HY, ZHANG JY. A review on immobilization matrix of polyphenol oxidase[J]. Journal of Tea Science, 2009, 29(4): 319-324. (in Chinese)
[10] ZHOU XY. Enzymology principles and enzyme engineering[M]. Beijing: China Light Industry Press, 2005. (in Chinese)
[11] BICKERSTAFF JR G F. Immobilization of enzymes and cells[M]. Humana Press, 1997.
[12] CARUSO F, TRAU D, MΔHWALD H, et al. Enzyme enc apsulation in layer-by-layer engineered polymer multilayer capsules[J]. Langmuir, 2000, 16(4): 1485-1488.
[13] PAN XR, WANG Y, ZHOU PG. Comparative studies of chitosanase immobilization by cross-linking and entrapment methods[J]. Periodical of Ocean University of China, 2007, 37(3): 419-422. (in Chinese)
[14] SONG JB, REN XX. Studies on immobilization of penicillin acylase to chitosan as larrier[J]. Chemical Industry and Engineering Progress, 2004, 23(2): 181-184. (in Chinese)
[15] ZHU YH, WANG QB. Preparation of surface functional magnetic microspheres and their application in nucleic acid separation and enzyme immobilization[J]. Acta Academiae Medicinae Sinicae, 2002, 24(2): 118-123. (in Chinese)
[16] HUANG HY, HAN CR, LI Y, et al. Improvement of the determination of polyphenol oxidase activity in mushroom ( Lentinus edodes (Berk.) sing)[J]. Academic Periodical of Farm Products Processing, 2016(21): 32-37. (in Chinese)
[17] YUAN B, ZHOU SY, LIU CW, et al. Study on extraction process and activity determination of polyphenol oxidase[J]. Tea Communication, 2020(2): 291-296. (in Chinese)
[18] SHEN JY, HUANG JY, LI XL. Study on characteristics of polyphenol oxidase from aloe[J]. Food and Fermentation Industries, 2005(3): 25- 29.
[19] HANG JH, REN SY, ZHANG CG, et al. Optimization of immobilizing conditions for chitin deacetylase with sodium alginate by response surface methodology[J]. Food Research and Development, 2020, 41(20): 101-107. (in Chinese)
[20] LUI YH, ZHAO XX, SHU L, et al. Immobilization of polyphenol oxidase and its enzymatic properties[J]. China Condiment, 2020, 45(5): 33-41. (in Chinese)
[21] ZHAN JF, HU XM, WANG WX, et al. Immobilization of phospholipase A1 using polyvinyl alcohol-alginate matrix[J]. Food Science and Technology, 2016(7): 8-13. (in Chinese)
[22] FENG XF. Study on the enzymatic properties of polyphenol oxidase and peroxidase of Lonicera japonica [D]. Tai’an: Shandong Agricultural University, 2014. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Rice Blast Resistance-associated Genes Based on Different RNA-seq Resources
- Research Progresses on QTLs for Main Grain Shape Genes in Rice
- Effects of Raising Chickens Under Moringa oleifera
- Preliminary Research on Radiation Breeding of Pteroceltis tatarinowii Maxim
- Comparison of Spring Radish Varieties with Entire Leaves
- Occurrence and Chemical Control Techniques of Rice Black-streaked Dwarf Disease in Rongshui County

