Current protocols and outcomes of ABO-incompatible kidney transplantation
Maurizio Salvadori,Aris Tsalouchos
Abstract One of the principal obstacles in transplantation from living donors is that approximately 30% are immunologically incompatible because of the presence in the recipient of antibodies directed against the human leukocyte antigen system of the donor or because of the incompatibility of the ABO system.The aim of this review is to describe the more recent data from the literature on the different protocols used and the clinical outcomes of ABO-incompatible kidney transplantation.Two different strategies are used to overcome these barriers:desensitization of the recipient to remove the antibodies and to prevent their rebound after transplantation and the exchange of organs between two or more pairs.The largest part of this review is dedicated to describing the techniques of desensitization.Even if the first reports of successful renal transplantation between ABO-incompatible pairs have been published by 1980,the number of ABO-incompatible transplants increased substantially in this century because of our improved knowledge of the immune system and the availability of new drugs.Rituximab has substantially replaced splenectomy.The technique of apheresis has improved and more recently a tailored desensitization proved to be the more efficient strategy avoiding an excess of immunosuppression with the related side effects.Recent reports document outcomes for such transplantation similar to the outcomes of standard transplantation.
Key words:ABO-incompatible transplants;Desensitization strategies;Immunological aspects;B cell depletion;Immunomodulation;Apheresis techniques;Kidney paired donation
INTRODUCTION
Renal transplantation is considered the best therapy for patients affected by end-stage renal disease.
To date,the major problems to receiving renal transplantation,in addition to cadaveric renal donor shortage,are that many patients on the wait list have problems because of advanced age,immunological reactivity,or issues related to surgery or cardiovascular disease.For such patients,to avoid a long time on dialysis,transplantation from living donor is the best option.However,approximately 30% of such transplantations are considered incompatible from the immunological point of view either because of the presence in the recipient of antibodies directed against the human leukocyte antigen (HLA) antigens of the donor or because of the incompatibility of the ABO system between the donor and recipient or because of both[1].
There are two principal strategies to overcome these barriers:(1) To desensitize the recipient to remove the antibodies and modify the immunological status,allowing the transplant;and (2) To exchange the organs between two or more pairs to exchange the organs between different donors.
The aim of this review is to give a general overview of ABO incompatibility (ABOi),the techniques used to overcome ABOi,the removal of the immunological barriers,and the outcomes obtained.In the last part of the review,kidney paired donation(KPD) will also be briefly discussed.
IMMUNOLOGICAL ASPECTS
The antigens of the ABO system are glycoproteins expressed on erythrocyte membranes as well as on epithelial and endothelial cells.At the renal level,these glycoproteins are also expressed on the collecting and distal tubules and on the vascular endothelial cells.The intensity of antigen expression on the tissues is also related to the acute rejection rate in patients with low isoagglutinin levels in the blood[2].
The ABO blood groups consist of four categories (A,B,AB and O).The formation of anti-blood group antibodies occurs against the antigens that are not native to the host.In addition,group O individuals have higher antibody titers to both the A and B antigens.As a consequence,recipients of blood type O have a higher incidence of antibody-mediated rejection (ABMR) after transplantation[3].
In addition to the membrane-linked antigens,circulating epitopes A and B,both soluble and linked to von Willebrand factor exist in some patients.These antigens,when free,are responsible for ABMR[4].Indeed,better results in transplantation involving ABOi are obtained when transplanting kidneys from A2donors to O recipients.The A2subtype does not have circulating antigens linked to von Willebrand factor[5].
The reaction of isoagglutinins with the antigens of the ABO group induces complement activation,as documented by the presence of C4d[6].
In addition to its relation with the development of ABMR,the presence of C4d on the tissues may also be associated with the development of chronic rejection[7,8].
DEVELOPMENT OF THE PRACTICE OF KIDNEY TRANSPLANTATION IN ABO-INCOMPATIBLE PAIRS
For a long time,ABOi has been considered an absolute contraindication to kidney transplantation.
For the first time,Bryngeret al[9]reported the clinical outcomes of 21 renal transplantations from A2donors to O recipients.Since then,several authors have tried to perform kidney transplantations in pairs with ABOi[10-13].
Principally after 1998,there was a worldwide increase in the rate of kidney transplantations from living donors that involved ABOi.
This fact may be principally ascribed to four factors.(1) Since 1998,our knowledge of the diagnosis and treatment of ABMR has substantially improved.(2) By the beginning of 2000,Japanese authors published excellent results in renal transplantations involving ABOi[14],although the main limitation of the Japanese strategy was the splenectomy associated with their pretransplantation protocol.(3)Later,Johns Hopkins University and the Mayo Clinic in the United States documented the possibility of performing such transplantation without splenectomy with the administration of an anti-CD20 monoclonal antibody (rituximab [RTX])[7,15].(4) Finally,Swedish authors developed a new technique that demonstrated outcomes in renal transplantation involving ABOi that were similar to the outcomes of standard renal transplantation[16-19].
The main steps in the evolution of desensitization are presented in Figure 1.
TECHNIQUES OF ABO DESENSITIZATION AND DIFFERENT PROTOCOLS USED OVER TIME
The most frequent protocols applied to obtain desensitization in pairs with ABOi are a mixture of the following strategies.
Removal of circulating ABO antibodies
The removal of circulating ABO antibodies is obtained principally by plasmapheresis or immune-adsorption (IA).The aim of these strategies,principally applied several days before transplantation,is to reduce the circulating anti-A/B antibody levels to achieve titers between 1:8 and 1:32.
Among the techniques used to induce desensitization,the apheretic techniques are those with the most relevant evolution since the beginning.
The simpler,cheaper,but less effective technique has been the therapeutic plasma exchange using highly permeable membranes.The procedure eliminates approximately 20% of the antibodies by session.Additionally,removes protective antibodies and coagulation factors.
The double filtration plasmapheresis is performed by two plasma filters,and the second one allows smaller molecules to return back to the patient,avoiding many complications associated with the therapeutic plasma exchange.
A further evolution has been made by the use of columns for the selective removal of humoral factors by IA.
There are different types of columns for IA.
IA with immobilized antibodies:are the most widely used.A Therasorb column contains polyclonal sheep anti-human IgG antibodies and is effective in removing IgG antibodies.A different IA technique uses immobilized staphylococcal protein A.These Immunosorba columns,that contains staphylococcal protein A bound to sepharose is effective in removing IgG of different classes.Additionally,are able to induce a B cell apoptosis,so enhancing the immunosuppressive effect.Another IA technique uses immobilized antigens and synthetic epitopes and is the most specific technique in removing only the undesirable antibodies[20].
Immunomodulation
This technique consists in the administration of high doses of polyclonal intravenous immunoglobulin (IVIG) to the patient before transplantation.The aim is to replace the patient’s immunoglobulins lost because of apheresis techniques.In addition,IVIG blocks the Fc receptor and has immunoregulatory properties.
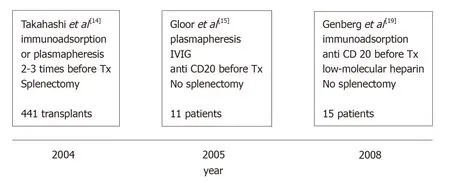
Figure 1 Main steps in the development of desensitization.
B cell depletion
B cells produce isoagglutinins,and their reduction is essential to obtain effective desensitization.
Historically,splenectomy has been used principally in Japan for B cell depletion.To date,RTX,a humanized monoclonal antibody that binds to CD20 expressed on the B cell membrane,is the drug used to obtain B cell depletion.
Independent of which strategy is used,the common aim is to reduce the agglutinin titer to a predetermined level.
When exposed to a low ABO antibody titer,the transplanted kidney develops in approximately 2 wk the capacity to resist complement-mediated damage.The phenomenon of lack of rejection in the presence of circulating ABO antibodies complement activating is known as accommodation[21,22].Accommodation is also responsible for kidney protection over a long period of time.
In the literature different protocols exist that have been changed over time.Initially,the most frequently used technique was the removal of circulating anti-ABO antibodies by plasmapheresis[11,12].The technique is effective but has the disadvantages of removing blood components that are useful to the patient and causing coagulation disorders.More recently,principally in Europe,plasmapheresis has been replaced by the technique of the IA of isoagglutinins using specific protein A or anti-human Ig columns[23-25].The main advantages of this technique are to its high capacity to induce ABO agglutinin removal with high biocompatibility and without complement activation.
Since then,almost all desensitization strategies used the administration of IVIG to improve immunomodulation and the administration of RTX to avoid splenectomy.
By 2007,Genberget al[26]reported 15 transplants between ABOi pairs that were compared with 27 transplants between ABO compatible (ABOc) pairs.Incompatible recipients were treated with IA and a single dose of RTX (375 mg/m2of body surface)30 d before transplantation.The day before transplantation,the patients were given IVIG at a dose of 0.5/kg body weight.
There was no significant difference in patient and graft survival rates or in the rejection episode rates.The patient follow-up was as long as 3 years,and at 3 years,the same estimated glomerular filtration rate was observed in both groups.
However,in a different subsequent study,the same authors[27]observed a harmful antibody rebound after the interruption of IA and suggested that patients should be carefully monitored both before and after transplantation.
Similar results with the same preconditioning therapy were reported by Donaueret al[28]in 11 transplants involving ABOi and by Oppenheimeret al[29]in 11 transplants involving ABOi.
Figure 2 represents the scheme of the treatment generally adopted.
Japanese physicians were the only researchers who continued with splenectomy as a preconditioning protocol up to 2005[30],because in Japan the use of RTX was not allowed by the National Health Service.
Since 2005,the preconditioning protocol in Japan includes RTX,and two studies,among others,reported excellent results[31,32].
As the number of renal transplantations involving ABOi increased with time,several attempts were made to modify the desensitization protocols to obtain better results,to reduce the desensitization-related complications or to reduce the costs.Attempts to reduce the RTX doses were made[33,34],as well as attempts to completely omit RTX administration[35,36].Similarly,the number of IA sessions given pre or posttransplantation were reduced,the former according to the initial titer of anti-ABO antibodies,and the latter not following a preformed scheme but performed according the real clinical need,the so-called on-demand strategy[37].In addition,Morathet al[38],in an attempt to reduce the costs,successfully used reusable non-antigen specific IA devices on 12 patients.The authors obtained excellent results,with an additional depletion of HLA antibodies when present,at reduced costs.
More recently,at the Guy’s Hospital,Barnettet al[39]adopted a tailored desensitization strategy in pairs with ABOi.The different strategies were adopted according to the initial anti-ABO antibody titer (Figure 3).With a titer of 8,only RTX was used;with a titer between 16 and 64,plasmapheresis was added to RTX therapy;and with a titer >64,IA and RTX were used.In total,62 kidney transplants involving ABOi were performed using this strategy,and the results were compared with 167 ABOc transplants.No difference was observed between the two groups in allograft and patient survival rates at 1 and 3 years or in the ABMR rates (Figures 4 and 5).These results highlight that a tailored desensitization strategy obtains good results with personalized therapy and lower costs.
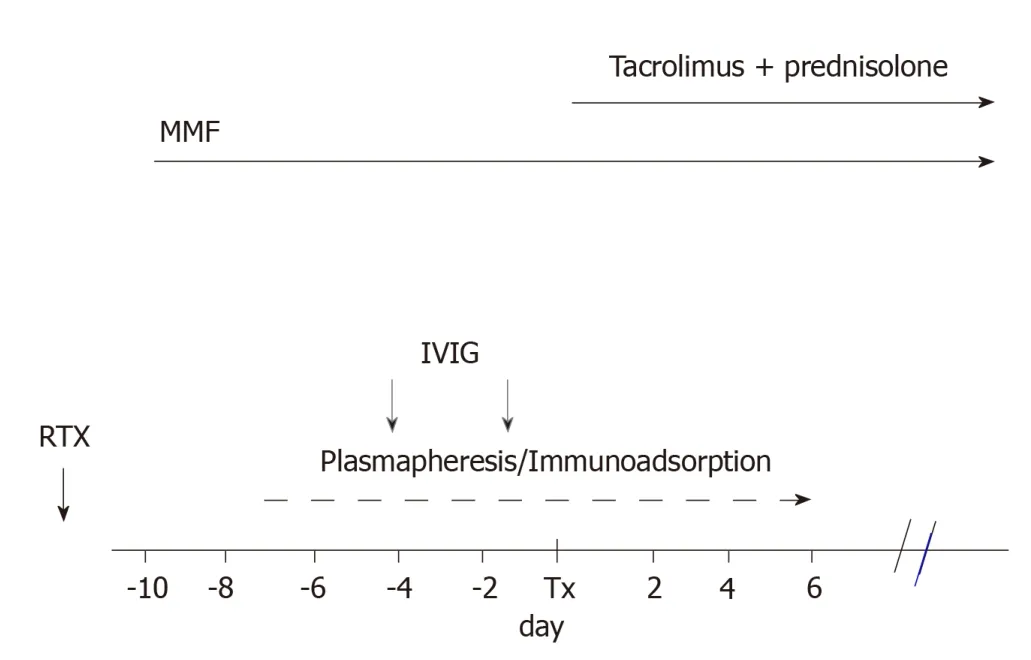
Figure 2 Scheme of desensitization treatment.
CLINICAL OUTCOMES AFTER RENAL TRANSPLANTATION INVOLVING ABOI
The results obtained after renal transplantation involving ABOi and reported in this review are generally good and overlap with the results obtained in ABOc renal transplantation.However,the results reported generally refer to studies with a low number of patients,with the exception of Barrett’s study[39].
In recent years,two systematic reviews and meta-analyses have been published and shed more light on the clinical outcomes of kidney transplantation involving ABOi.The review by Loet al[40]examined 83 studies (54 case reports and series,25 cohort studies,2 case control studies and 2 registry studies).Overall,4810 kidney transplants involving ABOi were examined.
The overall results are reported in Table 1.The main limitation of the review is the lack of randomized controlled trials.As a consequence,the systematic review does not allow us to compare the different therapies.The review only highlights the possibility that RTX and IA could have a superior efficacy,although this point remains to be confirmed by randomized controlled trial.Further research should be conducted on which preconditioning therapies should be adopted in patients with low pretransplant anti-ABO antibody titers[41,42].
The same study evaluates the grade evidence of the studies for patient and graft survival according the preconditioning therapies (Table 2).
Overall,the evidence of the benefits of the different preconditioning therapies is low,although it seems that patients receiving the newest therapies have a better outcome with fewer severe side effects.
A more recent and wider systematic review gives different results[43].This review examines 7098 renal transplantations involving ABOi.

Table 1 Meta-analysis of 4810 ABO incompatibility kidney transplants:Clinical characteristics and outcomes of recipients[40]

Table 2 Meta-analysis of 4810 ABO incompatibility kidney transplants,grade evidence profile of studies for patient and graft survival after preconditioning therapies[40]
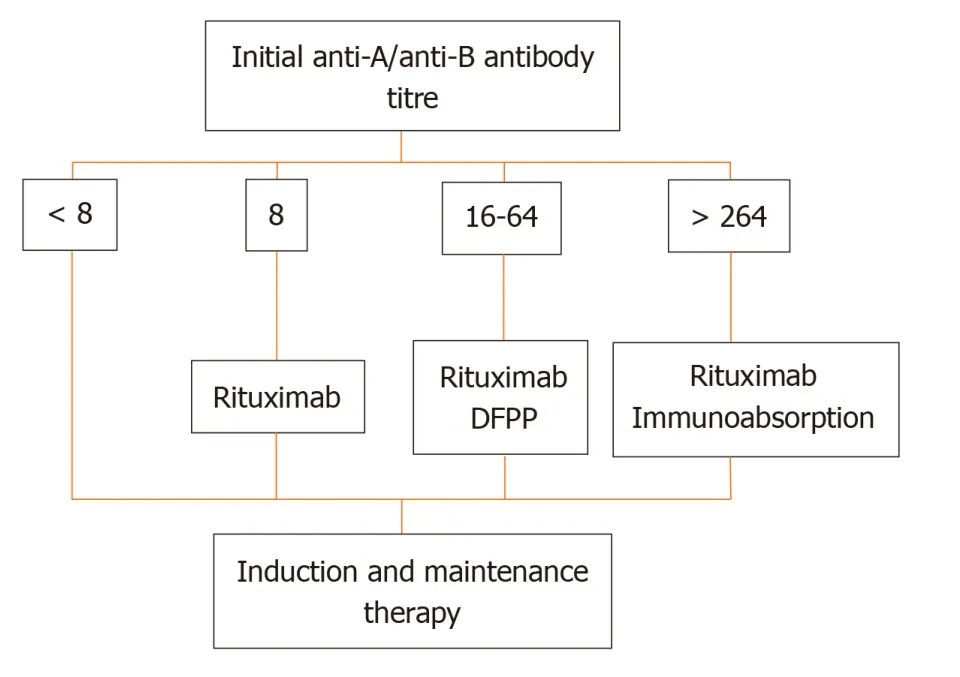
Figure 3 Tailored desensitization strategy.
Overall,transplants involving ABOi compared to those involving ABOc had a significantly higher mortality rates at 1,3 and 5 years (odds ratio 2.17,1.89 and 1.47,respectively;P<0.0001).
Both graft losses and mortality in transplants involving ABOi became equivalent to ABOc transplants at 8 years after transplantation.
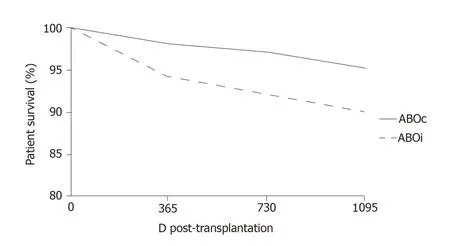
Figure 4 Kaplan–Meier survival curve of patient survival at 3 yr.
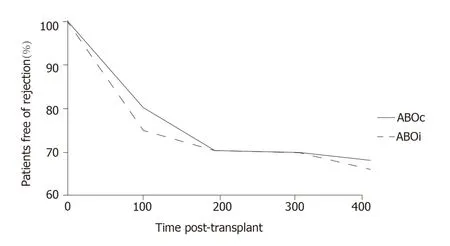
Figure 5 Kaplan-Meier survival curve of rejection free survival post-transplant.
The higher mortality rate is probably a consequence of the high immunosuppression related to the therapy.Excess immunosuppression is the cause of severe bacterial or viral infections[44-47].
Coagulation disorders were also frequent in transplant involving ABOi.This complication is probably related to the modifications in the coagulation system induced by plasmapheresis or IA[48,49].The use of a high dose of RTX or the use of other immunosuppressants to reach a higher desensitization was also associated with a higher mortality rate due to infectious disease.
Polyoma virus nephropathy was also more frequent in patients receiving RTX,as documented by several authors[50,51].
All of these findings on the higher mortality rate in transplants involving ABOi,probably related to the side effects of high immunosuppression are in favor of tailored immunosuppression.Indeed,some studies in which selected patients received a lower,tailored desensitization therapy reported an improved survival rate,with fewer infections and without ABMR[52-54].
OPEN CONTROVERSIES IN KIDNEY TRANSPLANTATION INVOLVING ABOI
The different and discordant results reported by the different authors highlight the controversies that are still open in kidney transplantation involving ABOi.The vast majority of controversies concern the strategies of desensitization.As mentioned above,desensitization aims to modify the immunological reaction in different ways:(1) Antibody reduction by plasmapheresis or IA;(2) Inhibition of antibody production by splenectomy (old system) or by RTX;(3) Pleiotropic action of IVIG;and (4)Complement inhibition by eculizumab.The most common controversies are shown in Table 3.
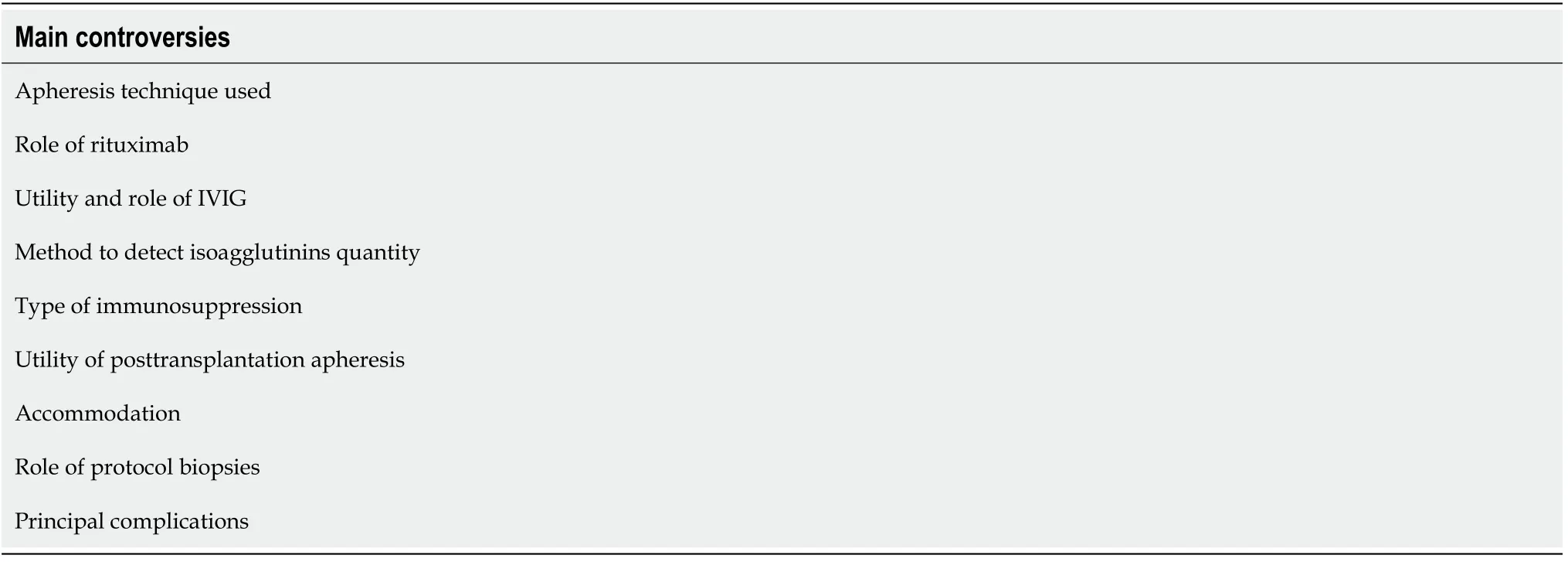
Table 3 Main controversies encountered in ABO incompatibility renal transplantation
Technique of apheresis
Specific columns used for IA are more efficient with fewer side effects than nonspecific columns[55,56],whereas non-specific columns for IA are less efficient but have a lower cost[57].The costs of IA are 2-3 times higher than those of plasmapheresis.Some studies[58]have suggested the reuse of columns to reduce the costs.
The number of apheresis treatments differs according to the basal levels of isoagglutinins and the center.Almost all centers try to reach a titer of 1:8 before transplantation.
A relevant question is what to do with patients with a low titer of isoagglutinins.Several centers have reported good results in these patients with few or no apheresis treatments[59].However,patients with low isoagglutinin titer could have beneficial effects from apheresis treatment because the reduction in inflammatory molecules inflammation present in the circulation.
Role of RTX
The use of RTX has allowed the avoidance of splenectomy since 2002.The use of RTX in kidney transplantation involving ABOi does not seem to be necessary in all patients.In the meta-analysis already mentioned[40],only 35% of patients were treated with RTX.The use of RTX reduces the risk of isoagglutin rebound and the risk of ABMR.In addition,the risk of chronic rejection seems to be reduced by the use of RTX[60].
The RTX dose is generally 375 mg/m2and its major effect occurs between 3 wk and 6 mo after administration[61].RTX administration generally begins 1 mo before the transplantation.As RTX may be removed by plasmapheresis,this technique should be avoided after RTX administration.
IVIG
Among the various effects of IVIG on the immune reaction,the most common are:(1)Blockage of the Fc receptor on the leukocyte membrane;(2) Inhibition of the complement activation;and (3) Inhibition of circulating antibodies against HLA.
In transplants involving ABOi,the IVIG administration reduces the antibody rebound after transplantation and the risk of ABMR[62].
The most common method of administration is to give 500 mg IVIG/kg body weight on the day before transplantation.Some authors prefer to divide the IVIG dose and to administer IVIG in two different sessions 4 d and 1 d before transplantation to avoid an excessive volume charge and the possibility of administering isoagglutinins together with IVIG[63,64].
Isoagglutinin quantification
To decide on the immunosuppression and in particular the apheresis technique to be used,it is necessary to know the isoagglutinin titer.
The best method is flux cytometry[65],even if the method has a high cost.The tube and gel techniques for ABO antibody titration are also frequently used[66].
However it should be highlighted that both the antibody basal titer before desensitization and the post-transplantation titer have a low predictive value for ABMR[67].
Immunosuppression
Apart from desensitization,the immunosuppression in kidney transplantation with ABOi is similar to that used for standard transplantation.The basal immunosuppression begins together with the desensitization.
The possibility of steroid withdrawal is discussed.Some studies[67]have documented a high risk of ABMR in the case of steroid withdrawal soon after transplantation.Other studies have documented a high risk for acute rejection even in the case of late withdrawal[68].Overall,patients receiving transplant involving ABOi are not allowed to reduce immunosuppression.
Post-transplantation apheresis
Apheresis treatments post-transplantation are reserved for patients who present abnormalities of graft function together with isoagglutinin rebound[69,70].According to several authors[71],the treatment by apheresis post-transplantation does not attain beneficial effects in patients without graft dysfunction.However,other authors perform post-transplantation apheresis in patients with isoagglutinin rebound or with high basal levels of antibodies[56].
Accommodation
We have mentioned earlier that,in the presence of circulating antibodies and antigens on the graft cell surface,the graft itself may not be rejected.This phenomenon is called“accommodation”.To date,accommodation is frequent in the case of transplantation with ABOi[72],even if its role is discussed[72,73].
Accommodation in renal transplantation involving ABOi has been defined as the presence of circulating isoagglutinins and antigens on the graft cells with normal renal function and normal histology[74].By 2006,the American Society of Transplantation established a consensus on the accommodation status and added the presence of C4d on the peritubular capillaries[72].
The pathogenesis of the accommodation seems to be due to the presence of a low titer of antibodies,with low affinity and with the blockage of complement activation[22,75,76].Under these conditions,endothelial cells develop a phenotypic change according to which they become resistant to antibody damage.
In the transplantations involving ABOi,the accommodation is facilitated by the reduction of the isoagglutinin level,by the blockage of complement activation and by RTX.IA-specific columns may facilitate the phenomenon[77].
Eculizumab,a monoclonal antibody against C5,may facilitate accommodation.In addition,eculizumab may allow a safe transplantation in patients with ABOi with high antibody titers[78]and may represent a rescue therapy in the case of severe ABMR[79,80].
Role of protocol biopsies
In one study of 33 patients with ABOi undergoing transplantation and with a protocol biopsy at 1 and 2 years,C4d in peritubular capillaries was found with no sign of ABMR or transplant glomerulopathy[81].Similarly,a different study compared 226 ABOc transplantations with 101 transplants involving ABOi.All patients received a protocol biopsy at 3 and 12 mo after transplantation.No difference was found in the histological aspects[82].
In summary,the presence and the role on protocol biopsies of C4d in transplantations involving ABOi seems to be related to accommodation when found in the absence of clinical or other histological abnormalities.
Complications
Surgical complications in transplantations involving ABOi are similar to complications of standard transplants,with the exception of hemorrhages.Hemorrhages are likely to be ascribed to the apheresis treatments[46].In one study[49],29% of patients needed transfusions,and 3 patients needed a second surgical intervention.
The hemorrhages were significantly associated with the number of IA sessions.
The incidence of infectious complications is different across the studies.The difference is probably related to the intensity of desensitization strategies (the number and type of apheresis treatments and the dose of RTX,IVIG and other immunosuppressants).
ABMR is the first cause of graft loss in transplantations involving ABOi[83].The risk for ABMR is related to the isoagglutinin level at transplantation and to the presence of anti-HLA antibodies[84].
The incidence of ABMR ranges between 10% and 30%[72].In the meta-analysis of Loet al[40],the incidence of acute rejections was 32.9%,most of which were ABMR.ABMR principally occurs in the early post-transplantation,usually in the first 2 wk.
KPD
The hypothesis of overcoming the immunological barriers in a different way without the desensitization of the recipient was first proposed by Rapaport[85]in 1986.
The simplest model of the KPD is the two-way,in which two pairs of donors and recipients with ABOi exchange the kidneys,thereby resolving the incompatibility[86-88].The model may also include a higher numbers of pairs realizing the KPD at three-way,four-way,and so on[89].
Several programs exist worldwide.A recent paper from Toewset al[90]compared the experience of Australia,Canada and the United States,but to date,other countries such as Holland have a well-developed KPD national program.
With the increasing number of KPD programs,it has been possible to achieve a global kidney exchange.Starting from a single-center experience,a state wise experience was realized followed by a national program and an international program[91-94].
A further increase in kidney exchanges was realized by the domino KPD,which start from an altruistic donor[95]and finish with the donation of a kidney to a recipient on the waiting list.A variant of domino KPD is the Never Ending Altruistic chain,which allows a higher number of recipients to be transplanted by the use of bridge donors[96-98].
KPD programs have several points that yet need to be better clarified.The principals are as follows:
Transportation of kidneys vs donor travel:This happens when two or more transplant centers are involved.The travel of the donor has a cost,and the donor will be operated on by an unknown surgical team.
The travel of the organ minimizes the costs and allows the continuity of donor care.
Simultaneous vs no simultaneous exchanges:Simultaneous donation has the advantage that no donor will change his mind.The drawback of simultaneous donations is logistical,principally if multiple organ donors will be operated on in the same center.
Closed chains vs open chains:The channels of transplantations initiated by a nondirected donor may be extended but should end with donation to a recipient on the waiting list[99].
MAKING A CHOICE BETWEEN DESENSITIZATION AND KPD
An answer to this question is difficult to be given.Indeed,given the fact that KPD is generally cheaper with respect to desensitization,it remains to be answered the availability of KPD in a short time.This fact depends by the hospital or the national organization.The kidney exchange may base on a single center strategy,on a regional strategy on a national or international strategy[100].According the strategy adopted and how it functions may be predicted the time lapse for receiving a transplant.This is indeed what really the patient likes to know.According our experience a single center or a regional exchange may facilitate finding a transplant by KPD.It should also be highlighted that looking at the data of the National Kidney Registry in the United States,overall the KPD is growing,while the desensitization strategy is dropping[101].
CONCLUSION
To date,the ABOi renal transplantation is possible because of the reconditioning strategies available.Many reports document outcomes similar to the standard transplantations.
The immunological barriers represented by the different ABO groups have been overcome.The two mechanisms responsible for this success are the accommodation and the achievement of some humoral tolerance.The most important techniques are those of desensitization and of isoagglutinins removal.In this field a relevant role have the immunoadsorption and the use of anti CD20 antibodies that allowed avoiding the splenectomy.
Several questions remain to be answered as which is the best apheresis technique to be used and which is the exact role of RTX.In addition,it remains to be better understood the significance of C4d deposition in protocol biopsies.However,this seems to be an aspect and the consequence of the accommodation.
Finally,it should be also considered that though the ABOi transplants are more expensive than the ABOc transplants,however they are less expensive than dialysis.In particular a study from Medicare in the United States compared the costs for living donor in the case of ABOc pairs and ABOi pairs with desensitization[102].The costs were obviously higher in the case of ABOi transplant and evaluated in $ 65080 per transplant episodevs$32039 per transplant episode for ABOc transplant.The difference was principally in the transplant cost and in the 1styear follow-up.We may argue that the costs in the case of KPD are similar to that of ABOc transplant,also depending from the organization:localvsnational.
For this reason also,programs of paired kidney donation are worldwide evolving.However,it should be highlighted that for the paired donation only 31% of the pairs are encountering a possible exchange in optimal conditions.Clearly this percentage represents a mean of different organizations and represents the possibility to find a donor in optimal conditions[103].As aforementioned,KPD is differently organized worldwide,but should be highlighted that KPD has substantially increased and has the potential to increase the number of transplants.In a recent conference has been highlighted that a higher number of transplants could be reached if only one wide national registry would be used instead of smaller registries[104].

