Particle-Stabilized Interfaces and Their Interactions at Interfaces
Guanqing Sun , Zonglin Yi , To Ngai ,,*
1 The Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, Jiangnan University, Wuxi 214122, Jiangsu Province, P.R.China.
2 Department of Chemistry, The Chinese University of Hong Kong, Shatin, NT, Hong Kong SAR, P.R.China.
Abstract: Particle-stabilized dispersions such as emulsions, foams and bubbles are catching increasing attentions across a number of research areas.The adsorption mechanism and role of these colloidal particles in stabilizing the oil-water or gas-water interfaces and how these particles interact at interfaces are vital to the practical use of these dispersion systems.Although there have been intensive investigations, problems associated with the stabilization mechanisms and particle-particle interactions at interfaces still remain to explore.In this paper,we first systematically review the historical understanding of particle-stabilized emulsions or bubbles and then give an overview of the most important and well-established progress in the understanding of particle-stabilized systems, including emulsions, foams and liquid marbles.The particle-adsorption phenomena have long been realized and been discussed in academic paper for more than one century and a quantitative model was proposed in the early 1980s.The theory can successfully explain the adsorption of solid particles onto interface from energy reduction approaches.The stability of emulsions and foams can be readily correlated to the wettability of the particles towards the two phases.And extensive researches on emulsion stability and various strategies have been developed to prepared dispersion systems with a certain trigger such as pH and temperature.After that, we discuss recent development of the interactions between particles when they are trapped at the interface and highlight open questions in this field.There exists a huge gap between theoretical approaches and experimental results on the interactions of particles adsorbed at interfaces due to demanding experimental devices and skills.In practice, it is customary to use flat surfaces/interfaces as model surfaces to investigate the particle-particle at interfaces although most of the time interfaces are produced with a certain curvature.It is shown that the introduction of particles onto interfaces can generate charges at the interfaces which could possibly account for the long range electrostatic interactions.Finally, we illustrate that particle-stabilized dispersions have been found wide applications in many fields and applications such as microcapsules, food, biomedical carriers, and dry water.One of the most investigated areas is the microencapsulation of actives based on Pickering emulsion templates.The particles adsorbed at the interface can serve as interfacial stabilizers as well as constituting components of shells of colloidal microcapsules.Emulsions stabilized by solid particles derived from natural and bio-related sources are promising platforms to be applied in food related industries.Emulsion systems stabilized by solid particles of the w/w (water-in-water) feature are discussed.This special type of emulsion is attracting increasing attentions due to their all water features.Besides of oil-water interface, particle stabilized air-water interface share similar stabilization mechanism and several applications reported in the literature are subsequently discussed.We hope that this paper can encourage more scientists to engage in the studies of particle-stabilized interfaces and more novel applications can be proposed based on this mechanism
Key Words: Interface; Particle adsorption; Pickering emulsions; Particle-particle interaction; Dry waters;Microcapsules

1 Introduction
At present, it has been well-established that solid nanoparticles, either being hard or soft can serve as interfacial stabilizers as effective as or even better than small molecular and polymeric surfactants for emulsions, foams, and bubbles1.Solid nanoparticles can adsorb at liquid-liquid or liquid-gas interfaces due to the reduction of interfacial energies and the formation of mechanical barriers composed of single or multiple layers of nanoparticles2,3.First reported on scientific journals by Ramsden and Pickering in the beginning of the last century4,5,the research of this field has basically been forgotten except for a few scattered researches.Most of the advanced instruments and theories that we are familiar with now are not available to colloidal scientists at that time6-8.Until the 1980s this area began to catch increasing attentions of a range of researchers both from the traditional colloidal research community as well as scientists from other fields and various industrial sectors and this research area has enjoyed considerable renaissance since then9-12.The absorption of colloidal nanoparticles onto interfaces is important for stabilization of fluid-fluid interfaces and thus it is crucial phenomena in understanding a number of industrial applications such as pharmaceuticals, cosmetics,foods, mineral purificationetc.Besides of this, the adsorption of colloidal nanoparticles at planar or curved interfaces can also serve as platforms to fabricate 2D (two-dimensional) or even 3D(three-dimensional) structures which find applications in optical,magnetic, and electronic devices13.In all of these specific research directions, the underlying principle that governs colloidal nanoparticle adsorption and interactions at the fluidfluid interfaces plays the essential role.Although using particle as particulate emulsifier, or so-called Pickering stabilization has been reported for over a century and intensive research endeavors have been contributed to from various aspects during the last decades, many problems about colloidal nanoparticle adsorption and interactions at interfaces still remain unanswered.Herein we tried to summarize and comment on recent progress in this field with focus on particle adsorption mechanism,emulsion stability, particle interactions at the interface and finally to highlight some of its use in various industrial sectors.We hope to encourage further investigation on these questions,which would be beneficial to extension of the knowledge to all other related colloidal materials.
2 Colloidal nanoparticle adsorption mechanism
Human has used nanoparticle stabilized disperse systems such as emulsions and foams long before the reports of Ramsden and Pickering.In the initial reports of Ramsden and Pickering,both of them had already realized that the adsorption of the“insoluble emulsifiers” had formed “mechanical surface aggregates” and this process may cause reduction of “surface potential energy” which was critical for interface stabilization4,5.However, it is not possible for them at that time to give clear pictures of why and how solid particles were adsorbed at emulsion or bubble interfaces.Finkleetal.were among the first to offer an illustration of solid particles at liquid-liquid interfaces6.They pointed that solid particles should behave like small molecular surfactants when served as interface stabilizers for emulsions.The solid particle should be better wetted by the liquid which forms the continuous phase and in this way the solid particles should straddle at the interface which makes a threephase contact angle (Fig.1).The solid particles behave similarly as small molecular surfactants and the phase that wets the solid particle more forms the continuous phase.This kind of description is very much close to the modern explanation of colloidal nanoparticle stabilization of interfaces but it lacks accuracy and rigor.

Fig.1 Finkle’s schematic presentation of particle at interface.
In 1980, Pieranski first derived a quantitative model to describe the detachment energy of colloidal particles adsorbed at air-water interface based on experimental observations9.It is observed that the polystyrene colloidal particles can form both 2D crystalline and disorder structures with high surface densities and the particles adsorbed at the interface, different from those in the bulk which undergo vibrant Brownian motion, can hardly move around under the microscope.This indicates that the solid particles adsorbed at the interface are trapped inside a surface energy well.From a simple calculation of surface energy changes due to particle adsorption onto interfaces, it is shown that the energy required to detach the solid particle from interface is much larger than thermal energy which fuels the Brownian motion.For a polystyrene microsphere adsorbed at air-water interface, the detachment energy is larger than thermal energy (kBT) by a factor of 106.This energy well is so large that particle irreversible adsorption at interface is naturally derived which is widely accepted in the research community for decades until new experimental evidence arises14.Although Pieranski did mention the electrostatic interactions and dipole-dipole interactions due to the asymmetric dissociation of polystyrene particles at air-water interface, he failed to point out other forms of interactions of solid particles at the interface.Later Levineet al.further calculated and verified the detachment energy is extremely large than thermal energy and other interacting forces at interfaces such as capillary, van der Waals and solid elastic forces are smaller by three or four orders of magnitude than this detachment energy which means that the energy well dominates over all other interactions11.As shown in Fig.2, a colloidal particle with diameterris sitting at an oil water interface with interfacial tensionγowand the contact angle the colloidal particle makes at the interface isθwhich is measured from the aqueous phase, the detachment energy can be expressed by the following equation.


Fig.2 Colloidal particles adsorbed at two phase interface.
As shown in the above equation, the detachment energy is proportional to the square of the radius of the colloidal particle and for typical oil-water and particle systems, it becomes so large that it makes the detachment basically impossible by thermal energy when the size of the particle is over several hundred nanometers.As small molecular surfactants only need a fewkBTto adsorb onto and detach from the interface and the emulsions stabilized by them usually experience stabilizer exchange at a relative fast rate(kBis Boltzmann constant andTis absolute temperature ).Therefore, it is expected that emulsions and foams stabilized by solid particle should have enhanced stability compared to those stabilized by small molecular surfactants.However, at the present stage there is still no method to measure the contact angle of particles at interfaces with adequate resolutioninsitu.In practice, most researches will prepare a pressed particle disc and then use the apparent water contact angle as an indicator of oil-water contact angle.Recently,Isaetal.have summarized the progress in characterizing the morphology and contact angle of particle at interfaces16.
3 Stability of solid particle stabilized interfaces
Emulsions are thermodynamically unstable and they are only kinetics stable, either stabilized by surfactants or solid particles,due to inherent increase of huge amount of interfacial energy during emulsification.It is worth notating that microemulsions are not within the scope of this paper and they are typically not considered as emulsions in this field.Stability of solid particle stabilized emulsions is of vital importance to many industrial applications and investigation on this topic can help achieve stability or instability depending on different application scenarios.For example, for the consumer products which longer shelf time is expected, the enhanced stability of the emulsions is desired while instability is wanted during the crude oil separation from sands and waters17.Although it is now well-accepted that higher detachment energy will generate more stable emulsions,it is very hard to determineinsituthe contact angle of the colloidal particles at interface16.This means that it is nearly impossible to obtain the exact detachment energy experimentally for a specific emulsion system and further difficulties will also arise when surface properties of colloidal particles changes in response to solution triggers such as pH18, salt concentration19,temperature20and so on.Recently Binks and Cuietal.reported the stabilization of Pickering emulsions in synergy with a responsive surfactant21.It is demonstrated the particle-stabilizer can inherit the CO2/N2trigger properties from the surfactants and a later paper shows that even phase inversion can be induced by similar strategy22.Furthermore, they demonstrated that the oppositely charged surfactant can also be used as a switchable agent to induce stability or instability23.It is also reported that surface modification can enhance the stability of pure inorganic stabilizers24.At the present stage, the results obtained in the measurement of colloidal particle detachment energy is still inadequate to predict the stability of emulsions subsequently prepared.
Another theory that argues the stability of particle-stabilized emulsions focuses on the mechanical barriers at the interface and the 3D gel-like networks formed by particles both at the interface and in the bulk phase can significantly retard the creaming and coalescence process which can improve the emulsion stability(Fig.3).Binksetal.have repeatedly mentioned that slightly aggregation of colloidal particles in the solution is beneficial for the stability of emulsions25-27.The aggregation of colloidal particles with high concentrations and under unfavorable conditions such as high salt concentration to obtain thick and viscous dispersion has been reported and investigated for quite a long time28.From the rheology data, it is quite straightforward to come the conclusion that there is a built-up of colloidal particles to form 3D connected structures.However, this often requires use of high concentrations of stabilizing particles which is undesirable in most applications.Therefore, at present it still lacks quantitative or semi-quantitative theory or method such as the hydrophilic-lipophilic balance (HLB) scale used for surfactant stabilized emulsions to predict the property and stability of particle stabilized emulsions.
During the last decades, a lot of researches have been carried out to investigate various experimental parameters such as particle concentration and surface modification, particle initial location, aqueous and oil phase conditionsetc., on the emulsions stability and some empirical rules have been found in the literature.It is worth noting that these are only general rules and they may not fit into some specific systems.First, the surface properties play crucial roles in the stabilization of emulsions.As discussed above, hydrophilic particles with a contact angle less than 90° will preferentially stabilize oil-in-water (o/w)emulsions andviceversa.In practice, colloidal particles that make a contact angle close to zero will be too hydrophilic and they cannot be used as good emulsions stabilizers either.Second,for inorganic colloidal nanoparticles slightly aggregation is usually required to prepare stable emulsions.Too much salt can induce the flocculation of the colloidal particles which is not wanted but experiments have shown that slightly aggregation can enhance the emulsion stability.It is shown that emulsions stabilized by silica particles and Laponite clays can have better stability when the particles are slightly aggregated26,31.Third,higher particle concentrations and appropriate particle sizes usually can produce more stable emulsion.In the literature, a single particle layer at the interface is usually assumed especially for schematic illustrations but this is not true in real case.It is reported that stable emulsion can be obtained even when part of the droplet surface is covered and increase of the coverage can improve the stability.This is why emulsions stability is usually enhanced with increasing concentrations of initial particle concentrations.In practice, multiple layer and even aggregated particles are often found at the interfaces which improve emulsions stability.It is generally believed that the size of emulsions droplets should be much larger than that of colloidal particles or no stable emulsions can be obtained.However, much smaller particle sizes down to the several nanometer range will also cause stability problems because of the decreasing detachment energy32,33.Listing all the parameters that influence the emulsion stability will make this section too bulky and some of the parameters can be specific only to certain emulsion systems34.
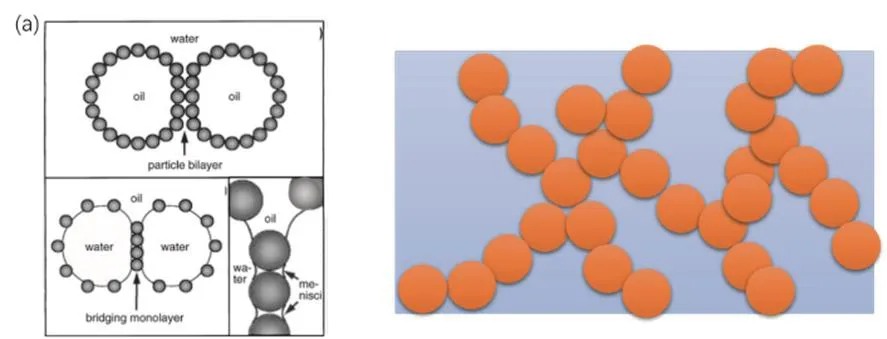
Fig.3 Stabilization mechanism of particle stabilized emulsions.
Besides of the stabilization of liquid-liquid interfaces to prepare emulsions, colloidal nanoparticles can also be used to stabilize gas-liquid interfaces and this can lead to the preparation of bubbles, foams, dry waters, or liquid marbles stabilized by solid colloidal particles (Fig.4)35.For hydrophilic colloidal nanoparticles, aqueous foams and bubbles are preferentially stabilized while dry water and liquid marble can only be stabilized by hydrophobic colloidal particles36,37.Particle stabilized foams and bubbles have long been explored in the separation of mineral of which the process is called froth flotation.Depending on the affinity of mineral particle to the interface, different minerals can be separated and refined in a series of the froth flotation process.In the past decade, there is a reoccurring interest to study the stability of particle stabilized foam and mechanism along with the Pickering emulsion research renaissance.
4 Particle-particle interactions at interface and measurement
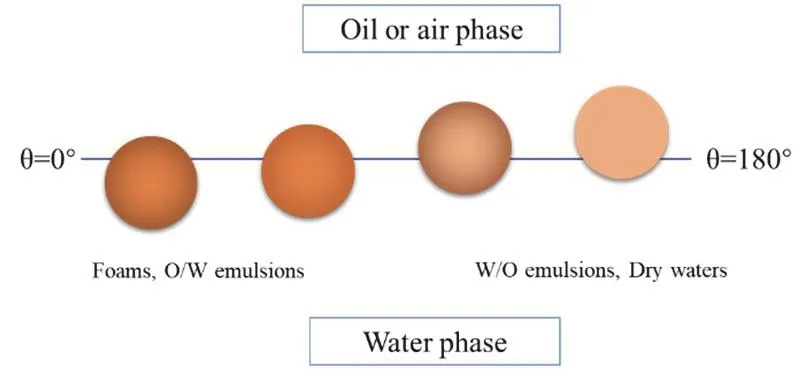
Fig.4 A variety of interfaces can be stabilized by colloidal particles.
Particles with size in the micrometer range trapped at oil/water or air/water interfaces are often visualizedviaa conventional optical microscope equipped with a video camera.These experimental conveniences offer researchers a quasi-twodimensional system to investigate many fundamental problems in condense matter physics, such as low dimensional phase transitions, jamming transition, crystal melting, and selfassembly because the particles can be viewed as “big atoms” and their trajectories can be used to elucidate the interactions,thermodynamics, and dynamics in those distinctive phases38.Aside from fundamental researches, understanding and precisely controlling the interfacial interactions among colloidal particles trapped at water/oil interfaces are crucial for Pickering emulsion related applications.Therefore, interfacial interactions among colloidal particles trapped at water/oil interface have been both theoretically and experimentally studied.As mentioned above,as early as in 1980, Pieranskietal.have shown that, at an air/water interface, interfacial particles can form a twodimensional colloidal crystal of which its lattice constant is much larger than the particle size, indicating that a long-ranged repulsive force exists among those interfacial particles9.They showed that the long-ranged repulsive force arises from longranged dipole-dipole interactions at the interface where asymmetrical dissociation of ion-pairs located at particle surfaces results in forming dipoles normal to the interfaces.Therefore, it is expected that adding salts can screen these interfacial dipoles and repress the repulsion.However, Averyardet.al.showed that charged colloidal particles produce extremely stable monolayers at an octane/water interface even at very high NaCl salt concentration, which cannot be explained by dipoledipole interaction alone39.They proposed this repulsive force lies in charge-charge repulsion, arising from a small fraction of residual charges existence in the oil phase, which result in unscreened charge-charge repulsion.A satisfying explanation for the origin of interfacial particle interaction still remains elusive39,40.
Recently, by combing various state-of-the-art microscopy techniques and microinjection, we have successfully investigated the interfacial behavior of colloidal particles trapped in an ultrathin, highly stable and clean oil-water interface41.We showed that instantaneous gradients of surface tension can induce particles to jet towards the oil-water interface with velocities as high as ≈ 60 mm·s-1when particle suspensions come in contact with the interface (Fig.5).We found that this high-speed interfacial jet and the resultant friction between the particles and oil lead to charges at the particle-oil interface.These residual charges dominate the longstanding, controversial,long-ranged electrostatic repulsions, and prevent the interfacial particles against aggregation.This in turn alters the interfacial tension and rheology.However, these residual charges are unstable, and can fade out in several hours, thus gradually attenuating the repulsions among interfacial particles.The charging and discharging of particles at oil-water interfaces thus play remarkable roles in interfacial interactions of particles (Fig.6).

Fig.5 The particles jet along the oil/water interface with extremely high speeds at the moment of contact with the interface.
We also found that the emergence of those unstable residual charges at particle/oil interfaces actually leads the system possessing time-dependent evolution features, such as the measured interaction potentials shown in Fig.7a.This phenomenon may limit their fundamental studies, especially when the required systems need to stay at thermodynamic equilibrium state.To further reveal the origin of the residue charges in affecting the interaction between charged colloidal particles at an oil/water interface, we further utilized the buoyancy of micro-sized particles to drive the particles floating to an oil/D2O interface (Fig.8)42.We then compared the difference between such spontaneously adsorbed particle monolayer to the spread monolayer obtained from commonly solvent spreading methods.We found that the additive-free spreading would not lead particles to slide along the interface,and thus did not result in time-dependent evolution of interaction potentials among those interfacial particles (Fig.7b).These findings further confirmed that those unstable residual charges actually arise from the violently rubbing of particles against the oil at the interface.Importantly, the additive-free spreading method enables to completely abolish the formation of those unstable residual charges at the oil phase, which is crucial for applying the system of colloidal particles trapped at oil/water interfaces to ongoing investigations that requires precise control of the interfacial interactions among particles.

Fig.6 Schematic representation of charging and discharging of colloidal particles when they are dispersed at an oil/water interface.
5 Applications based on particle-stabilized interfaces
5.1 Microcapsules and microparticles templated from emulsion and bubble templates

Fig.8 Schematic show of the utilizing pure buoyancy to drive the particles onto an oil/water interface without involving spreading solvent.

Fig.7 Time evolutions of pair potentials U(r) of polystyrene microspheres trapped at an octane/D2O arising from two different spreading methods.
Microcapsules fabricated by various methods are receiving great attentions and they have found applications in drug delivery, energy storage, heat and sound insulation materials,anti-reflection coatings, catalysis and so on.Different from solid particles, the unique properties of microcapsules all derive from their hollow core-shell structure.Of all the methods developed,template related preparation is quite easy and facile for different materials and intended uses.Using the nanoparticles or microparticles as templates, it is quite convenient to obtain hollow microcapsules through layer-by-layer or nanodeposition after removal of the hard template.However, this removal process often involves harsh solvents or high temperature which may cause breakup of the shell and result in unwanted shell failures for applications.Compared with hard templates, soft templates such as emulsion droplets and foam bubbles are quite easy to remove after the shell formation.To assembly colloidal nanoparticles at interfaces and after that, sintering or crosslinking these colloidal nanoparticles at the interface can produce hollow microcapsules with shells comprising of single or multilayer ordered colloidal nanoparticles.These microcapsules are called colloidosomes43or colloidal microcapsules as suggested by Bollhorstetal.in a recent review44.Velevetal.are among the first to study the interactions of colloidal particles at interfaces and to fabricate the microstructured hollow sphere as they called it in their publications (Fig.9a)45,46.By investigating the polystyrene microparticles adsorption at the oil-water interfaces, they have tried a series of locking methods to fix the microparticles at the interface.Later on, Dinsmoreetal.reported that it is possible to assemble polystyrene colloids at the oilwater interface and the interstices of shell can be tuned by sintering the colloids at suitable temperatures (Fig.9b)43.They also demonstrated that selective release can be realized depending the size of the encapsulated materials.Our group has embedded high molecular polymers inside the oil droplets and during the oil diffusion process the polymer will deposit on to the interface and lock the colloids at the interface subsequently which can form free-standing colloidosomes (Fig.9c)47.This work has provided a successful example that physical locking of colloids at interface is quite a feasible way to prepare colloidosomes.Controlled release upon changes of pH values has also been reported and it is shown that fluorescent dye encapsulated inside colloidosomes can be released when the pH is decreased from 10 to 3 (Fig.9d)48.To list all the reported examples is not necessary here and herein we recommend the readers to a book chapter written by Biggs and Cayre49.

Fig.9 Images of colloidosomes templated from emulsion droplets.
The emulsion droplets can also be used as place for assembly and formation of nanoparticles and in this way microparticles or microspheres can be obtained during this process.One advantage of microparticles over nanoparticles is facile processing and separation.Furthermore, the confined space of emulsion droplets is an ideal place to study the assembly of nanoparticles into supra-structures.Liuetal.used water-in-oil(w/o) stabilized by silica nanoparticle as microparticle formation platform and they prepared a series of mesoporous carbon microparticles with different internal structures50.These mesoporous carbon microparticles can be further decorated by N and Co which enable them very useful catalysts.Heetal.directly polymerized the emulsion droplets stabilized silica nanoparticles and silica particle armored microparticles.These results showed a new strategy to prepare hybrid polymeric microparticles51.Yangetal.use the emulsion droplets themselves as templates and fabricated microparticles directly from these templates52.By incorporation of bisphenol A in the oil phase, molecularly imprinted polymer microsphere were obtained and these microspheres showed good specific adsorption capacity and selectivity.Although these microparticles have been prepared from other methods, the particle stabilization method has provided some advantages such as incorporation ofinsituinorganic nanoparticles, avoidance of small molecular surfactants, and confined space of particle and micelle assembly and so on.
5.2 Emulsion stabilized by bioparticle and related applications
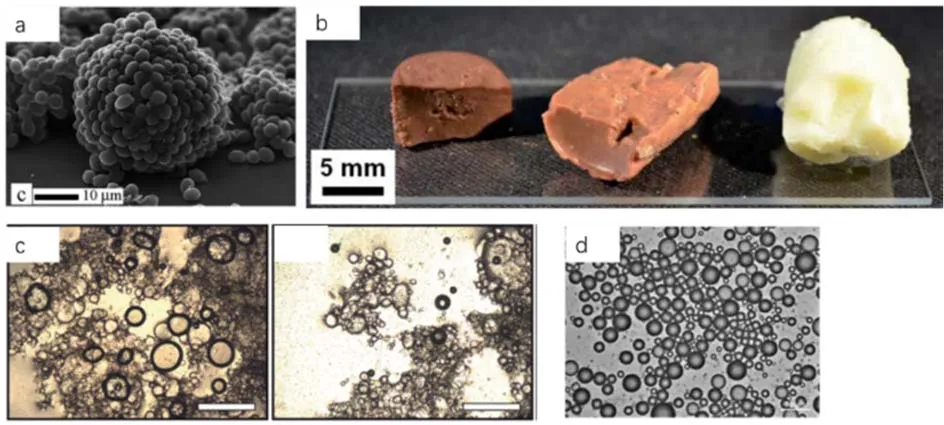
Fig.10 Examples of bioparticle stabilized interfaces.
Protein globules, fat crystals, polysaccharide particles and many more food hydrocolloids and colloids derived from biosources have been used in preparation of food and cosmetic dispersions and formulations for biomedical applications53-56.While previously this area is mostly application-oriented, the research focus is on the final emulsion stability or final product quality, less attention has been paid to the stabilization mechanism, interfacial structure, process and kinetics of adsorption and so on.During the past decade, along with the increasing interest in particle stabilized emulsions the studies on bioparticle stabilized dispersions have also gained significant progress52.Different from lyophobic colloids which the colloidal scientists classify as hard particles, most colloids derived from bio-related sources are relatively soft and can be swollen by the aqueous environment.A model colloidal system made from thermally responsive polymer, poly(N-isopropylacrylamide) (PNIPAM) microgel have been intensively investigated and these studies have provided insightful and fundamental knowledge into the understanding of adsorption of soft particles onto the interfaces and the stabilization mechanism and criteria57,58.In 2010, Brandyetal.reported the directed assembly of yeast cells and obtained microstructures made from yeast cells (Fig.10a)59.It was found that the yeast cells can act as particles and adsorb onto the air-water interface which was locked by polyelectrolytes subsequently.This structure is similar to evolutionary development of multicellularity and it has potential applications in advanced bioreactors and various biotechnology.Skelhonetal.showed that healthier chocolate confectionary can be prepared through silica and chitosan stabilized water-in-oil (w/o) emulsions59.The aqueous phase contains fruit juice and the oil phase contains sunflower oil,molten cocoa butter, and ultimately white, milk, and dark chocolate and in this way the 50% of the fat can be reduced (Fig.10b).Folteretal.first reported the stabilization of Pickering emulsions stabilized by zein protein particles and various factors that influence the stabilized of emulsion were systematically investigated (Fig.10c)61.Currently investigation of zein as stabilizers has become a hot research topic in food development62-64.Nanetal.reported the chitosan-coated alginate particles can be used to stabilize monodisperse emulsion and to obtain colloidosomes for oral insulin delivery65.Tanget al.modified cellulose nanocrystals to make them hydrophobic and suitable for stabilization of oil-in-water (o/w) emulsions (Fig 10)65.The emulsion can be stabilized for over four months and this emulsion is expected to be useful in food, cosmetics and pharmaceuticals.The research interest on bioparticle stabilized dispersion systems is growing, both from academia and industries due to a number of benefits this strategy can offer.Use of Pickering emulsions in foods, cosmetics and even pharmaceuticals are the most promising areas and some of the newly developed ideas have been employed in practical applications.We believe that the continued research in bioparticle stabilized emulsions and foams should have a huge impact on future development of food and cosmetic products.
5.3 Ultralow tension systems stabilized by particles
As discussed in the introduction section of this paper, the driving force that promote the adsorption of colloids at oil-water interfaces is mainly due to the replacement of the interface and thus the reduction of the interfacial energy.For oil-water or gaswater interfaces, the interfacial tension or interfacial energy is quite large compared to the interfacial energy between solidwater or solid oil.In the research community of particlestabilized emulsions, it was previously regarded that stable Pickering emulsions can be formed only when the interfacial energy reduction is adequately high.However, reports on waterin-water and oil-in-oil emulsions are accumulating in the literature13,67,68.To make clarifications, both of these aqueous phases and oil phases are basically incompatible and they are not pure water but usually contain thermodynamically incompatible polymers or the oil phase are immiscible.Solid colloids adsorbed onto interfaces with ultralow tension has long been observed in protein separation studies but it has not caught much attention in the emulsion research communities for decades69.Firoozmandetal.observed that polystyrene colloid particle can adsorb strongly onto the interfaces of aqueous gelatin and oxidized starch during the phase separation process (Fig.11a)70.For these water-water interfaces, the interfacial tension can be as low as 0.001-0.01 mN·m-1which means that the interfacial energy reduction due to colloids adsorption is significantly low compared to oil-water interfaces.The driving force to place the colloids at the interface is the osmotic repulsion between the particles and the nonadsorbed biopolymer molecules and this can lead to the accumulation of the colloids at the interface.This is similar to the conventional depletion phenomena that induce particle aggregation in the presence of noninteracting polymer chains71.Balakrishnanetal.found that solid nanoparticles with a radius of 100 nm can be trapped at two aqueous phases containing dextran and poly(ethylene oxide) (PEO) and stable emulsions can be formed (Fig.11b)72.From the confocal laser scanning microscopy (CLSM) images, it can be clearly seen that the solid nanoparticles are packed at the water-water interface with varying densities.However, the authors argued that the stabilization of the droplets is due to the reduction of interfacial energy even if the interfacial tension is only 10-6N·m-1and the estimate interfacial energy reduction is around 7kBT.The nanoparticle can only delay the phase separation a bit and eventually macroscopic phase separation can take place.Using protein particles as stabilizers, Nguyenetal.were able to stabilize the same water-water system with a stable period of several weeks (Fig.11c)73.It is found that the stability of the w/w (water-in-water) emulsions can be enhanced with increasing interfacial tension which indicates that the interfacial energy still plays a role in the stabilization of w/w emulsions.Furthermore, the use of protein particles makes it possible for this typical w/w emulsions useful in food, cosmetic and life sciences.Using all aqueous emulsion-based system, Cacaceet al.prepared a model w/w emulsions stabilized by lipid vesicles as artificial mineralizing vesicles (AMV) to study the biomimetic mineralization process and it showed that submicrometer amorphous CaCO3particles can be obtained in the microreactors which means that a wide variety of materials can be prepared by this process with changing of the precursors74.Furthermore, the pH-triggered destabilization can also be realized in w/w emulsions stabilized by pH-sensitive microgels and this can lead to the triggered release of this system75.Much more work have been carried out on the dextran and PEO system and now a number of researches on the mechanism and applications have been proposed76-78.Employing an all biomaterial system, de Freitasetal.studied the water-in-water(w/w) emulsions composed of xyloglucan (XG) and amylopectin(AMP) in aqueous solutions stabilized by β-lactoglobulin microgels (βLGm)79.Recently there are increasing research endeavors to use food-derived particles to stabilize w/w emulsions with a hope to develop a better and healthier food design options80-82.Recently, Binksetal.reported the phase inversion behavior of particle stabilized w/w emulsions83.It is shown that dichlorodimethylsilane-modified nanosilica particles with intermediate hydrophobicity can stabilize the w/w emulsions for over a year and catastrophic phase inversion happens by increasing the volume fraction the disperse phase.
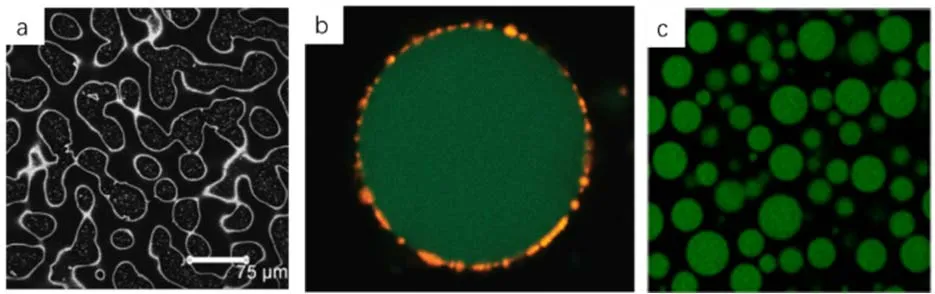
Fig.11 (a) CLSM image showing the stabilization of biopolymer mixtures by polystyrene latex (Reprint from reference 70 with permission, Copyright©2019 American Chemical Society);(b) CLSM image of an w/w emulsion droplet.The green phase is dextran (aq), the PEO (aq) phase is not labelled and the latex particle is in yellow (Reprint from reference 72 with permission, Copyright©2019 American Chemical Society); (c) CLSM image of w/w (dextran/PEO)stabilized by protein particles (Reprint from reference 73 with permission, Copyright©2019 American Chemical Society).
Besides of the w/w emulsions, o/o (oil-in-oil) emulsions can also be stabilized by solid colloids with immiscible oils and here we refer the readers to a recent review by Binksetal.84and we will not go into details in this paper.
5.4 Dry water and liquid marble
Increasing the hydrophobicity of the particle stabilizers, it generally induces a change of the emulsion type form o/w to w/o and this has been well established in the literature85.For gaswater interfaces, hydrophilic particles will stabilize foams and bubbles while it can easily lead to the idea that liquid-in-air type disperse system shall occur when the particles move towards more hydrophobic.When placed onto a dry particle bed of high hydrophobicity, the water droplets will quickly become encapsulated by the particles and this leads to the formation of dry waters or liquid marbles, depending on the size of the encapsulated water droplets35,86,87.In 2001, Aussillous and Quéré reported inNaturea new way to transport small amount of liquid on solid surface without leakage86.It shows that tiny water droplets can be wrapped automatically by hydrophobic particles and the liquid marble shows deformation during fast transportation but leakage is prohibited.In a research paper that follows this seminal paper, the authors investigated a series of properties of liquid marbles such as surface structures, shapes under gravity, effective surface tension, motion under electric and magnetic fields and so on88.Binksetal.showed that silica nanoparticles can stabilize foams and bubbles and dry waters can be obtained when the particles are hydrophobically modified and this has great potentials for on-site release of water or gas components35.Using particles made from perfluoroalkyl particles (oligomeric OTFE) and polymeric tetrafluoroethylene(PTFE), Gaoetal.prepared liquid marbles encapsulating ionic liquids88.Different from water marble which evaporates fast90,91,ionic liquid are nonvolatile and some chemical reactions can be carried out inside the droplets which makes them suitable microreactors.Bormashenkoetal.have carried a series of investigations on the properties and applications of liquid marbles92-94.They first carried out systematic studies on the surface tension or effective surface tension of liquid marbles and liquid marbles can stable for quite a long time over clean water surface but it readily collapses in the presence of oil which they claim could server as pollution indicator.However, this is only a demonstration in the lab but there is no practical use of it at present.Bormashenkoetal.also showed that liquid marbles can serve as micropumps due to difference of Laplace pressure (Fig.12a)95.For two liquid marbles with the same size but stabilized with different particles, the effective surface tension will be different and the water encapsulated will flow from liquid marbles of high pressures to low pressure.Tianetal.reported that liquid marble can be used for gas sensing when the aqueous solution can change color upon absorption of ammonia or hydrogen chloride vapor (Fig.12b)96.Using magnetic particles as stabilizers, Zhangetal.prepared liquid marbles that can be remotely controlled which ca serve as smart microreactors97.Our group prepared liquid marbles stabilized by single layer of silica particles and then carried out reactions inside the marble98.It showed that the particles adsorbed at the interface can be selectively modified by the silver mirror reactions inside the marble and so that the Janus particles can be obtained after the breakup of the liquid marble (Fig.12c).Serranoetal.reported that liquid marbles show high potentials in cryopreservation of mammalian cells99.Arbatanetal.showed blood typing can be easily realized using liquid marble testing material100.A lot of work has been done regarding the use of liquid marble in various fields, especially in the biomedical related areas101.However most of the proposed applications are only lab-bench demonstration and there is still a very long way before real applications.

Fig.12 (a) micropump based the two liquid marbles (Reprint with permission from reference 95, Copyright© AIP Publishing); (b) A liquid marble of CoCl2 shelled with Teflon powder changed colour after being exposed to the vapour of water-based flexographic ink (left).A fresh CoCl2 liquid marble (right) was placed in the same Petri dish for comparison (Reprint from reference 96 with permission, Copyright©Royal Society of Chemistry); (c) Janus particles obtained from partial modification of silica particles at the interface of liquid marbles(Reprint from reference 98 with permission, Copyright©2015 WILEY-VCH Verlag GmbH & Co.KGaA, Weinheim).
6 Conclusion, perspectives and outlook
In this paper, we first reviewed the development of particlestabilized interfaces from a historical survey.In the beginning stage of the research, the phenomena of particle adsorption onto the interfaces and stabilization role of these colloidal particles are well documented and simple models are proposed.Due to the small size of colloidal particles, it is very hard for the researchers to give a quantitative description for decades and only sporadic reports can be found in the literature.In the past decades, we have witnessed an ever-increasing interest, more indepth fundamental researches and endless emerging proposed applications based on particle-stabilized interfaces.Based on the Pickering emulsions as an example, we systematically discuss the stabilization mechanism and a number of experimental factors that influence the stability of the emulsion dispersions.The interactions of particles adsorbed at the interface are briefly discussed and these results should contribute to the understanding of particle-stabilized interface properties, such as low dimensional phase transitions, jamming and self-assembly.One emerging application regarding the use of Pickering emulsions is catalysis and increasing research works are being published in this area.In the past decades, there are booming research efforts that report a great variety of dispersion systems that stabilized by colloidal particles and thousands of papers have been published.To avoid this paper being a lengthy summary of publications, we focus on the mechanism of stabilization when discussing the possible application of these particle-stabilized dispersion systems.Although particlestabilized systems have long been extensively used in human activities, the understanding of why and how the colloidal particles stabilize the interfaces has been the most crucial and fundamental research subject in the past decades.This will continue to be so in the future and it will form the foundation of controlling the stability or instability of various particlestabilized interfaces which is essential to all the applications based on this mechanism.
In the past two decades great progress toward better understanding of particle adsorption onto interfaces has been made and tons of experimental data have been accumulated.However, there still remain several unsolved problems.The contact angle is a crucial parameter in determining the wetting properties of the particle but the precise andinsitudetermination of it still remains unsolved.One possible challenge, at least in our opinion, is to measure the contact angle of adsorbed colloids precisely and to use this value for practical purposes such as stability prediction for emulsions and foams.There are many proposed applications but few of them have come into industrial uses.Except several established industrial processes such as froth flotation in mineral separation, many proposed applications in the literature are only demonstrative.Another challenge and opportunity is the use of the basic principle developed in this field to go to massive industrial production.As far as we can see,particle stabilized system may soon find its place in cosmetics,personal care products, food and beverages as well as other applications such as drug deliveries, catalysis, and coatings/inks and so on should also come into practical uses sooner or later.The understanding of particle’s adsorption onto fluid interfaces is an intriguing research topic and the application of basic principle obtained from the fundamental research will help it develop into more in-depth theoretical understanding of this phenomena.

