Effects of EGCG on Liver Antioxidation and Fat Metabolism in Heat-stressed Broilers
Han WU Zhengjun LI Ye YANG



Abstract This study aims to investigate the effects of EGCG on the lipid deposition and liver anti-oxidative capacity of broilers under heat stress. One hundred and ninety-two 2-week-old broilers were divided into four groups with 6 replicates per group and 8 chickens per replicate: one thermoneutral control group (28 ℃, TN group), which was fed the basal diet, and three cyclic high-temperature groups (35 ℃ from 7:00 to 19:00 h; 28 ℃ from 19:00 h to 7:00 h, heat stress (HS) group), which were fed the basal diet added with EGCG at doses of 0 (HS0 group), 300 (HS300 group), and 600 mg/kg (HS600 group), respectively. The liver metabolism and lipid deposition indices were performed at 35 d of age. The results showed that heat stress decreased the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and the Nrf2 mRNA expression in liver, and increase significantly the levels of malondialdehyde (MDA) and the expression of LITAF, NF-KB, FAS, SREBP1 mRNA and the lipid deposition compared with TN group. EGCG (HS300 and HS600 group) increased the activities of SOD and GSH-Px and catalase (CAT), increased the Nrf2 mRNA expression, decreased the MDA contents, and reduced the lipid deposition and expression of LITAF, NF-KB, FAS and SREBP1 mRNA. In conclusion, the results of this study show that EGCG can improve liver antioxidative capacity to alleviate oxidative damage caused by heat stress.
Key words Broiler; Heat stress; EGCG; Fat metabolism; Antioxidant capacity
Received: September 10, 2021 Accepted: November 12, 2021
Han WU (1996-), female, P. R. China, master, devoted to research about animal nutrition and feed.
*Corresponding author.
The liver of poultry not only plays an important role in regulating the digestion, absorption and metabolism of nutrients, but also plays an important role in the deposition and metabolism of protein, fat and carbohydrates in the body. In addition, it’s also an important endocrine tissue, so ensuring the health of the liver of poultry is extremely important for maintaining the health of poultry and ensuring normal production performance[1]. Due to the rise in global temperature, heat stress is the main environmental factor affecting the health of the liver, it also causes oxidative stress, causing lipid peroxidation and protein oxidative damage, and impairing the function of cells and mitochondria[2]. Therefore, antioxidant additives such as vitamins, trace elements or plant extracts are added to the feed to improve the antioxidant performance of poultry[3]. Tea polyphenols contain a variety of bioactive components with antioxidant and anti-inflammatory properties, which can scavenge free radicals and reduce the ROS formation. More importantly, tea polyphenols can improve the balance of cellular redox balance and exert antioxidant effects, thereby reducing the oxidative stress damage[4]. Meanwhile, the main active components of tea polyphenols also increase the antioxidant function of poultry liver by inhibiting inflammatory signal pathways (such as NF- κ B, AP-1), thus reducing the effect of oxidative stress on poultry[3]. At present, there are relatively few studies on the regulation of heat effect by EGCG in broilers. The main purpose of this experiment is to study the effects of EGCG on liver antioxidation and fat metabolism in broilers under heat stress, which provides a theoretical basis for the anti-heat stress effect of green tea extract in regulating animal production.
Materials and Methods
This study was carried out in Changping Base, State key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences (Beijing, China).
Experimental design
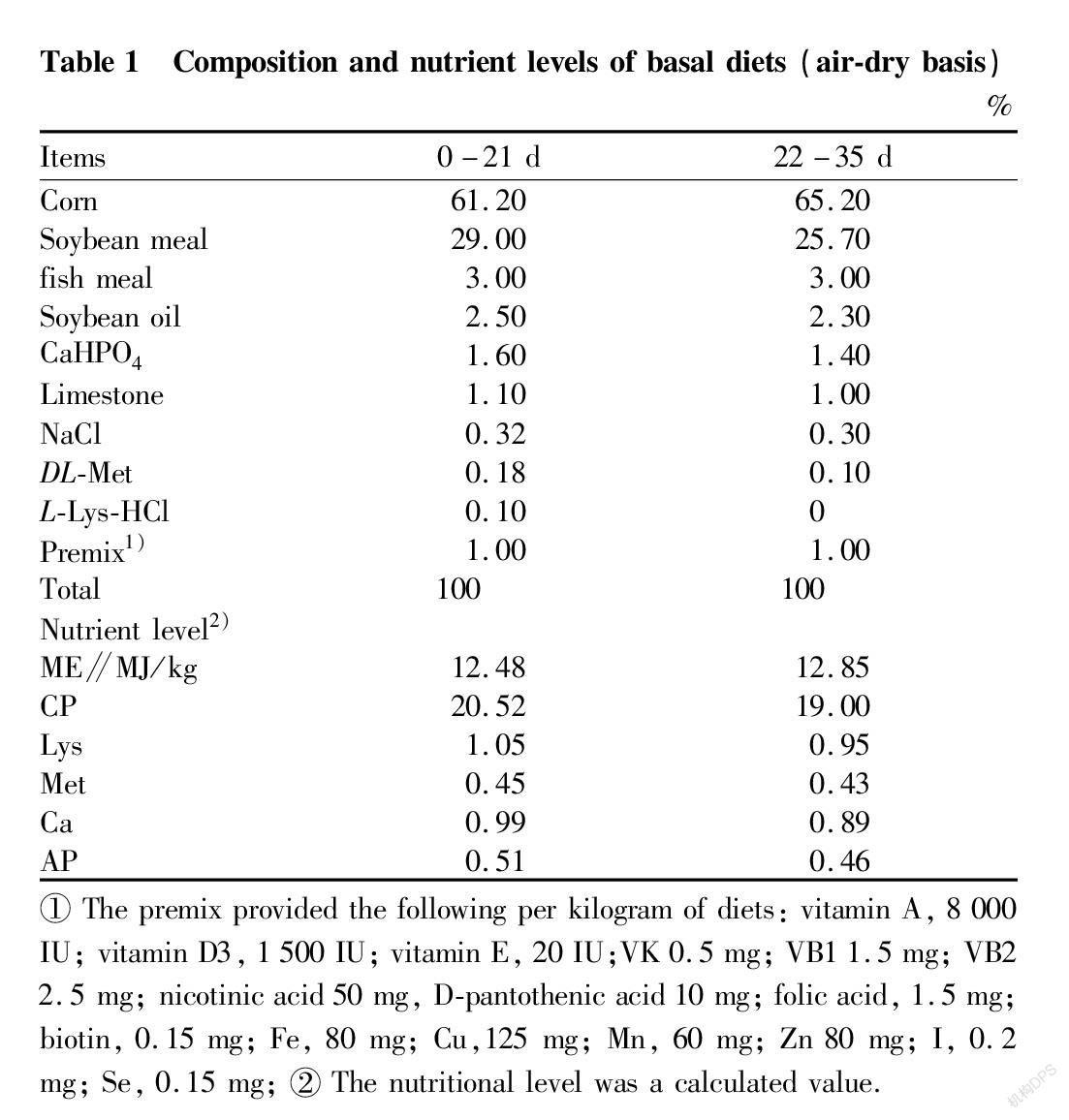
After 1-day-old male Arbor Acres broilers (purchased from Huadu Broiler Company, Beijing, China) were pre-fed for 2 weeks, 192 healthy broiler chicks of similar weight were randomly divided into 4 groups. Each group had 6 replicate cages with 8 chickens per replicate. Among them, one group (thermoneutral group) was housed in an environmentally controlled chamber at 28 ℃ (Group TN) and was fed with the basal diet, a commercial diet provided by Huadu Feed Company (Beijing, China). Other 3 groups were housed in a cyclic high-temperature environmentally controlled chamber (35 ℃ from 07:00 to 19:00 h; 28 ℃ from 19:00 to 7:00 h). The basal diets for the HS group were supplemented with EGCG at the following doses: 0 (Group HS0), 300 (Group HS300), and 600 mg/kg (Group HS600). The diets were based on corn-soybean meal (Table 1). The trial was last 3 weeks. The chickens were raised in cages and supplied diet and water ad libitum.
Sample collections
At 35 d, one chicken from each replicate was randomly selected and killed. The left lobe of the liver was collected. The liver samples were snap-frozen in liquid nitrogen, and then stored at -80 ℃ to analyze liver antioxidant enzymes’ activity and gene expression. Blood was collected by vein, centrifuged at 3 000 rpm at 4 ℃ for 15 min to separate the serum, and stored at -20 ℃ to analyze the serum biochemical indexes.
Determination of fat deposition
The abdominal fat was stripped and weighed to determine the percentage of abdominal fat. Muscle (thigh and leg) fat content was determined by Soxhlet extraction.
Determination of serum biochemical indexes
Serum biochemical indexes of aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), creatine kinase (CK), serum cholesterol (CHOL), triglycerides(TG), glucose (GLU) and uric acid (UA) were determined by a Toshiba 120 automated biochemical analyzer (Toshiba, Tokyo, Japan) as previously described[5].
Measurement of the activity of antioxidant enzymes and lipid peroxidation
The activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), catalase (CAT), and the contents of malondialdehyde (MDA) were determined according to the commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China)[5].
Quantitative real-time polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the liver tissues and fat tissues using the TRIzolTM Reagent (Ambion, CA, USA), and cDNA reverse transcription according to Primer Script TM RT Master Mix (Perfect Real Time) kit’s (Takara, Japan) manufacturer’s instructions. qRT-PCR was conducted on the LightCycler480 real-time system (Roche, Switzerland) using QuantiNova SYBR Green PCR Kit (QIAGEN, Germany). Sequences of the primer we used in this study are listed in Table 2. The relative level of gene expression was calculated according to the comparative (2-ΔΔCT) method[6].
Statistical analysis
The data were analyzed by using the one-way analysis of variance (ANOVA) procedure (SAS Version 8). Means were compared with one-way ANOVA followed by Duncan’s multiple tests. Statistical significance was considered at P <0.05.
Results and Analysis
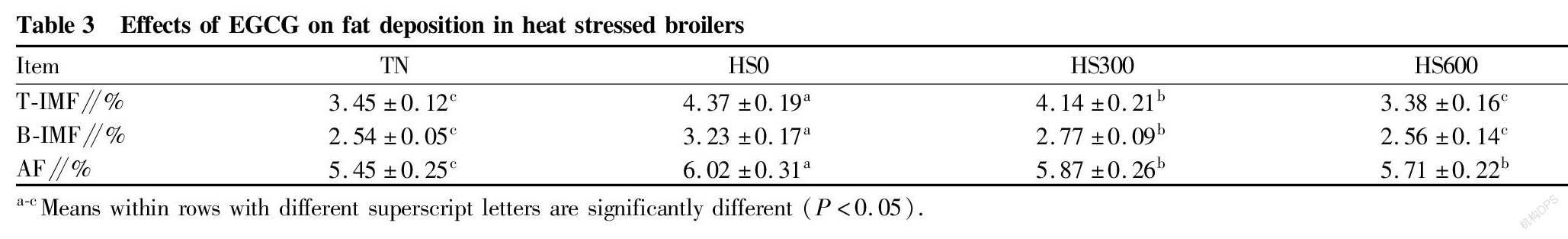
Fat Deposition
Compared with the Group TN, heat stress increased ( P <0.05) the fat contents of leg muscle and breast muscle and abdominal fat percentage (Table 3). Compared with the Group HS0, EGCG (Group HS300 and Group HS600) decreased ( P <0.05) the fat contents of leg muscle and breast muscle and abdominal fat percentage (Table 3).
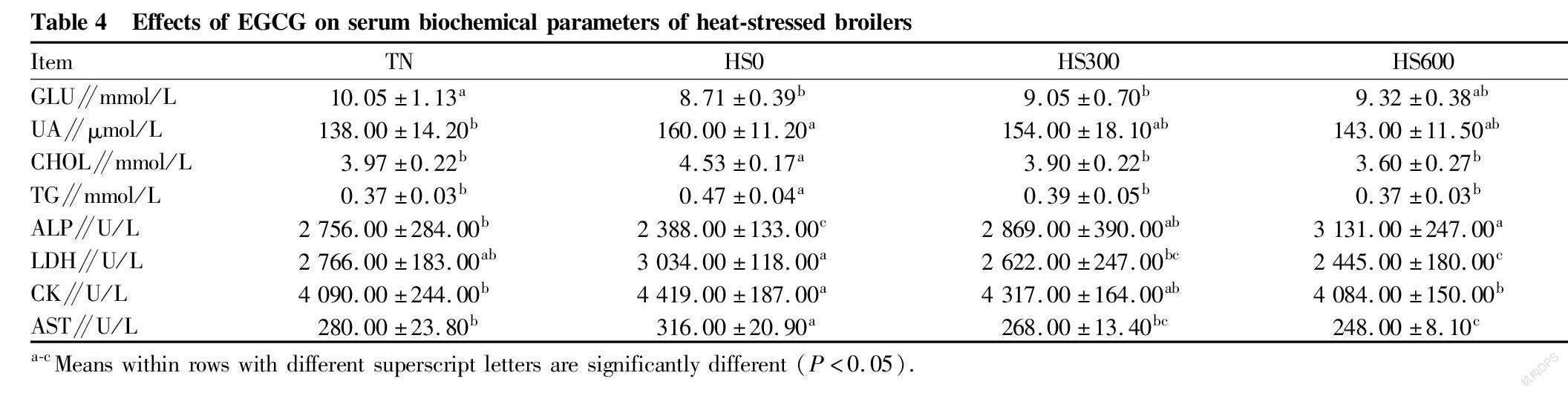
Serum biochemical parameters
The effects of heat stress and EGCG on serum biochemical parameters are shown in Table 4. Compared with Group TN, heat stress reduced ( P <0.05) the serum GLU and ALP activity, but increased ( P <0.05) the contents of CHOL, TG and UA, as well as the activities of the CK, LDH and AST ( P <0.05) in Group HS0. In the heat stress groups, an increase ( P <0.05) was observed in the activity of serum ALP and the content of GLU in Group HS600 compared with Group HS0, as well as the serum CHOL, TG and UA contents and LSH, CK and AST activities were decreased ( P <0.05).
Serum antioxidant capacity
The effects of EGCG on liver antioxidant capacity of heat-stressed broilers is presented in Table 5. Compared with Group TN, heat stress (Group HS0) decreased the liver GSH-Px and SOD activity ( P <0.05), but increased the MAD contents were increased ( P <0.05). The activities of GSH-Px, SOD and CAT were increased ( P <0.05) and MDA was reduced ( P <0.05) in Group HS600 compared with Group HS0 when dietary EGCG was added to the heat-stressed broilers.
Han WU et al. Effects of EGCG on Liver Antioxidation and Fat Metabolism in Heat-stressed Broilers
The mRNA relative expression in liver of heat stressed broilers
Compared with the Group TN, heat stress increased the mRNA relative expression of NF-κB, LITAF, FAS and SREBP1 ( P <0.05), but decreased the mRNA relative expression of Nrf2 ( P <0.05) (Fig. 1). In the Groups HS, the mRNA relative expression of Nrf2 ( P <0.05) increased with the increase of EGCG content. In contrast, the mRNA relative expression of NF-κB, LITAF, FAS and SREBP1 ( P <0.05) decreased gradually with the increase of EGCG content (Fig. 1).
Conclusions and Discussion
Effects of heat stress and EGCG on liver antioxidation
Heat stress is the main factor affecting the production performance of broilers. Many studies have shown that heat stress can significantly reduce the production performance of broilers. The main effect of heat stress on poultry comes from the tissue and cell damage caused by oxidative stress caused by heat stress[7-8]. This study also showed that EGCG increased the activities of SOD, GSH-Px and CAT in the liver of heat-stressed broilers, and enhanced the antioxidant activity of broilers, which indicated that EGCG played a certain role in alleviating the heat effect of broilers. Meanwhile, it can also increase the antioxidant function of poultry liver by inhibiting inflammatory signal pathways (such as NF- κ B, AP-1), so to reduce the effect of oxidative stress on poultry[3].
Effects of heat stress and EGCG on liver metabolism
The changes of serum metabolites reflect the liver metabolism in poultry under heat stress. Changes in the activities of serum glutamic oxaloacetic transaminase (AST), lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and creatine kinase (CK) reflect the healthy state of liver. To some extent, the level of serum glutamic oxaloacetic transaminase (AST) can reflect the degree of liver damage. Continuous heat stress increased the oxidative damage of liver tissue, and when the permeability of cell membrane increased, the activity of these enzymes in serum increased[9]. Creatine kinase (CK) reflects the damage of muscle cells and is a sensitive indicator that animals are in a state of heat stress. Lactate dehydrogenase (LDH) is a glycolytic enzyme, which can catalyze the conversion of pyruvate to lactic acid, the final product of anaerobic glycolysis, so its activity is closely related to the degree of glycolysis. Alkaline phosphatase (ALP) is involved in the transport of phosphate groups, which is closely related to Ca and P metabolism. Under the condition of heat stress, the liver is damaged, which will decrease the content of active form of vitamin D3, thus affect the metabolism of Ca and P, and decrease the activity of ALP[1]. This study showed that EGCG changed the activities of these serum enzymes in heat-stressed broilers, which can significantly reduce the activities of AST, LDH and CK in
serum, while increasing the activity of ALP, indicating that EGCG can alleviate the tissue damage caused by heat stress.
Effects of heat stress and EGCG on fat metabolism
Liver is the main place of fat metabolism in poultry. Heat stress can increase the activity of fatty acid synthase (FAS) in liver, which leads to a significant increase in muscle fat content and abdominal percentage[10-11]. The results also showed that heat stress significantly increased the expression of FAS mRNA in liver, fat content in muscle. The increase of fat deposition will cause a series of inflammatory reactions and increase the expression of inflammatory factors such as IL-6 and TNF-α. This study also showed that heat stress increased the expression of NF-kB and LITAF. EGCG can reduce the expression of key genes of fat synthesis such as PPAR γ, C-/EBP α and SREBP1, and increase the expression of fat decomposition genes such as HSL and CPT1, thus reducing fat deposition. On the other hand, EGCG can also increase the phosphorylation level of AMPK to promote fat oxidation[12]. The results also showed that EGCG could reduce the fat deposition and the expression of liver inflammatory factors in heat-stressed broilers.
In summary, the results of this study confirmed that heat stress significantly decreased the antioxidant capacity of the liver, increased fat deposition and the expression of inflammatory factors in the liver, and had an adverse effect on the meat quality of broilers. Dietary addition of EGCG improved the antioxidant performance of the liver, decreased the fat deposition and the expression of inflammatory factors in the liver, improved the muscle quality, and alleviated the heat stress effect of broilers.
References
[1] ZAEFARIAN F, ABDOLLAHI MR, COWIESON A, et al. Avian liver: The forgotten organ[J]. Animals, 2019, 9(2): 63. doi:10.3390/ani9020063.
[2] YANG L, TAN GY, FU YQ, et al. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens[J]. Comparative Biochemistry and Physiology, Part C, 2010, 151: 204-208.
[3] ORHAN C, TUZCU M, GENCOGLU H, et al. Epigallocatechin-3-gallate exerts protective effects against heat stress through modulating stress-responsive transcription factors in poultry[J]. Brit. Poult. Sci., 2013, 54: 4, 447-453.
[4] DEMBINSKA-KIEC A, MYKKNEN O, KIEC-WILK B, et al. Antioxidant phytochemicals against type 2 diabetes[J]. Br J Nutr, 2008, 99: ES109-ES117.
[5] XUE B, SONG J, LIU LZ, et al. Effect of epigallocatechin gallate on growth performance and antioxidant capacity in heat stressed broilers[J]. Arch Anim Nutr., 2017,71(5): 362-372
[6] LIVAK KJ, SCHMITTGEN TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25: 402-408.
[7] GU XH, HAO Y, WANG XL. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress[J]. Poult Sci., 2012, 91: 790-799.
[8] ZHANG ZY, JIA GQ, ZUO JJ, et al. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat[J]. Poult. Sci., 2012, 91: 2931-2937.
[9] XIE JJ, TANG L, LU L, et al. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders[J]. Poult. Sci., 2015, 94 (7): 1635-1644.
[10] QU H, DONKIN SS, AJUWON KM. Heat stress enhances adipogenic differentiation of subcutaneous fat depot-derived porcine stromovascular cells[J]. J. Anim. Sci., 2015, 93: 3832-3842.
[11] LU Z, HE X, MA B, et al. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism[J]. J. Agric. Food Chem., 2017, 65: 11251-11258.
[12] WANG S, MOUSTAID-MOUSSA N, CHEN L, et al. Novel insights of dietary polyphenols and obesity[J]. J Nutr Biochem, 2014, 25(1): 1-18.
- 农业生物技术(英文版)的其它文章
- Rice Blast Resistance-associated Genes Based on Different RNA-seq Resources
- Research Progresses on QTLs for Main Grain Shape Genes in Rice
- Effects of Raising Chickens Under Moringa oleifera
- Preliminary Research on Radiation Breeding of Pteroceltis tatarinowii Maxim
- Comparison of Spring Radish Varieties with Entire Leaves
- Occurrence and Chemical Control Techniques of Rice Black-streaked Dwarf Disease in Rongshui County

