Probing Molecular Structures of Antifouling Polymer/Liquid Interfaces In Situ
Chengcheng Zhang , Ralph Crisci , Zhan Chen
Department of Chemistry, University of Michigan, Ann Arbor, Michigan 48109, USA.
Abstract: Marine organisms such as plants, algae or small animals can adhere to surfaces of materials that are submerged in ocean.The accumulation of these organisms on surfaces is a marine biofouling process that has considerable adverse effects.Marine biofouling on ship hulls can cause severe fuel consumption increase.Investigations on antifouling polymers are therefore becoming important research topics for marine vessel operations.Antifouling polymers can be applied as coating layers on the ship hull, protecting it against the settlement and growth of sea organisms.Polyethylene glycol (PEG) is a hydrophilic polymer that can effectively resist the accumulation of marine organisms.PEG-based antifouling coatings have therefore been extensively researched and developed.However, the inferior stability of PEG makes it subject to degradation, rendering it ineffective for long-term services.Zwitterionic polymers have also emerged as promising antifouling materials in recent years.These polymers consist of both positively charged and negatively charged functional groups.Various zwitterionic polymers have been demonstrated to exhibit exceptional antifouling properties.Previously,surface characterizations of zwitterionic polymers have revealed that strong surface hydration is critical for their antifouling properties.In addition to these hydrophilic polymers, amphiphilic materials have also been developed as potential antifouling coatings.Both hydrophobic and hydrophilic functional groups are incorporated into the backbones or sidechains of these polymers.It has been demonstrated that the antifouling performance can be enhanced by precisely controlling the sequence of the hydrophobic-hydrophilic functionalities.Since biofouling generally occurs at the outer surface of the coatings, the antifouling properties of these coatings are closely related to their surface characteristics in water.Therefore,understanding of the surface molecular structures of antifouling materials is imperative for their future developments.In this review, we will summarize our recent advancements of antifouling material surface analysis using sum frequency generation (SFG) vibrational spectroscopy.SFG is a surface-sensitive technique which can provide molecular information of water and polymer structures at interfaces in situ in real time.The antifouling polymers we will review include zwitterionic polymer brushes, mixed charged polymers, and amphiphilic polypeptoids.Interfacial hydration studies of these polymers by SFG will be presented.The salt effect on antifouling polymer surface hydration will also be discussed.In addition, the interactions between antifouling materials and protein molecules as well as algae will be reviewed.The above research clearly established strong correlations between strong surface hydration and good antifouling properties.It also demonstrated that SFG is a powerful technique to provide molecular level understanding of polymer antifouling mechanisms.
Key Words: Antifouling materials; Sum frequency generation vibrational spectroscopy; Interfacial hydration;Zwitterionic polymer; Salt effect; Protein interaction
1 Introduction
Biofouling can cause a variety of issues related to applications of biomedical implants, marine antifouling coatings,biomolecule separation membranes, etc.Extensive researches have been performed to develop fouling-resistant materials to prevent biofouling1-4.Many different kinds of polymers have been designed and synthesized for antifouling purposes,examples of which include super-hydrophobic polymers,hydrophilic polymers, and amphiphilic polymers.A hydrophilic or amphiphilic material’s applicability as an antifouling coating has been proven to be linked to its strong surface hydration―this strong hydration plays a key role in the repulsion of protein and other biological molecules to adsorb5-7.For example,poly(ethylene glycol) (PEG) based materials have been widely used and demonstrated to be excellent antifouling materials with low protein and cell adsorption8.It has been shown that PEG materials can be strongly hydrated.Even though PEG materials exhibit excellent antifouling activity, they do have some weaknesses such as easy degradation at high temperatures9.
In recent years, extensive research has demonstrated that zwitterionic polymer materials are excellent candidates for antifouling coatings10-13.Zwitterionic materials, even though overall are neutral, have positively and negatively charged functionalities on their surfaces, which can bind water molecules extremely strongly.The strength of this binding makes it difficult for biological molecules such as proteins and biological systems such as cells and marine organisms to replace these water molecules at the surface, therefore preventing them from sticking14-19.In this review, we will discuss recent researches into surface hydration of common zwitterionic materials such as polysulfobetaine and polycarboxybetaine as well as recent developments in mixed charged polymers15,16,20,21.
In addition to incorporating zwitterionic components, many other polymers can also achieve antifouling properties through special designs22-25.The properties of polymeric materials are largely determined by their sequences26.Incorporation of different functionalities into the polymer structure will usually yield different properties27,28.Therefore, precise control of polymer sequence has garnered various research interests in recent years29.Amphiphilic polymers have been the focus of numerous recent research interests, because of the simultaneous incorporation of hydrophobic and hydrophilic components into such polymers30.Commonly encountered hydrophilic and hydrophobic entities are poly(ethylene glycol) and poly(dimethylsiloxane) or fluorinated functionalities respectively3,31,32.
Among the emerging classes of such specifically controlled polymers, polypeptoids (N-substituted glycines) have been developed, targeting their applications as antifouling coatings33-35.Polypeptoids are very accessible materials through easy synthetic methods.They possess great functionality diversity and biological compatibility.Sequences with varied hydrophilicities can be systematically incorporated into the backbones or sidechains of polypeptoids, making the entire polymer amphiphilic36,37.Studies have shown that this amphiphilic property can effectively enhance the antifouling performance of polymer coatings38-41.Precise control of length scales of hydrophilic and hydrophobic components is critical for the success of polypeptoids as antifouling materials.It was found that the surface hydration of polypeptoid materials can be well controlled by the variation of the hydrophilic and hydrophobic components in the polypeptoids41,42.
In this article, we will summarize our recent research on surface hydrations of zwitterionic polymers, mixed charged polymers, and amphiphilic polymers such as polypeptoids.We applied a nonlinear optical vibrational spectroscopy, sum frequency generation (SFG) vibrational spectroscopy, to examine polymer/liquid interfacesinsituin real time.Understanding of the interfacial molecular behavior and mechanisms of these polymer materials in aqueous environments is necessary for the development of antifouling materials with improved performance.
2 Sum frequency generation vibrational spectroscopy
SFG is a second-order nonlinear optical process which combines two input photons to generate an output photon with the sum frequency (or sum energy) of the two input photons43,44.For our SFG experiments, two input optical beams: a visible beam with a fixed wavelength of 532 nm and a wavelength tunable infrared (IR) beam are spatially and temporally overlapped at a surface/interface of interest, to generate the SFG signal beam.This output SFG beam is then collected and analyzed45.Due to the selection rule of a second-order nonlinear optical process, SFG signal can only be generated from a medium with no inversion symmetry.Most bulk materials have inversion, but surfaces and interfaces do not.Therefore, usually SFG is intrinsically surface and interface selective with submonolayer sensitivity46,47.Additionally, SFG experiments can be carried out under ambient conditions, without the requirement of high vacuum.It can therefore access solid/liquid interfacein situ, monitoring the interfacial change of antifouling coatings in real time (Fig.1)48-51.
3 Zwitterionic polymers
3.1 Interfacial hydration
Recently, we have investigated surfaces of zwitterionic polymer brushes in different chemical environments, such as air,water, salt solutions, and protein solutions.Surfaces of these polymer brushes are of interest due to their potentials for antifouling coating design using such polymers.Theinsitustudy of these surfaces could provide important information regarding their antifouling capabilities under a variety of conditions,leading to the better design of polymers with improved antifouling performance.The molecular formulae of the investigated zwitterionic and mixed charged polymers are illustrated in Scheme 1.
The zwitterionic polysulfobetaine methacrylate (pSBMA) and polycaroboxybetaine acrylate (pCBAA1 and pCBAA2)15,16were investigated with SFG at the polymer brush/air and polymer brush/water interfaces (Fig.2).In the SFG spectra collected from the polymer brush surfaces in air, each of the samples has SFG peaks at 2845 and 2920 cm-1, which are contributed by the symmetric and asymmetric stretching of the methylene groups in the side chains and/or backbones.The strong SFG signals from the zwitterionic polymer surfaces in air indicate that the polymer brushes are well ordered in air.Upon contact with water, the new peaks at 2985 and 2950 cm-1appeared in the SFG spectra collected from the polymer/water interface.The SFG signal change from the polymer/air interface to polymer/water interface indicates a likely reorientation of the polymer surface upon exposure to water, which was observed from SFG studies on other polymers as well44,45.

Fig.1 (a) Energy level diagram for an SFG process;(b) SFG experimental designs of two optical geometries.Top: prism geometry; bottom: window geometry.

Scheme 1 Molecular formulae of the zwitterionic polymer brushes (A)and mixed charged polymers (B) reviewed in this article.
In addition to the C―H stretching signals from the polymer brushes, the SFG signal centered at 3180 cm-1from the ordered interfacial water O―H stretching was observed at the polymer brush/water interface (Fig.2B)15.Previous research on the SFG water spectrum reveals that interfacial water has O―H stretching peaks center at 3200 and 3400 cm-1, which are associated with strongly and weakly hydrogen bonded water respectively.The dominating 3180 cm-1SFG signals observed here indicate the water molecules are strongly hydrogen bonded at the zwitterionic brush/water interface for all the three zwitterionic polymer materials.This strongly hydrogen bonded water layer is key for antifouling behavior.
Besides zwitterionic polymers, mixed charged polymer brushes were also of interest as a potential antifouling coating option, which are easier and cheaper to prepare compared to zwitterionic polymers.We investigated mixed charged polymer brushes with different ratios of positive quaternary amine and negative sulfonate compositions to determine the trend between charges and interfacial hydration, and therefore potential antifouling behavior (Fig.3)20.We also examined the interfacial hydration of these polymers at different pH values.Based on the water signals in the SFG spectra collected from the mixed charged polymer/water interfaces, only the 1 : 1 mixed charge polymer had a significantly strong 3200 cm-1(strongly hydrogen bonded) peak without a coupled weak 3400 cm-1(weakly hydrogen bonded) shoulder peak.The presence of the dominating 3200 cm-1peak indicates the presence of a large amount of strongly hydrogen bonded water at the interface,which we have previously shown is an indicator of strong antifouling behavior for polymer surfaces14-16,20.Upon exposure to different pH solutions, there is minimal change in the SFG water signal of the 1 : 1 mixed charged polymer, indicating that this polymer brush is a promising candidate for future research and development for antifouling20.

Fig.2 SFG spectra of pCBAA1, pCBAA2, and pSBMA in air (A) and water (B).

Fig.3 SFG spectra collected from the interfaces between mixed charged polymers with different charge ratios and solutions with pH at 5, 7, and 9.
We further compared interfacial hydration of different zwitterionic polymer brushes.Specifically, we compared the water orientations at the pSBMA and polycaroboxybetaine methacrylate (pCMBA) polymer/water interfaces to further elucidate the mechanism of their antifouling behavior21.The SFG study resulted in the conclusion that water molecules on the pSBMA and pCMBA surfaces have different absolute orientations at a neutral pH (Fig.4).The absolute orientations were determined by monitoring SFG signals at different pH values (see details below), confirmed through phase sensitive SFG measurement, which accesses the phase component ofχ(2).By accessing this information, we can determine the absolute orientation (i.e.does the dipole point up or down?) of the interfacial water molecules.From the results of these experiments we learned that at the pCBMA interface, the water molecules point their oxygen atoms towards the interface (Fig.4C).Oppositely, at the pSBMA interface, the water molecules point their hydrogen atoms towards the interface (Fig.4D)21.
SFG spectra were collected from the interface between pCBMA and water with various pH values (water at pH = 2 or pH = 12 is actually a dilute HCl or NaOH solution while we refer these two solutions to water for simplicity).The SFG signal change as a function of time upon pH change of the water(solution) in contact with pCBMA was also monitored21.At pH =2 and 12, solutions are still very dilute, therefore we believe that pCBMA should have a similar surface structure or similar SFG C―H signals.Based on the SFG signals in the C―H stretching frequency regime obtained at different pH values, we can see that the C―H signals from pCBMA are similar at pH = 2 and 7.At pH = 2, the pCBMA surface has majority of ―COOH groups instead of COO-groups.Therefore, the pCBMA surface is positively charged due to the surface quaternary amine groups.At pH = 2, the water molecules at the pCBMA interface must have their oxygen atoms face the pCBMA surface.The SFG C―H stretching signals are similar at pH of 2 and pH of 7 cases,indicating that they have similar interference with water O―H signals.Therefore, at pH = 2 and 7, the water O―H stretching signals should have the same phase.That is, the oxygen atoms in water molecules face towards the pCBMA surface at the pCBMA/water (pH = 7) interface.At pH = 12, the pCBMA surface is dominated with ―COO-groups instead of ―COOH groups.Since ―COO-groups have stronger water binding ability compared to the quaternary amine groups, the hydrogen atoms in water molecules face towards the pCBMA surface at pH of 12.This is the reason why C―H signals at pH of 12 have different interference patterns with water compared to those of pH of 2 and 7.Fig.5C shows the SFG signal changes at 3200 cm-1collected from the interface between pCBMA and water as a function of time, before and after the aqueous phase (pH = 12)in contact with pCBMA was replaced by water (pH = 7).At pH of 12, strong O―H signals were detected.As we mentioned,hydrogen atoms in water molecules face the pCBMA surface.When water with pH of 12 was replaced with water with pH of 7, water molecules at the interface started to change their orientations to have their oxygen atoms face the pCBMA surface.The overall SFG O―H signal intensity decreased as a function of time.When half of the water molecules changed the absolute orientation, 50% water molecules faced the pCBMA surface with oxygen atoms, while the other 50% faced the pCBMA surface with hydrogen atoms, leading to the disappearance of the SFG O―H stretching signal, which was observed21.
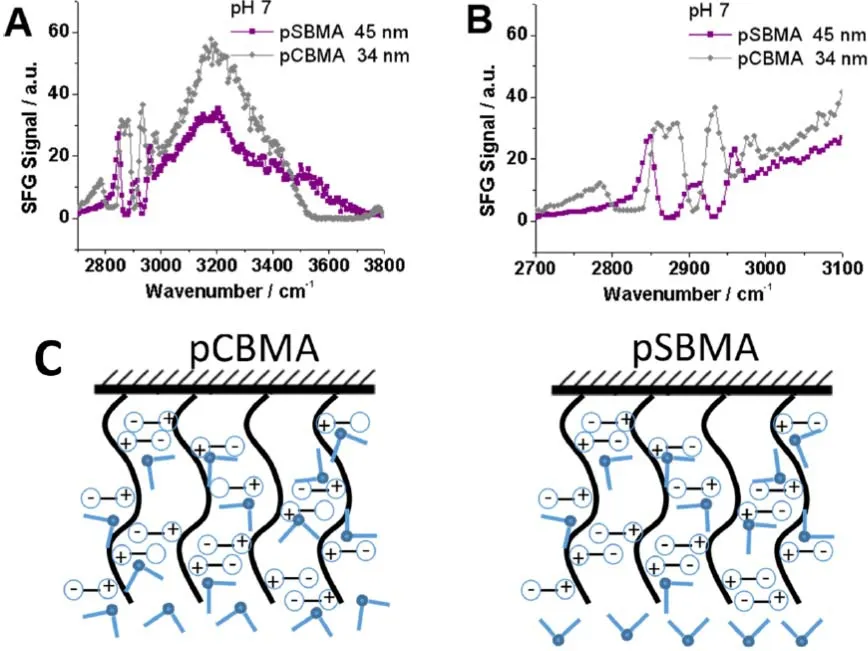
Fig.4 (A) SFG spectra collected from two zwitterionic polymers, pCBMA and pSBMA, in water (pH ~7).(B) Replotted SFG spectra in the C―H stretching frequency range from (A).(C) Schematic plots showing that water molecules have different absolute orientations on the pCBMA and pSBMA surfaces.
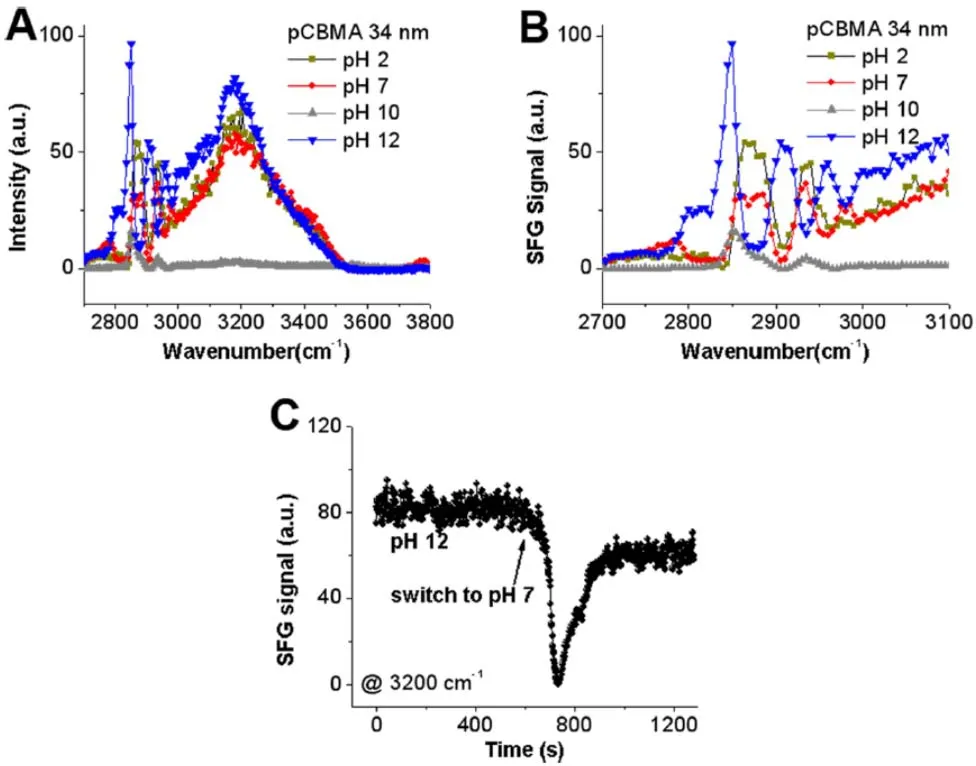
Fig.5 (A) SFG spectra collected from the interfaces between pCBMA and water with different pH values.(B) Replotted SFG spectra in the C―H stretching frequency range from (A).(C) Time-dependent SFG signal intensity (detected at 3200 cm-1) from the interface between pCBMA and water before and after the contacting aqueous phase (pH = 12) was changed to water (pH = 7).
Similar experiments were then performed with pSBMA to compare the differences between the functional COOH and SO3H group behaviors21.With the pSBMA, we did not observe the similar different interference patterns between C―H and O―H signals at different water pH values (Fig.6A).The timedependent SFG signals did not decrease when water with pH of 12 in contact with pSBMA was replaced with water with pH of 7 (Fig.6B).SO3H is a strong acid, therefore at different pH values, the surface is always dominated by SO3-groups instead of SO3H groups.Since SO3-groups bind water more strongly than quaternary amine groups, the hydrogen atoms in water face the pSBMA surface at various water pH values, including the pH of 7 case.Therefore, at pH of 7, water molecules have opposite average absolute orientations on pCBMA and pSBMA.Here we can relate the orientation of water molecules to the pKaof the acid group in the zwitterion.
3.2 Salt effect

Fig.6 (A) SFG spectra collected from the interface between pSBMA and water at different pH values.(B) Time-dependent SFG signal intensity(detected at 3200 cm-1) from the interface between pSBMA and water before and after the contacting aqueous phase (pH = 12) was changed to water (pH = 7).
Many antifouling materials are designed to be used in marine environment, where sea water has a high salt concentration.In addition to studies of hydration at these zwitterionic polymer/water interfaces, we have investigated the effects common salts have on the interfacial hydrations of these polymers15.Particularly, we examined how different monocations Na+and K+in the forms of chloride salts NaCl and KCl and dications Mg2+and Ca2+influence the surface hydration of pSBMA, pCBAA1 and pCBAA2 (Fig.7)15.Upon exposure to these salts, all three zwitterion brushes exhibited a significant loss of surface hydration, and therefore most likely a significant loss of antifouling efficacy.Interestingly, these zwitterion brushes responded to the salts differently.The surface hydrations of pCBAA1 and pCBAA2 brushes were more significantly disrupted by NaCl than KCl, while the surface hydration of pSBMA brushes was more significantly disrupted by the KCl than the NaCl.This means that different functional groups and differently designed zwitterions will respond differently to different monocation salts.However, all three of the brushes were more significantly disrupted by CaCl2than MgCl215.
We also compared the effect of salt on the interfacial hydration on the 1 : 1 mixed charged polymer with that of pSBMA20.In these tests we can see that the mixed charged polymer outperforms the pSBMA upon exposure to 0.02, 0.1 and 0.5 mol·L-1NaCl solutions (Fig.8)20.It is also significant that both pSBMA and the mixed charged polymer are able to recover their surface hydration after exposure again with water.An important point to note is that the mixed charged polymer exhibits a signal enhancement of surface hydration after changing from salt solution to water.It is believed this enhancement is due to trapping of ions within the mixed charged polymer.These trapped ions lead to an increase in order of the polymer and therefore an increase in order of the water at the surface.It is important to investigate salt effect on surface hydration for the above potential antifouling coating materials, as the coatings will have to perform in sea water conditions.

Fig.7 SFG spectra of (A, D) pCBAA1, (B, E) pCBAA2, and (C, F) pSBMA in contact with (A–C) NaCl or KCl solutions and (D–F) MgCl2 or CaCl2 solutions.The NaCl solutions were prepared with various concentrations.Inset in panel C: enlarged portion of the water spectra in panel C for a clear comparison.(G) Corresponding normalized H2O signal intensities.

Fig.8 SFG spectra collected from the interfaces between the 1 : 1 mixed charged polymer or pSBMA and water or NaCl solutions.
In addition, we investigated the time dependent changes at the zwitterionic polymer (pCBMA and pSBMA)/0.2 mol·L-1NaCl solution interfaces21.We were able to observe that upon replacing water in contact with the polymer (pCBMA or pSBMA) with 0.2 mol·L-1NaCl solution, SFG water signal reduces, indicating the loss of ordering of the water molecules at the pCBMA and pSBMA surfaces.However, as time passes, the pCBMA polymer recovers some SFG water intensity and therefore some amount of ordering of the water molecules at the surface (Fig.9A, D).It is believed that this happens because after the salt rushes to the surface and disorders the interfacial water,the polymer reorients and then water is able to again bind with the surface.These results are supported by the changes of the CH region of the spectra taken at different times during the CBMA salt exposure.(Fig.9A).Also later water spectrum (Fig.9A) has different spectral features compared to the original spectrum collected from pCBMA/water interface (Fig.5A), showing that interfacial water hydration changed substantially because of the salt effect.Different from the pCBMA behavior, the pSBMA does not observe the time-dependent water signal change, as the SFG spectrum is identical at the 5 minute and 75 minute time marks (Fig.9C).This shows that the effect of salt on the change of pSBMA surface hydration is much faster, occurred immediately21.
The above studies clearly indicate that salt molecules can reduce surface hydration of zwitterionic polymers and mixed charged polymers, showing that these polymers may lose antifouling capability in marine environment.Recently, new zwitterionic polymers have been developed, and we found that some newly synthesized zwitterionic polymers can prevent surface hydration loss induced by salt exposure, which will be published in the future.
3.3 Protein Interactions
Marine organisms are known to secrete adhesive proteins to adhere to immersed surfaces in sea water52-56.Therefore, the antifouling property of the newly designed coatings can be mediated by their interactions with proteins.Protein adsorption can be probed by SFG signal change at the coating surface.The excellent surface/interface sensitivity enables SFG to detect submonolayer protein coverage, which usually is difficult by using regular IR spectroscopy.We have applied SFG to investigate the interactions between pSBMA and three model protein molecules(Fig.10)14.Fig.10 shows that the symmetric stretching and asymmetric stretching modes of methylene group at 2845 and 2920 cm-1respectively were detected at the pSBMA/air interface (black spectrum, Fig.10A).The signals might come from either the backbone or the sidechains of pSBMA polymer brush.The same sample was then subject to BSA, lysozyme and fibrinogen protein solution contact respectively.After brief water rinsing, the sample was dried and analyzed again by SFG in air.Very similar spectra were obtained after the three protein contact experiments (red, blue and pink spectra in Fig.10A),indicating that no protein residues remained at the pSBMA surface.This result demonstrates the antifouling property of the pSBMA coating14.

Fig.9 (A) After the contacting water was replaced by a 0.2 mol·L-1 NaCl solution, SFG spectra were collected from the interface between pCBMA and 0.2 mol·L-1 NaCl solution at different time points.(B) Time-dependent SFG signal intensity (detected at 3200 cm-1)from the interface between pCBMA and solution before and after the contacting water was changed to 0.2 mol·L-1 NaCl solution.(C) Similar SFG spectra were observed from the interface between pSBMA and 0.2 mol·L-1 NaCl solution at 5 and 75 min after the contacting water was changed to the 0.2 mol·L-1 NaCl solution.(The spectra collected between 5 and 75 min are not shown because they have the same O―H stretching signal.) (D) Time-dependent SFG signal intensity (detected at 3200 cm-1) before and after water in contact with pSBMA was replaced by the 0.2 mol·L-1 NaCl solution.
To gain more insights into polymer coating/protein interactions, pSBMA/protein solution interfaces were also studiedinsitu.Time-dependent SFG analysis was conducted to monitor water molecule signal change before and after replacing water contacting the pSBMA with protein solutions (as proteins approached pSBMA surface, Fig.10B)14.It was revealed that the interfacial water SFG signal was not affected by protein interactions, indicating that the strongly hydrogen-bonded water molecules at pSBMA surface can effectively resist protein molecules’ disturbance.The full spectra at the pSBMA/protein solution interfaces were also collected and shown in Fig.10C,demonstrating the same conclusion.This study further confirms that the strong hydration on pSBMA surface is the key for antifouling.
The same experiments were repeated for a 1 : 1 mixed charged polymer surface20.As shown in Fig.11, SFG spectra were collected from polymer/water interface (black), polymer/protein solution interface (red) and then polymer/water interface again after water rinsing (blue).Similar to the pSBMA coating, BSA and fibrinogen did not substantially perturb mixed charged polymer surface structure.The spectra feature also almost completely recovered after protein solution contact and water washing, showing the antifouling property of the mixed charged polymer.However, when this polymer was subject to contact with lysozyme solution, an obvious shoulder peak at 3300 cm-1was detected, which is assigned to the N―H vibrational mode of lysozyme molecules at the interface.Additionally, slight spectral change was observed after lysozyme contact and water rinsing, indicating irreversible protein adsorption at the polymer surface.This indicates that the mixed charged polymer surface did not completely recover after lysozyme contact, different from the previous result collected for the zwitterionic pSBMA surface.We believe that this variance was caused by the slight positive-negative charge imbalance that existed in the mixed charge polymer.XPS analysis of this coating showed that the ratio of the positively and negatively charged components is 0.97 : 1, which made the overall polymer negatively charged.Positively charged lysozyme in the solution could therefore preferably interact with the mixed charged polymer, making resisting this protein more difficult.
4 Polypeptoid

Fig.10 (A) SFG signals of pSBMA collected in air before and after contacting proteins.(B) Time-dependent water SFG signals as the contacting aqueous phase was switched from water to protein solutions at 200 s.(C) SFG signals of pSBMA/water and pSBMA/water and pSBMA/protein solution interfaces.

Fig.11 SFG spectra collected from the mixed charged polymer/water and polymer/protein solution interfaces.(a1), (b1) and (c1) are the beginning mixed charged polymer/water interface SFG spectra.(a2), (b2) and (c2) are polymer/protein solution interface spectra for BSA, fibrinogen and lysozyme respectively.(a3), (b3) and (c3) are polymer/water interface spectra collected again after protein contact and washing.
The antifouling properties of polypeptoid coatings, similar to other materials discussed above, are largely dependent on their surface structures in water.For sequence-dependent polymers such as polypeptoids, surface/interface characterization can provide more insights into their sequence-property relationships.We therefore applied SFG to study sequence-specific polypeptoids to gain molecular understandings of their surfaces in waterinsitu57.The molecular formulas of the analyzed polypeptoids are shown in Fig.12A.The amphiphilicity of these polypeptoids was tuned by varying the sidechain sequences:hydrophilic poly(ethylene glycol) (Nme, green) and hydrophobic perfluorinated carbon chain (NF, yellow) moieties were differently arranged into seven different sequences.
To study the interfacial behavior of different polypeptoids, we carried out SFG studies on these polymers in water,supplemented by contact angle measurements.Time-dependent water contact angle measurement was conducted to monitor the surface restructuring of polypeptoids from air to water (Fig.12B)57.For 5mer and 15mer-1NF, which contain only one NF unit, very fast contact angle decrease with time was observed(Fig.12B).This is correlated to their previously reported faster reorganization and crystallization rates.This fast surface reorganization behavior is well correlated to the fouling-release property of the polypeptoid surfaces.Fig.13 shows that the spore removal percentages on these two surfaces are much higher than other surfaces (Fig.13, green curve).Other surfaces indeed have much slower time-dependent contact angle changes(Fig.12B).

Fig.12 (A) Molecular structures of the polypeptoid samples studied here.(B) Time-dependent water contact angles at polypeptoid sample surfaces.(C) SFG signal intensity of strongly hydrogen-bonded water at 3200 cm-1 collected from polypeptoids/water interfaces.

Fig.13 Correlation between SFG signals and antifouling/fouling release properties of the polypeptoid samples.
15mer-1NF, 15mer-2NF, and Seq1 which contain one, two,and three NF units at outer edge of the sequence respectively,show very different water contact angles: The more NF units, the higher the measured water contact angle57.This is reasonable because the NF unit is hydrophobic, meaning more NF units in the molecule led to a more hydrophobic surface which results in a higher water contact angle.This trend is well correlated to the SFG water signal intensity57.The SFG water intensity collected from the 15mer-1NF/water interface is higher than that from the 15mer-2NF/water interface, which are both higher than that from the Seq1/water interface.This shows that 15mer-1NF has the strongest hydration.This trend also matched the antifouling performance of these three polypeptoids.Fig.13 shows that Seq1 has the highest spore attachment amount.We then compare the polypeptoids Seq1, Seq2, and Seq3, when the three NF units are moving from the edge to the middle positions of the side chain.The water contact angles measured decreased from Seq1 to Seq2 to Seq3, showing that NF units at the edge enabled the surface to be more hydrophobic, while the NF units inside the chain led to a more hydrophilic surface.This observation is also well correlated to SFG data and antifouling behavior.The SFG water signal intensity observed from the Seq1/water interface is weaker than that from the Seq2/water interface than that from the Seq3/water interface, showing that the Seq3 surface has the strongest hydration in water while the Seq3 surface has the weakest hydration57.Fig.13 shows that among Seq1, Seq2, and Seq3, Seq1 has the highest spore attachment (worst antifouling activity), while Seq3 has the lowest spore attachment (best antifouling activity).This research demonstrated the similarly to the hydrophilic materials discussed above, for amphiphilic materials strong hydration leads to better antifouling.This study also indicates that surface reorganization rate is correlated to the fouling-release performance.In addition, it is clearly shown that it is feasible to control the antifouling behavior of polypeptoids by controlling the surface hydration through manipulating the peptoid sequence.
In addition to the hydrophilicity (amphiphilicity) of the polymer coating surfaces, hydrogen bonding also plays an important role regarding polymer-biological system interactions,and therefore in the antifouling property of the polymer coating.To gain fundamental understanding of the effect of the surface hydrogen bond formation capability on the antifouling/foulingrelease activity, SFG analysis was carried out to characterize the interfacial behavior of two polypeptoid samples, one with hydrogen-bonding functionality and the other without it42.As shown in Fig.14A, the sample (HB) which can form interfacial hydrogen bonds contains a hydrogen-bonding-donating amide group, while the non-hydrogen-bonding formation sample(NHB) contains a N-substituted peptoid group.The other parts of the two polymers are identical.The water contact angle of HB and NHB were determined to be very similar, indicating that the surface energies of the two coatings are very similar.Despite of their slight structural and minimal surface energy differences,SFG spectra collected from these two polymers in water exhibit drastic differences (Fig.14C)42.The SFG water signal collected from the HB/water interface is substantially stronger than that collected from the NHB/water interface, dominated by a peak centered at ~3150 cm-1.This indicates that water molecules are much more ordered at the HB surface, due to the hydrogenbonding formation between water and HB.On the other hand,the removal of this hydrogen bonding formation functionality on the NHB surface makes NHB-water interaction very weak without hydrogen bonding formation, leading to much less ordering of the interfacial water molecules42.The interfacial hydration analysis of these polypeptoid coatings can be correlated with their different antifouling performances against algae attachment.As shown in Fig.14B, HB has substantially less settledU.linzaamount, showing better antifouling activity than NHB.Again, the results here show that strong surface hydration leads to better antifouling properties of polypeptoid coatings.

Fig.14 (A) Molecular structures of the PDMS-based block copolymers, incorporating either hydrogen-bonding peptoids or nonhydrogen-bonding peptoids.(B) Biofouling assay settlement data for U.linza on different polypeptoid sample surfaces.(C) SFG spectra collected from hydrogen-bonding peptoid/water interface and nonhydrogen bonding peptoid/water interface.
5 Conclusions
In this article we summarized our recent SFG studies on novel antifouling materials, including zwitterionic polymers, mixed charged polymers and polypeptoid polymers.Particularly, we focused on the surface hydrations of these polymers in water.Even though structures of such polymers are very different, we found that strong surface hydration is the key for antifouling.Zwitterionic polymers and the 1 : 1 mixed charged polymers exhibit strong surface hydration, leading to their antifouling capability.For polypeptoids, surface hydration probed using SFG can be well correlated to their antifouling activity -Stronger surface hydration is well correlated to better antifouling performance.Therefore, for amphiphilic and hydrophilic polymers, we believe that to design a good antifouling coating,it is necessary to ensure that the polymer material has strong surface hydration.
Here we also showed that salt can reduce the surface hydration of zwitterionic polymers and the 1 : 1 mixed charged polymer by surface charge screening, which may induce loss of antifouling capability of such materials in the marine environment.Therefore, it is necessary to design zwitterionic or mixed charged polymers which can bind water more strongly to prevent hydration loss induced by salt.Interestingly, it was found that protein molecules cannot disrupt the strong hydration on the pSBMA surface, but lysozyme can influence the surface hydration of the 1 : 1 mixed charged polymer surface, possibly due to the imbalance of the surface charge.We also found that surface hydrations on polypeptoid surfaces are well correlated to their antifouling performance, similar to those of zwitterionic and mixed charged polymers.The surface reorganization capability and dynamics measured using contact angle goniometer can be correlated to the fouling release ability of polypeptoids.Such information revealed in this study provides important knowledge for developing antifouling and foulingrelease coatings using polymers.
It is worth noting that the SFG spectroscopy can be used to probe solid/liquid interfacesinsituin real time.The above conclusions were obtained directly from polymer/water or aqueous solution interfaces.Suchtcharacterization capability makes SFG an important and powerful tool to study surface hydration of various polymer materials, providing important understanding on the mechanisms of fouling and antifouling.We believe that SFG results can be used to guide the design and development of advanced polymer coating materials.SFG studies can also facilitate the general evaluation of antifouling products in practical applications.

