Determination of Residual Amount of 15 Plant Growth Regulators in Bean Sprouts by HPLC-MS/MS
Lei WANG Yi ZHANG Baiqin ZHENG Shuai WANG Kai GE Sining TANG Shuhong ZHANG Liwu HAO

Abstract [Objectives] This study was conducted to effectively monitor PGR residues in bean sprouts to provide guarantee for the food safety of agricultural products.
[Methods]A high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) method for the determination of residues of 15 plant growth regulators (PGRs) in bean sprouts was established using bean sprouts as an experimental material. Samples were extracted with a solution containing 5% acetic acid-acetonitrile (1∶99, V/V ), purified with anhydrous magnesium sulfate, and diluted with methanol solvent to constant volume. The solutions were filtered through 0.22 μm filtering membrane and the target analytes were separated on a Phenomenex H18 column. The identification of each compound was established by retention time matching along with the accurate mass measurement of the precursor ions and their main fragment ions. The quantification was carried out using matrix-matched external standard method.
[Results] The retention time of the 15 PGRs were found in the range from 5.8-11.7 min under the optimized conditions. The linear relation was good in the concentration range of 0.005-0.050 μg/ml, and the correlation coefficients of the 15 PGRs were ≥0.999 0. The limits of detection were in the range of 0.03-0.92 g/kg, and the limits of quantification were in the range of 0.50-2.10 μg/kg. The average recovery in the recovery test at 3 concentration levels was 80%-110%, and the relative standard deviations were in the range of 2.8%-7.5%.
[Conclusions]This method is simple and accurate, and can quickly qualitatively and quantitatively analyze the residues of 15 PGRs in bean sprouts. The proposed procedure was simple, quick and accurate for the simultaneous determination of the 15 PGRs in bean sprout.
Key words High performance liquid chromatography-tandem mass spectrometry; Bean sprout; Plant growth regulator
Received: August 1, 2021 Accepted: October 8, 2021
Supported by Research Projects Funded by Talent Project Training Funds in Hebei Province (A201901128); Key R&D Project of Tangshan City (20150210C).
Lei WANG (1982-), male, P. R. China, associate researcher, devoted to research about utilization of agricultural by-products.
Yi ZHANG (1980-), male, P. R. China, veterinarian, devoted to research about agricultural product inspection and function analysis.
#These authors contributed equally to this work.
*Corresponding author.
Plant growth regulators (PGRs) are a class of substances that have similar physiological and biological effects to plant hormones and are also called plant natural hormones or plant endogenous hormones. They can affect and effectively regulate the growth and development of plants, including the whole process of plant life from cell growth and division, to rooting, germination, flowering, fruiting, maturation and falling. In recent years, abuse and improper use of PGRs have occurred from time to time, which has affected the food safety of agricultural products and threatened people’s health[1-5].
China’s basic research on multi-residue detection technology and related limit standards for plant growth regulators is still in its infancy, and the national standard for simultaneous multi-residue detection has not yet been established. At present, the detection methods for plant growth regulator residues mainly include enzyme-linked immunoassay (ELISA)[6], gas chromatography (GC)[7], gas chromatography-tandem mass spectrometry (GC-MS/MS)[8], high performance liquid chromatography (HPLC)[9], liquid chromatography-mass spectrometry (LC-MS) and high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS)[10]. In this study, QuEChERS method combined with HPLC-MS/MS were used to determine the residues of 15 PGRs in bean sprouts, aiming to provide a reference for the revision of standards and the effective monitoring of PGRs residues in bean sprouts.
Materials and Methods
Instrument and equipment
Agilent 6460 HPLC-MS/MS, Agilent Technologies (China) Co., Ltd.; 099ADPM12 vortex mixer, Shanghai Equl Corp.; 3-15 high-speed centrifuge, Hettich, Germany; EVA50A nitrogen blowing instrument, American Organization Associates; T-200 electronic balance, Changshu Shuangjie Instrument Manufacturing Factory; SZ13022-NY 0.22 μm organic filter membrane, Tianjin Linghang Experimental Equipment Co., Ltd.
15 standard PGRs: forchlorfenuron, gibberellic acid, cyclanilide, abscisic acid, indolepropionic acid, indoleacetic acid, p-chlorophenoxyacetic acid, thidiazuron, 6-benzylaminopurine, 4-iodine phenoxyacetic acid, triapenthenol, 2,4-D, naphthylacetic acid, uniconazole and diamine (purity ≥99.0%, national standard material center); methanol, formic acid, acetonitrile and NH4AC (chromatographically pure, American ASC Company); anhydrous magnesium sulfate, sodium chloride, acetic acid (analytical pure, Shanghai Sinopharm Chemical Reagent Co., Ltd.).
Experimental methods
Chromatographic conditions
Column: Phenomenex H18 (50 mm×3.00 mm, 2.60 μm); injection volume: 5 μl; column temperature: 35 ℃; mobile phase: A 5 mmol/L ammonium acetate solution containing 0.1% formic acid, and B methanol; gradient elution: 0.5-5.0 min: 10% B, 5.0-8.0 min: 95% B, 8.0-12 min: 10% B; and flow rate: 0.25 ml/min.
Mass spectrometry conditions
Electrospray ion source (ESI), positive (ESI+), negative (ESI-) ion scanning mode; atomizing gas flow: 3.00 L/min; drying gas flow: 15.00 L/min; DL tube temperature: 250 ℃; electrospray voltage (IS): 2 500 V; scanning time: 0.1 s; multiple reaction monitoring mode (MRM).
Standard solution preparation
From each of 1 000 μg/ml single PGR standard substances, 100 μl was drawn accurately, and the obtained mixture was diluted to 10 ml with methanol, giving a mixed standard stock solution with a concentration of 10 μg/ml, which was stored in a refrigerator at 4 ℃. Matrix-containing mixed standard working solutions with concentrations of 0.005, 0.01, 0.02, 0.04 and 0.05 μg/ml were prepared with the sample blank solution. The matrix-containing mixed standard working solutions should be used freshly.
Sample pretreatment
A 5.0 g of bean sprout sample was weighed into the outer tube of a centrifuge tube and added with the mixed standard solution (external standard method). After standing for 30 min, 10 ml of 5% acetic acid-acetonitrile (1∶99, V/V ) was added, followed by the addition of an extraction salt packet (containing 5.5 g of anhydrous magnesium sulfate, 1.5 g of sodium chloride, 0.5 g of disodium hydrogen citrate, 1.0 g of sodium citrate) and 10 zirconium beads. Then, centrifugation was performed by alternating shaking after tightening the inner tube (containing 5 zirconia beads with a size of 4 mm, 500 mg of anhydrous magnesium sulfate) for 25 min. Next, 1.0 ml of the supernatant was accurately drawn and blow-dried with 10% methanol solution. The residue was diluted with 10% methanol solution and filtered through a 0.22 μm organic microporous membrane. The filtrate was used for HPLC-MS/MS determination.
Results and Analysis
Optimization of mass spectrometry conditions
The standard solutions of the 15 PGRs of 1 000 μg/L were injected into the mass spectrometer system through a needle pump for parameter optimization. Full scanning was performed in positive and negative ion mode, within a scan range of m/z 50-500, obtaining first-order mass spectra of the 15 kinds of PGRs. Due to the different properties of the 15 PGRs, their ionization methods were also different, so the appropriate ionization methods were determined to achieve the highest ionization effect. The ion with the highest response was selected as the quasi-molecular ion, and the declustering voltage (DP) was optimized to obtain the strongest precursor ion signal. And the two ions with stronger response were selected as the quantitative ion and the qualitative ions, and the collision energy (CE) was optimized in the multiple reaction monitoring (MRM) mode. The MS parameters are shown in Table 1.
Optimization of chromatographic conditions
The separation effects of Phenomenex H18 column (50 mm×3.00 mm, 2.60 μm), Zorbax SB-C18 column (100 mm×2.1 m, 3.5 μm) and Waters Acquity BEH C18 (50 mm×2.1 mm, 7.0 μm) on the analytes were compared. The 15 kinds of PGRs had the best separation effect and peak shape on Phenomenex H18 chromatographic column. Therefore, in this study, the Phenomenex H18 column was selected to analyze the analytes.
The separation effects of acetonitrile-water and methanol-water as mobile phases were compared, and their elution effects on the 15 PGRs were investigated. The results showed that when methanol-water was used as the mobile phase, the 15 analytes showed the shortest retention time on the chromatographic column, and the separation effects and peak shapes were better, because when methanol was used as the organic phase, the analytes had higher response values and sensitivity. The results showed that adding formic acid to the mobile phase would ionize the analytes, and ammonium acetate could promote the ionization effects of the targets in the negative ion mode. Following concentrations of the mobile phase were prepared: ① 0.1% formic acid-1 mmol/L ammonium acetate-methanol, ② 0.1% formic acid-5 mmol/L ammonium acetate-methanol, ③ 0.1% formic acid-10 mmol/L ammonium acetate-methanol, and ④ 0.3% formic acid-5 mmol/L ammonium acetate-methanol. The results showed that when 0.1% formic acid-5 mmol/L ammonium acetate-methanol was used as the mobile phase, the response and peak shape of each target were the best. Under the optimal elution conditions, the retention time of the 15 PGEs was between 5.8-11.7 min.
Methodology validation
Linearity range, detection limit and quantification limit
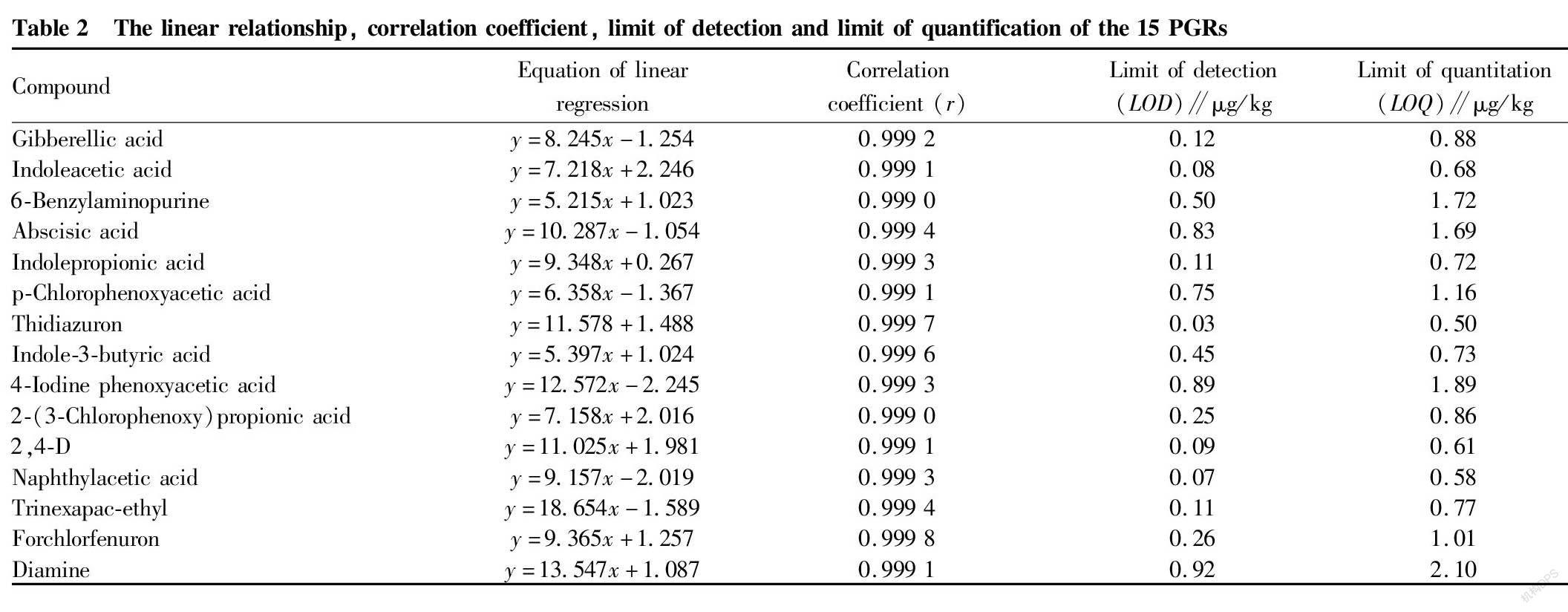
Matrix effect is a common interference factor, which means that the matrix of sample co-extraction will affect the ionization of the target, and the resulting co-extract has a matrix enhancement or inhibition effect, which can be reduced or eliminated by appropriate methods to improve the accuracy and precision of the determination. In this study, the bean sprout samples without the target substance were used as the blank matrix, and the blank matrix solution was used to prepare the standard solution to eliminate the influence of the matrix effect. The mass concentrations of the series matrix standard solutions were 0.005, 0.010, 0.020, 0.040 and 0.050 μg/ml, respectively. A standard curve was drawn with mass concentration as the abscissa and peak area as the ordinate. The linear regression equation and correlation coefficient are shown in Table 2. The results showed that the 15 PGRs had a good linear relationship in the concentration range of 0.005-0.050 μg/ml, and the correlation coefficients ( r ) were all≥0.999 0. The limit of detection ( LOD ) was calculated by 3 times the signal-to-noise ratio ( S/N =3), and the limit of quantification ( LOQ ) was calculated by 10 times the signal-to-noise ratio ( S/N =10). The results showed the LOD of the 15 PGRs was 0.3-1.2 μg/kg, and the LOQ was 1.0-5.0 μg/kg (Table 2).
Method recovery and precision
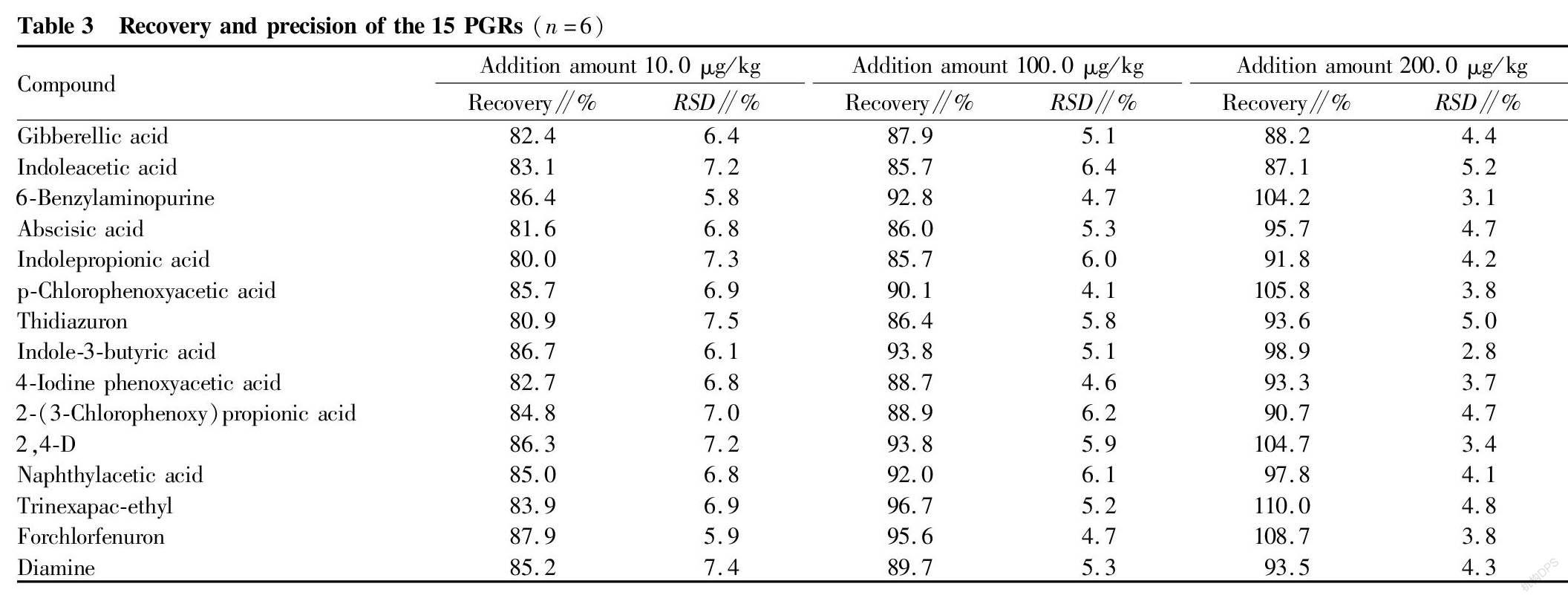
A 5.0 g of bean sprout sample without the target components was used as a blank matrix, which was added with the 15 kinds of PGRs standards at concentration levels of 10, 100 and 200 μg/kg, respectively, and each concentration was repeated 3 times. The measurement was repeated 6 times for each concentration level, and the relative standard deviations (RSDs) of the measured values were calculated. It can be seen from Table 3 that the average recovery of each additive concentration level was 80%-110%, and the RSDs were 2.8%-7.5%. The accuracy and precision of the method met the requirements of residue analysis.
Analysis of actual samples
The established analytical method was used to detect 30 batches of bean sprout samples randomly purchased on the market. Among the tested samples, 3 batches of samples were detected with 4-chlorophenoxyacetic acid (4-CPA), but its content was below the limit of quantification, and 6-benzyl adenine (6-BA) was detected in 2 batches of samples, with contents of 2.4 and 10.6 μg/kg, respectively, which were unqualified samples.
Conclusions
In this study, 5% acetic acid-acetonitrile (1∶99, V/V ) was used as the extraction solvent. By optimizing the chromatographic and mass spectrometry conditions, an HPLC-MS/MS method for simultaneous determination of 15 PGRs in bean sprouts was established. The method is simple in operation, high in sensitivity, good in reproducibility, and its precision and accuracy meet the methodological indexes. It can be used to simultaneously determine the residues of the 15 PGRs in bean sprouts.
References
[1] ZHANG LW, JIAO GR, WANG K. Simultaneous determination of 26 kinds of plant growth regulator residues in fruits and vegetable by QuEChERS-high performance liquid chromatography-tandem mass spectrometry[J]. Journal of Food Safety & Quality, 2016, (7): 2677-2688. (in Chinese)
[2] KONG DX, SHI LL, SHAN ZJ, et al. Determination of α-naphthylacetic acid by ASE-GPC coupled with HPLC[J]. Journal of Instrumental Analysis, 2010, 29(4): 382-385. (in Chinese)
[3] HUANG HH, ZHANG J, XU DM, et al. Determination of 21 plant growth regulator residues in fruits by QuEChERS-high performance liquid chromatography-tandem mass spectrometry[J]. Chinese Journal of Chromatography, 2014, 7(7): 707-716. (in Chinese)
[4] SHI XM, JIN F, HUANG YT, et al. Simultaneous determination of five growth regulators in Fruits by modified quick, easy, cheap, effective rugged and safe (QuEChERS) extraction and Liquid chromatography-andem mass spectrometry[J]. J Agric Food Chem, 2012, 60(1): 60-65.
[5] SHI XM, JI NF, HUANG YT, et al. Simultaneous determination of five plant growth regulators in fruits by modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction and liquid chromatography-tandem mass spectrometry[J]. Journal of Agricultural and Food Chemistry, 2012, 60(1): 60-65.
[6] DENG ZZ, WANG LH, WANG QL. Effects of plant growth regulators on growth and endogenous hormones of in vitro plantlets of manchurian ash[J]. Journal of Northeast Forestry University, 2009, 37(12): 10-13. (in Chinese)
[7] GONG RR, JIN YM. Determination of 2,4-dichlorophenoxyacetic acid residues in bean sprout by gas chromatography[J]. Agricultural Engineering, 2016, 6(3): 46-49. (in Chinese)
[8] WU PG, TAN Y, ZHANG J, et al. Determination of 10 plant growth regulators in bean sprouts by sequential cleaning-gas chromatography-mass spectrometry[J]. Chinese Journal of Analytical Chemistry, 2014, 42(6): 866-871.
[9] LI GL, LIU SC, SUN ZW, et al. A simple and sensitive HPLC method based on pre-column fluorescence labeling for multiple classes of plant growth regulator determination in food samples[J]. Food Chemistry, 2015, 170: 123-130.
[10] lIU YM, LIU HL, JI WL, et al. Simultaneous determination of 7 plant growth regulators residues in bean sprout by QuEChERS-HPLC-MS/MS[J]. Chinese Journal of Health Laboratory Technology, 2015, 25(12): 1880-1883. (in Chinese)
- 农业生物技术(英文版)的其它文章
- Rice Blast Resistance-associated Genes Based on Different RNA-seq Resources
- Research Progresses on QTLs for Main Grain Shape Genes in Rice
- Effects of Raising Chickens Under Moringa oleifera
- Preliminary Research on Radiation Breeding of Pteroceltis tatarinowii Maxim
- Comparison of Spring Radish Varieties with Entire Leaves
- Occurrence and Chemical Control Techniques of Rice Black-streaked Dwarf Disease in Rongshui County

