Study on the Optimization of Extraction Technology of Rose laevigate Michx. Polysaccharides and Its in vitro Antioxidant Activity
Guochen HU Han LIU Qianqian TANG Li CHENG Chenzhong JIN Yunyun ZHOU Xuejiao ZHANG Guiying LIU
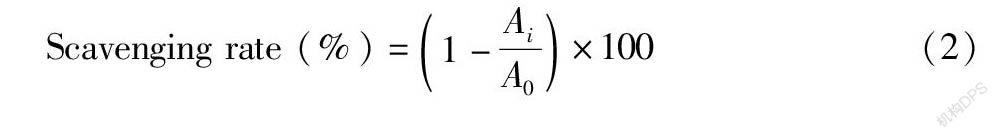
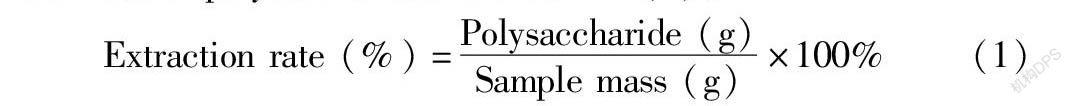


Abstract [Objectives] This study was conducted to optimize the optimal process conditions for extracting polysaccharides from Rose laevigate Michx. with hot water.
[Methods]The extraction rate of R. laevigate was used as an evaluation index, and extraction temperature, material-to-liquid ratio, extraction time and extraction times were selected as influencing factors. On the basis of single factor tests, L9 (34) orthogonal tests were carried out for optimization, and the content of polysaccharides was determined by the phenol-sulfuric acid method. The in vitro antioxidant activity of the extracted R. laevigate polysaccharides was studied.
[Results] The effects of various influencing factors on the extraction rate of R. laevigate polysaccharides ranked as material-liquid ratio>extraction temperature>extraction times>extraction time. The optimal extraction process was extraction temperature 80 ℃, extraction time 2 h, and material-to-liquid ratio 1∶25, extraction times 3 times, under which the extraction rate was (9.55±0.12) %. R. laevigate polysaccharides had certain scavenging capacities of DPPH free radicals and hydroxyl free radicals, and its scavenging capacity of DPPH free radicals was better than that of hydroxyl free radicals.
[Conclusions]This study provides a theoretical basis for the optimization of the extraction process of R. laevigate polysaccharides and the in-depth development and application of polysaccharides.
Key words Rose laevigate Michx.; Polysaccharides; Extraction; Antioxidation
Received: August 2, 2021 Accepted: October 9, 2021
Guochen HU (1994- ), male, P. R. China, assistant agronomist, devoted to research about food safety supervision.
*Corresponding author.
Rose laevigate Michx. is a perennial shrub of the Rosaceae family, and its fruit is widely used as a food and medicine in Asia. Its fruit is rich in vitamin C, polysaccharides, triterpene acids, steroids, polyphenols, saponins and flavonoids[1-2]. In China, they are used to brew fruit wine, to extract brown pigment, and to prepare vinegar beverages[3]. In recent years, it has been reported in the literature that R. laevigate has a variety of pharmacological functions, such as anti-oxidation[4], anti-apoptosis[5], reducing inflammation[2], inhibiting arteriosclerosis[1] and liver protection[6]. The polysaccharides, flavonoids and saponins in R. laevigate fruit have been confirmed as the main active ingredients by previous pharmacological studies[7-8]. Among them, polysaccharides are the main component, with a total content of about 260.5 mg/g, which is of great significance to human health[1]. The extraction methods of polysaccharides mainly include water extraction and alcohol precipitation, acid or lye extraction, and internal boiling extraction. The water extraction and alcohol precipitation method is by far the most widely used traditional method, and the hot water extraction and alcohol precipitation method is used more often than room temperature water extraction. The hydroxyl groups of polysaccharides can easily form hydrogen bonds with water, so that the polysaccharides are dissolved in water, while adding an appropriate amount of ethanol can precipitate the polysaccharides[9-10]. Although the hot water extraction method of polysaccharides is low in cost, simple in operation, environmentally friendly and non-toxic, it is also time-consuming and low in yield, so it is particularly important to optimize the extraction method. In recent years, there have been few literature reports on the extraction, properties and biological activity of polysaccharides from R. laevigate .
Therefore, orthogonal tests were carried out to optimize the process of R. laevigate polysaccharides, and the in vitro antioxidant activity of the extract was evaluated, so as to provide a reference for the development and utilization of R. laevigate polysaccharides, which is of great significance to the comprehensive utilization of R. laevigate resources.
Materials and Instruments
Experimental materials
Raw materials and reagents
R. laevigate used in the experiment was wild mature R. laevigate fruit grown in Wugang, Hunan, purchased at the local market in Wugang, Hunan. It was identified as R. laevigate fruit by Associate Professor Liu from Loudi Institute of Agricultural Sciences, Hunan Province. Anhydrous ethanol, chloroform, n-butanol, concentrated sulfuric acid, and phenol were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd., and glucose standard product and DPPH were purchased from Sigma Company in the United States.
Instruments and equipment
723 visible spectrophotometer, Shanghai Spectrum Instrument Co., Ltd.; FW100 high-speed universal pulverizer, Guangzhou Huruiming Instrument Co., Ltd.; CP214 electronic analytical balance, Shanghai Ohaus Instrument Co., Ltd.; KQ3200 ultrasonic cleaner, Kunshan Ultrasonic Instrument Co., Ltd.; DZF-6020 vacuum drying oven, Shanghai Yiheng Technology Co., Ltd; DK-8D three-hole electrothermal constant temperature water tank, Shanghai Yiheng Scientific Instrument Co., Ltd.; YRE2000B rotary evaporator, Gongyi Yuhua Instrument Co., Ltd.; 101 electric blast drying oven, Beijing Zhongxing Weiye Instrument Co., Ltd.; refrigerated centrifuge, Hunan Xiangyi Laboratory Instrument Development Co., Ltd.
Methods
Drawing of glucose standard curve
First, 10 mg of dry glucose with constant weight was weighed and diluted to a 100 ml volumetric flask to obtain a 100 μg/ml reference solution. Then, 0, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 ml of the reference solution were precisely pipetted into different test tubes, which was added with distilled water to 1.0 ml. Next, 1 ml of 5% phenol was added, and after shaking well, 5 ml of concentrated sulfuric acid was added, followed by shaking well. The mixtures were heated in boiling water for 15 min, and measured for the absorbance at 490 nm after they were completely cooled. A standard curve was drawn with the concentration of reference solution as the abscissa x and the measured absorbance as the ordinate y[11]. According to the phenol-sulfuric acid method, the polysaccharide content was determined, and the linear regression equation was obtained as Y=15.738x+0.000 1 (R2=0.999 2) .
Single factor tests for hot water extraction
After removing the seeds, the R. laevigate fruit was dried to constant weight at 60 ℃, crushed by a Chinese medicine pulverizer, sieved through a 60-mesh sieve, and stored in a drier at low temperature for later use. Then, 100.0 g of powder was weighed and soaked with 90% ethanol solution (material-to-liquid ratio 1∶20) with shaking at 60 ℃ for 5 h; and the ethanol solution was replaced with new 90% ethanol solution, and the above operation was repeated until the soaking solution was almost colorless. Finally, the pretreated sample was centrifuged in a centrifuge (4 000 × g, 20 min) to collect the precipitate, which was freeze-dried for later use. Next, 1 g of the pretreated R. laevigate powder was weighed, respectively, and the effects of extraction times (1, 2, 3, 4, 5, 6) and the extraction temperature (50, 60, 70, 80, 90, 100 ℃), extraction time (1, 2, 3, 4, 5, 6 h) and material-to-liquid ratio (1∶5, 1∶10, 1∶15, 1∶20, 1∶25, 1∶30, 1∶35 g/ml ) on the extraction rate of polysaccharides were investigated, respectively. An appropriate amount of deionized water was added to each sample, and the mixtures were stirred and extracted at a constant temperature at different temperatures and centrifuged (4 000 × g) for 20 min. The supernatants were merged and concentrated to 100-120 ml (60 ℃) by rotary evaporation. Sevag reagent was used to remove denatured protein, and the operation was repeated until there was no obvious protein denaturation layer. The upper aqueous solution was concentrated and dialyzed with a dialysis bag with a molecular weight cut-off of 3 500 Da for 48 h (to remove oligosaccharides). After the samples in the dialysis bags were combined, and absolute ethanol was added to adjust the final concentration of ethanol to 80%. After standing overnight, centrifugation was performed at 4 000 × g for 15 min to collect the precipitate, which was freeze-dried to obtain the crude polysaccharides of R. laevigate , which was determined with phonel-sulfate method to determine the content of polysaccharides[11]. The formula for calculating the extraction rate of polysaccharides is shown in (1):
Extraction rate (%)=Polysaccharide (g)Sample mass (g)×100%(1)
Orthogonal test
According to the single-factor test results, the hot water extraction process of R. laevigate polysaccharides adopted a four-factor three-level design, with extraction times, extraction temperature, extraction time and material-to-liquid ratio as reference factors. According to the L9 (34) orthogonal table (as shown in Table 1), the orthogonal tests of the hot water extraction process of R. laevigate polysaccharides were carried out.
In vitro antioxidant activity test
Hydroxyl radical scavenging rate: The determination of the scavenging rate of hydroxyl radicals by samples referred to Feng et al. [12], with some modifications. Various sample solutions (1.0 ml each) with concentrations of 0.2, 0.4, 0.6, 0.8, 1.0 and 0.2 mg/ml were mixed well with 1 ml of ferrous EDTA sulfate (1.5 mM), 1 ml of H2O2 (10 mM) and 1 ml salicylic acid (9 mM). Each mixture was incubated at 37 ℃ for 60 min. With ascorbic acid as a positive control, the absorbance was measured at 560 nm. The formula for calculating the scavenging rate of hydroxyl radicals is shown in (2):
Scavenging rate (%)=1- AiA0 ×100(2)
Wherein A 0 is the blank absorbance, and Ai is the absorbance of the sample or ascorbic acid.
DPPH free radical scavenging rate: The determination of the scavenging rate of DPPH radicals referred to Feng et al. [12], with some modifications. A certain amount of the polysaccharide sample was accurately weighed to prepare polysaccharide solutions of different concentrations (0.2, 0.4, 0.6, 0.8, 1.0, 1.2 mg/ml), respectively. Then, 1 ml of DPPH solution (0.1 mM, prepared with absolute ethanol) was mixed with 1 ml of one polysaccharide sample solution in a test tube. After shaking, each mixture was incubated for 30 min (25 ℃, in dark). An ultraviolet-visible spectrophotometer was used to measure the absorbance of the reaction solutions at 517 nm, with ascorbic acid as a positive control and 95% ethanol as a blank control. The calculation formula of DPPH radical scavenging rate adopted (2).
Data statistics and analysis
Microsoft Excel 2013 was used to calculate the experimental data, which were represented by the mean±standard deviation (Mean± SD ).
Results and Analysis
Single factor test results and analysis
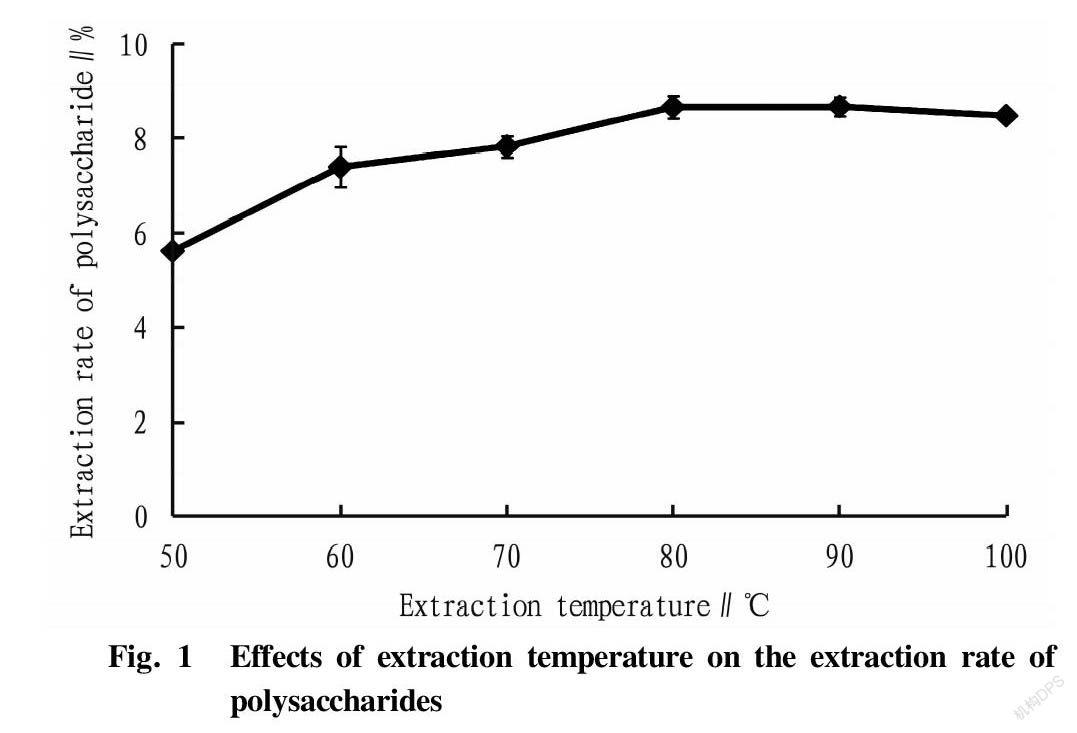
Effects of extraction temperature on the extraction rate of polysaccharides
A curve was drawn based on the extraction temperature and the extraction rate of polysaccharides (Fig. 1). As shown in Fig. 1, the extraction rate of polysaccharides from R. laevigate was the highest when the extraction temperature was 90 ℃, and then began to stabilize and showed a slight downward trend, indicating that a too-high extraction temperature might lead to the hydrolysis of polysaccharides[13-14], which led to the decrease of the extraction rate of R. laevigate polysaccharides. Therefore, 80, 90, and 100 ℃ were selected as the three levels of extraction temperature in the orthogonal tests.
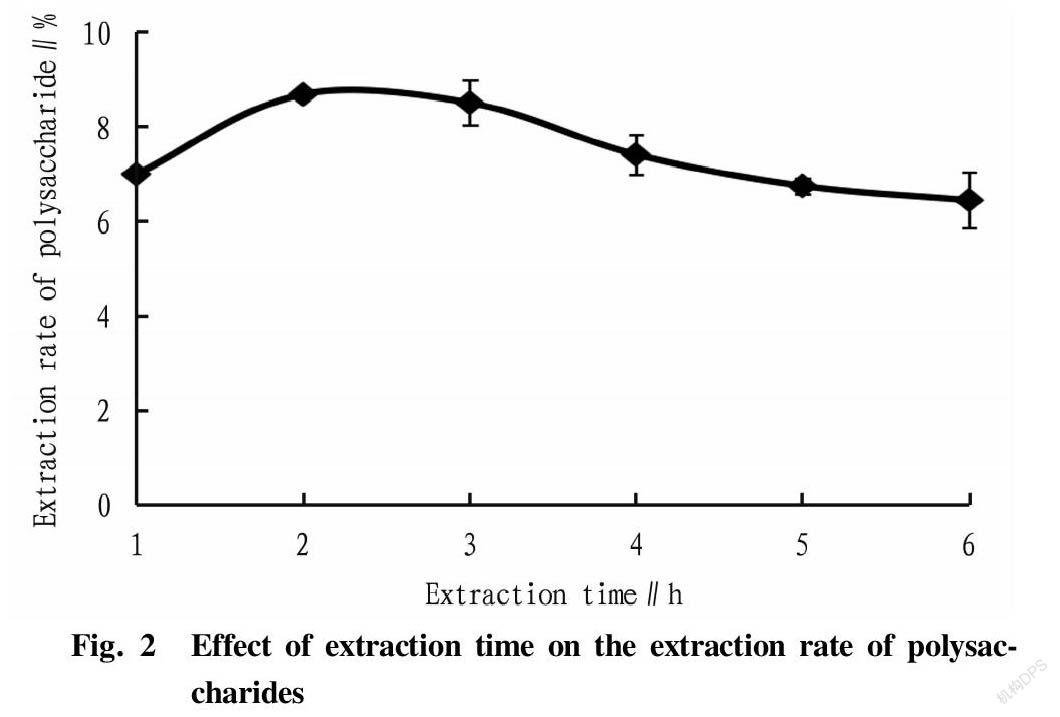
Effects of extraction time on the extraction rate of polysaccharides
A curve was drawn based on the extraction time and the extraction rate of polysaccharides (Fig. 2). As shown in Fig. 2, the extraction rate of polysaccharides from R. laevigate increased first and then decreased with the extension of the extraction time. The extraction rate was highest when the extraction time was 2 h, and then began to show a downward trend, indicating that continuous high-temperature extraction might lead to the gradual hydrolysis of polysaccharides. Therefore, 1, 2, and 3 h were selected as the three levels of extraction time in the orthogonal tests.
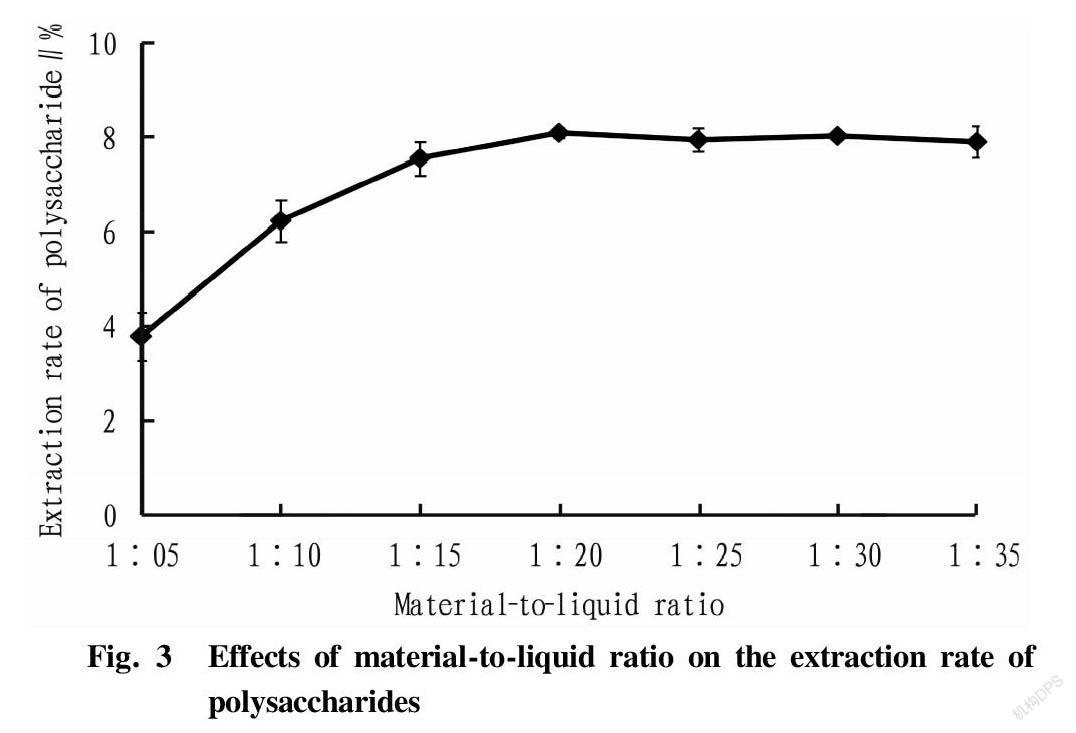
Effects of material-to-liquid ratio on the extraction rate of polysaccharides
A curve was drawn based on the ratio of material to liquid and the extraction rate of polysaccharides (Fig. 3). As shown in Fig. 3, the extraction rate of R. laevigate polysaccharides increased first and then stabilized at seven different material-liquid ratio levels. It reached the maximum when the material-liquid ratio was 1∶20, and with further increase of the material-to-liquid ratio, the extraction rate did not change significantly. Taking into account the saving of extraction solvent and subsequent concentration time, 1∶15, 1∶20, and 1∶25 were selected as the three levels of the material-to-liquid ratio in the orthogonal tests.
Agricultural Biotechnology2021
Effects of extraction times on the extraction rate of polysaccharides
A curve was drawn based on extraction times and the extraction rate of polysaccharides (Fig. 4). As shown in Fig. 4, the extraction rate of polysaccharides from R. laevigate was the largest when the extraction times were 3 times. Before reaching the extraction times of 3 times, the extraction amount of polysaccharides increased with the increase of the extraction times; and after the extraction times reached 3 times, the extraction rate of polysaccharides changed slightly. Taking into account the saving of extraction solvent and subsequent concentration time, 2, 3, and 4 times were selected as the three levels of extraction times in the orthogonal tests.
Orthogonal test design analysis
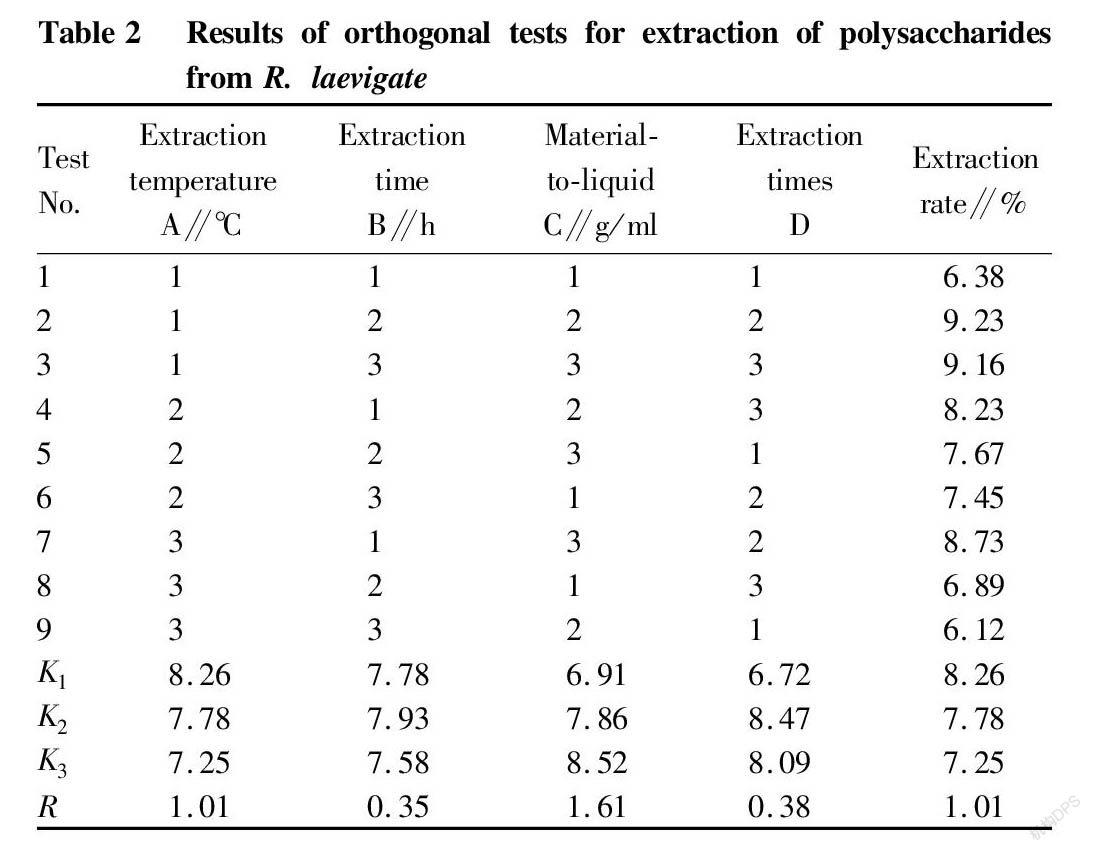
The R. laevigate polysaccharide extraction results of the orthogonal tests are shown in Table 2. It can be seen from the range analysis in Table 2 that the material-to-liquid ratio had the greatest effect on the extraction rate of R. laevigate polysaccharides, followed by the extraction temperature. The order of the effects of various influencing factors on the extraction rate of R. laevigate polysaccharides was the material-to-liquid ratio>extraction temperature>number of extractions>extraction time. From the analysis results in Table 2, it could be seen that the optimal combination was A1B2C3D2, that is, extraction temperature 80 ℃, extraction time 2 h, material-to-liquid ratio 1∶25, and extraction times 3 times, with which the extraction rate was the highest.
Repeatability verification test
According to the optimal extraction process conditions: extraction temperature 80 ℃, extraction time 2 h, material-to-liquid ratio 1∶25, and extraction times 3 times, 5 parallel tests were performed to investigate the process stability. The results showed that the extraction rate of polysaccharides in the R. laevigate fruit could be as high as 9.55±0.12% under the optimal technological conditions.
In vitro antioxidant activity
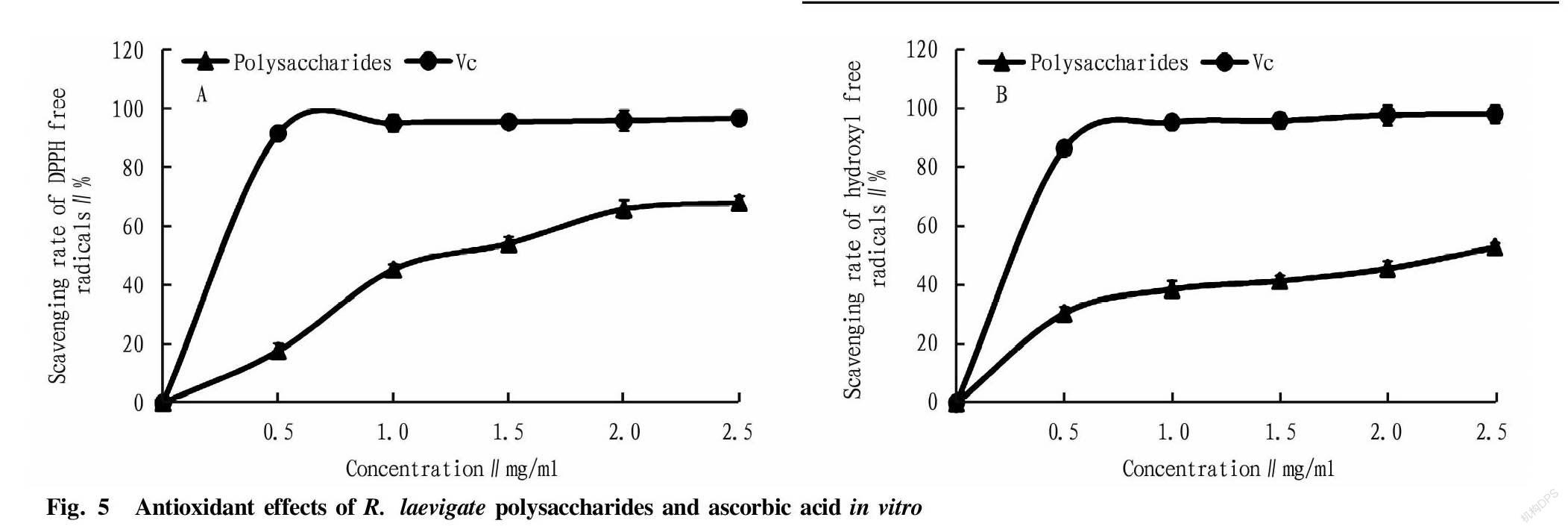
The antioxidant activity of the compounds attributed to various reactions and mechanisms, such as free radical scavenging and reducing capacity. In this study, we studied the scavenging capacities of R. laevigate polysaccharides on DPPH and hydroxyl free radicals. As shown in Fig. 5, the scavenging effect of R. laevigate polysaccharides on DPPH free radicals was concentration-dependent and was lower than the activity of ascorbic acid in the test concentration range. When the concentration was 2.5 mg/ml, the scavenging rate of R. laevigate polysaccharides was 65.7%, and the scavenging rate of ascorbic acid was 96.5%. The scavenging effect of R. laevigate polysaccharides on hydroxyl free radicals was also concentration-dependent. When the concentration was 2.5 mg/ml, the scavenging rate of R. laevigate polysaccharides was 52.8%, and the scavenging rate of ascorbic acid was 98%. Although R. laevigate polysaccharides had good DPPH and hydroxyl free radical scavenging capacities, they were significantly lower than the scavenging activity of ascorbic acid.
Conclusions
In this study, the hot water extraction process of R. laevigate fruit polysaccharides with R. laevigate as a raw material was optimized, and the optimal extraction process conditions were 80 ℃, extraction time 2 h, material-to-liquid ratio 1∶25, and extraction times 3 times, under which the extraction rate reached (9.55±0.12)%. The extraction process is simple and easy to operate, high yield in crude polysaccharides and good in stability, which provides a research basis for the further development and utilization of R. laevigate polysaccharides. In this study, the in vitro antioxidant activity test of the extracted R. laevigate polysaccharides found that although R. laevigate polysaccharides had lower scavenging capacities on DPPH and hydroxyl free radicals than ascorbic acid, it also had a better scavenging effect, which provides basic research data support for R. laevigate polysaccharides as a raw material for the development of functional foods.
References
[1] YU C, DAI X, CHEN Q, et al. Hypolipidemic and antioxidant activities of polysaccharides from Rosa laevigatae Fructus in rats[J]. Carbohydrate Polymers, 2013, 94(1): 56-62.
[2] ZHAN Q, WANG Q, LIN R, et al. Structural characterization and immunomodulatory activity of a novel acid polysaccharide isolated from the pulp of Rosa laevigata Michx fruit[J]. International Journal of Biological Macromolecules, 2020(145): 1080-1090.
[3] HARTEMINK R, ROMBOUTS FM. Comparison of media for the detection of bifidobacteria, lactobacilli and total anaerobes from faecal samples[J]. Journal of microbiological methods, 1999, 36(3): 181-192.
[4] LI X, CAO W, SHEN Y, et al. Antioxidant compounds from Rosa laevigata fruits[J]. Food Chemistry, 2012, 130(3): 575-580.
[5] ZHANG S, QI Y, XU Y, et al. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation[J]. Neurochemistry International, 2013, 63(5): 522-532.
[6] CHIRUMBOLO S. Hormetic effect of Rosa laevigata Michx in CCl4-induced hepatotoxicity and the presumptive role of PPARs[J]. Food and Chemical Toxicology, 2013(57): 387-388.
[7] ZHANG S, ZHENG L, DONG D, et al. Effects of flavonoids from Rosa laevigata Michx fruit against high-fat diet-induced non-alcoholic fatty liver disease in rats[J]. Food Chemistry, 2013, 141(3): 2108-2116.
[8] DONG D, ZHANG S, YIN L, et al. Protective effects of the total saponins from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice[J]. Food and Chemical Toxicology, 2013(62): 120-130.
[9] NIU YC, LIU JC. Polysaccharide pharmacology[M]. Beijing: People’s Medical Publishing House, 2008.
[10] LI ZJ, CAI MS. Sugar chemistry: Basics, synthesis, isolation and structure[M]. Beijing: Chemical Industry Press, 2007.
[11] HOU XJ, CHEN W. Optimization of extraction process of crude polysaccharides from wild edible BaChu mushroom by response surface methodology[J]. Carbohydrate Polymers, 2008, 72(1): 67-74.
[12] FENG K, CHEN W, SUN LW, et al. Optimization extraction, preliminary characterization and antioxidant activity in vitro of polysaccharides from Stachys sieboldii Miq. tubers[J]. Carbohydrate Polymers. 2015(125): 45-52.
[13] SHU RG, JIANG YP, CAI YH. Discussion on extraction and separation methods of plant polysaccharides[J]. China Pharmacy, 2011, 22(11): 1052-1055.
[14] LI J, LIU Y, LIU DJ, et al. Optimization of extraction process of water chestnut polysaccharides by orthogonal test[J]. Journal of Agriculture, 2021,11(4): 51-55.
- 农业生物技术(英文版)的其它文章
- Rice Blast Resistance-associated Genes Based on Different RNA-seq Resources
- Research Progresses on QTLs for Main Grain Shape Genes in Rice
- Effects of Raising Chickens Under Moringa oleifera
- Preliminary Research on Radiation Breeding of Pteroceltis tatarinowii Maxim
- Comparison of Spring Radish Varieties with Entire Leaves
- Occurrence and Chemical Control Techniques of Rice Black-streaked Dwarf Disease in Rongshui County

