Cytomegalovirus infection after liver transplantation
Blanca C Lizaola-Mayo,Eduardo A Rodriguez
Abstract Human cytomegalovirus (CMV) represents the most common opportunistic infection in liver transplant recipients.CMV infections in post liver transplant patients cause significant morbidity and mortality,directly affecting posttransplant outcomes.This review will provide the framework for the surveillance,diagnosis,prophylaxis and treatment of CMV in the liver transplant population.
Key words:Cytomegalovirus;Liver transplant;Immunosuppression;Serostatus
INTRODUCTION
Cytomegalovirus (CMV) or human herpes virus 5 (HHV 5) is a member of theHerpesviridaefamily.It is a double stranded DNA virus that gives the appearance of intranuclear inclusions in infected cells[1].The primary infection tends to happen during the first 2 decades of life[2-4].In immunocompetent patients,the infection can be asymptomatic or self-limited.After the primary infection,the virus remains latent in the body,serving as a reservoir[5].Reactivation and transmission are higher in susceptible individuals,either due to acquired immunodeficiency syndrome (AIDS) or secondary to induced immunosuppression like in the case of liver transplant recipients[5].In developed countries,the seroprevalence of CMV is around 50%,and in developing countries this percentage increases up to 97%[1,3,6].CMV is the most common opportunistic infection in liver transplant recipients,directly affecting graft survival and patient mortality[7,8].Despite the development of diagnostic,therapeutic and prophylactic measures,up to 29% of liver transplant recipients develop CMV disease jeopardizing posttransplant outcomes[9-11].
INFECTION PATHOPHYSIOLOGY
Among adults,transmission occurs through exposure of bodily fluids and tissue including saliva,tears,blood and transplanted grafts[12].The primary infection happens in mucosal epithelial cells and the virus disseminates through circulating CD14+monocytes[13].After the primary infection,the virus remains latent in lymphoid and myeloid cells.In liver transplant recipients without prophylaxis,CMV infection tends to occur within the first three months after transplantation[4].CMV reactivation or infection is directly affected by the serologic status of the donor and recipient,degree of immunosuppression and prophylaxis[14].CMV has a predilection to attack the transplanted allograft amongst other organs – causing hepatitis in liver recipients(mainly infecting hepatocytes and macrophages)[15].It is unclear why CMV tends to attack the transplanted graft,however,it is believed that this occurs as the organ serves as a reservoir that harbors latent CMV which is undetected by the impaired immune system[9].Following transplantation,CMV infection reactivates from latency,starts viral replication mainly in lymphoid-rich organs,and ultimately disseminates through the blood stream to other organs[16].
RISK FACTORS
The biggest risk factor in liver transplant recipients that increases the likelihood of developing CMV disease is the recipient’s CMV-seronegative status[17].CMV infection has a reported incidence of 78%-88% in seronegative recipients (R-) obtaining a seropositive organ (D+) without prophylaxis.This percentage decreases to 13% in seronegative recipients from seronegative donors[8,18].Immunosuppressant drugs block T-cell function and cause severe lymphopenia,increasing the risk of CMV after liver transplantation[19,20].A higher risk has been seen in patients receiving elevated doses of maintenance immunosuppression[21].The use of lymphocyte-depleting agents like alemtuzuman,muromonab-CD3 and anti-thymocyte globulins has been identified as a risk as well[22,23].In contrast,the lowest risk has been seen when utilizing sirolimus and everolimus (mTOR inhibitors)[21,24].The use of lymphocyte-depleting antibodies to treat allograft rejection,has been associated with an increased risk for CMV infection too[25].The risk of CMV disease in liver transplant recipients is higher than in kidney recipients and lower than lung,small intestine and simultaneous heart-lung recipients[26-29].Other risk factors include:donor and/or recipient advanced age,HLA mismatch,immediate graft rejection by itself,impaired humoral immunity and coinfection with other herpes viruses (HSV 6 and 7)[10,30,31].
SEROLOGIC STATUS
Prior to transplantation,all candidates and donors must be tested for CMV using CMV-IgG serology[7].The presence of CMV IgG antibodies through previous exposure catalogs recipients and donors as seropositive[7].Recipients who are CMV seronegative have the highest risk of CMV infection when receiving a CMV-positive organ (D+/R-)with rates up to 88% without prophylaxis[32].A moderate risk is seen in seropositive recipients receiving a seropositive organ (D+/R+)[33].The lowest risk is in seronegative recipients receiving seronegative organs (D-/R-).This last group may still acquire the infection from person to person or from transfused blood products[17].Amongst CMVseropositive recipients,the risk is higher when receiving a CMV+ organ,likely due to superinfection (Table 1).
CLINICAL MANIFESTATIONS
Based on the recently published American Society of Transplantation CMV in solid organ transplant recipient guidelines[7],it is important to standardize disease definitions:CMV infection is the presence of CMV replication regardless of symptomatology.CMV replication can be detected by nucleic acid testing (NAT),antigen testing or viral culture[12].CMV disease is defined as CMV infection accompanied by clinical signs and symptoms.Asymptomatic CMV infection is CMV replication without symptomatology[12].CMV syndrome is characterized by the detection of CMV in blood plus at least two of the following:Fever ≥ 38 °C for at least 48 hours,new or increased malaise or fatigue,leukopenia or neutropenia on two separate measurements,5% atypical lymphocytes,thrombocytopenia and transaminitis three times the upper limit of normal (ULN)[7,9].
Most CMV cases (60%) in post liver transplant patients present as a mononucleosislike syndrome (fevers,myalgias,arthralgias and malaise) along with hematologic abnormalities and hepatitis[34].CMV hepatitis normally presents with jaundice,mixed hepatocellular and cholestatic elevation of liver chemistries (within 3 × ULN),and documented viral presence in the liver tissue[35].This presentation,may be confused with allograft rejection or other causes of hepatitis[36].Few cases of fulminant and granulomatous hepatitis in the setting of CMV infections have been described as well[37-39].Gastrointestinal CMV disease is also common in liver transplant recipients;it is defined as the presence of upper and or lower gastrointestinal (GI) symptoms with documented CMV in tissue.Serum presence of CMV alone is not sufficient for diagnosis of CMV GI disease.It tends to manifest with odynophagia,dysphagia,nausea,vomiting,epigastric pain,bloating,diarrhea and hematochezia[40].The colon is reportedly the most common GI site to be involved (94% of cases)[41-43].
CMV infection has been related with a four-fold increased risk of acute and chronic allograft rejection through the potentiation of alloantigens[44].CMV also has immunosuppressive effects through the impairment of CD4 T cells and macrophages-it also decreases the levels of interleukin 1 and 2 and increases the levels of interferon alpha.All these indirect effects increase the risk of invasive fungal infections,bacteremia,EBV-associated posttransplant lymphoproliferative disease and cardiovascular disease[44,45].Thrombosis of the graft has also been described due to the proinflammatory state that CMV infection causes[25,31].It may even facilitate reactivation and replication of hepatitis C virus[46].
DIAGNOSIS
Serologic diagnosis of the donor and recipient is paramount due to its significant implications in posttransplant outcomes.In the solid organ transplantation cohort,IgM and IgG antibodies have a sensitivity of 90%,antigenemia 80% and CMV PCR of 84% to 100%[47].PCR is the preferred CMV detection method in liver transplant patients[48].
The diagnosis of CMV disease in liver transplant recipients is made through the identification of clinical manifestations including fever,fatigue,malaise,leukopenia,thrombocytopenia and elevation of liver enzymes plus identification of CMV viremia and detection of the virus in target organs[12].Liver biopsy may be warranted due to the increased risk for allograft rejection in the setting of CMV infection[48].The main diagnostic tests utilized are viral culture in cells,detection of viral nucleic acid through PCR and identification of viral proteins through antigenemia essays[16].Urine CMV detection has a poor correlation with CMV disease as it usually represents viral shedding,however it has been identified as one of the first methods to identify CMV replication in CMV seronegative recipients from positive donors[49].Whole blood has been identified to be superior over plasma,peripheral blood leukocytes and peripheral blood mononuclear cells for the quantification of CMV DNA by PCR[50].
As mentioned before,the gastrointestinal tract is the second most common target for CMV infection in the liver transplant population.Endoscopic evaluation of the large intestine with terminal ileum intubation with biopsies is recommended as isolated ileal involvement in the setting of invasive gastrointestinal CMV infection has beenreported[51,52].Endoscopic findings vary from mucosal inflammation to severe ulcerations with the characteristic diagnostic inclusion bodies seen from biopsies taken from the ulcer base[40].
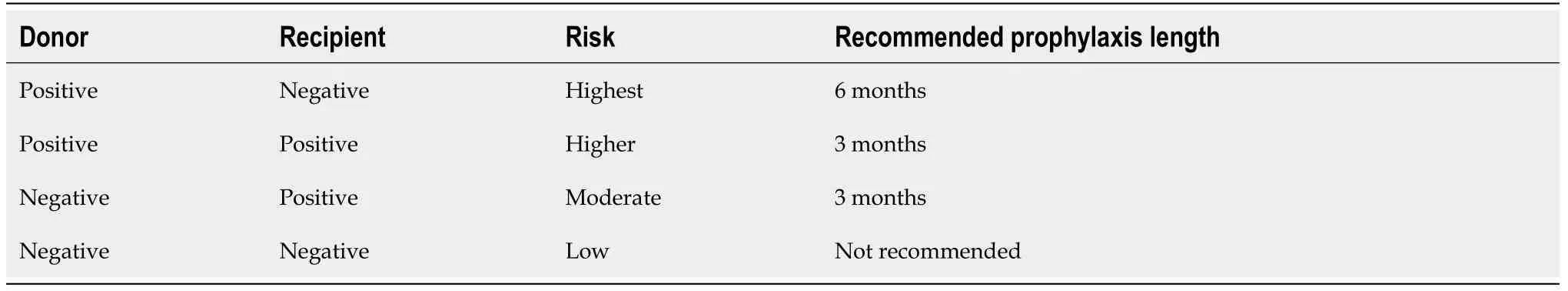
Table 1 Risk of cytomegalovirus infection based on donor and recipient serostatus
THERAPY
Due to its direct and indirect effects,CMV infection prevention is a major strategy in post liver transplant patients.With the use of prophylaxis against CMV,there has been a reported reduction of CMV infection up to 80% during the first 90 days after transplant[53].Amongst D+/R-liver transplant patients,CMV prophylaxis has shown to be superior to preemptive therapy[54,55].CMV prophylaxis should be started within the first 10 days after transplant[7].
Prophylaxis
Oral Valganciclovir and intravenous (IV) Ganciclovir are the antiviral drugs used for CMV prophylaxis[56].A novel viral terminase inhibitor called Letenovir has been recently approved as a prophylactic drug in hematopoietic stem cell transplantation and is under a clinical trial in kidney transplant CMV D+/R-patients(ClinicalTrials.gov NCT03443869)[57].To our knowledge,this drug has not been studied in the liver transplant cohort.Studies have shown comparable safety and efficacy between ganciclovir and valganciclovir[58].Valganciclovir has not been approved for CMV prophylaxis by the U.S.Food and Drug Administration due to a subgroup analysis revealing higher rates of tissue invasive CMV disease in liver transplant patients treated with this drug[56].Regardless,as an oral drug with improved bioavailability,Valganciclovir is still the preferred prophylactic drug over IV Ganciclovir in liver recipients[59].The recommended valganciclovir dose is 900 mg per day which needs to be adjusted in patients with kidney disease[60].The ideal duration in high-risk patients (D+/R-) has not been specifically studied in liver transplant patients,however due to the increased risk of postprophylaxis delayed-CMV disease seen during the first six months,and the decreased rate of CMV infection in a kidney transplant population receiving CMV prophylaxis;most transplant centers apply the prophylaxis for six months[56,60].In CMV R+ patients,prophylaxis is recommended to last 3 months and in D-/R-prophylaxis is not indicated.After conclusion of CMV prophylaxis,it is important to monitor for postprophylaxis delayed-onset CMV disease weekly for three months[7].
Preemptive therapy
Preemptive therapy should be started as soon as CMV viremia (>threefold increase in serum CMV PCR) is identified[61].Oral valganciclovir 900 mg twice a day to be adjusted for renal impairment or IV ganciclovir 5 mg/kg twice a day have a similar reported treatment efficacy of up to 85% in posttransplant patients[58](Table 2).Treatment is advised to be continued until there is a documented negative quantitative CMV PCR.Due to its high sensitivity it is not necessary to confirm two consecutive negative tests anymore[48,62].
CMV disease therapy
For stablished CMV disease,IV ganciclovir (5 mg/kg twice a day) is recommended for severe disease.For mild to moderate disease,oral valganciclovir 900 mg twice a day(renally-dosed) has shown to be equally effective to ganciclovir[58].Foscarnet and cidofovir are considered second line drugs due to its nephrotoxic effects[63].Therapy should be given for at least 2 weeks and discontinuation is based upon virological clearance and resolution of symptomatology[62,64].Persistent viremia after therapy and extensive involvement of the gastrointestinal tract have been identified as risk factors for CMV relapse[65].CMV seroconversion,viral load,treatment duration,maintenance therapy and endoscopic findings at the end of therapy are not significantly associated with CMV relapse[66].
CMV resistance to antiviral drugs is an emerging problem.Resistance happens through mutations of the viral DNA polymerase UL54 and the thymidine kinase UL97[67].The incidence of CMV resistance in liver transplant patients is not well defined,however it is believed that in solid organ transplants in general it is around 7% in high risk patients[68,69].When ganciclovir and valganciclovir are shown to be ineffective due to resistance,patients should be treated with combination therapy,foscarnet and cidofovir or experimental treatments[70].
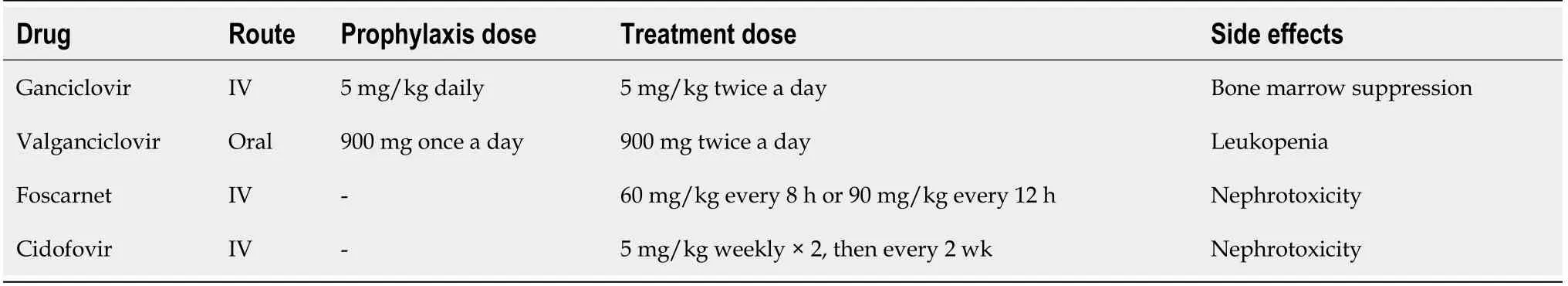
Table 2 Antiviral drugs for cytomegalovirus prophylaxis and treatment in liver transplant patients
CONCLUSION
CMV is the most common opportunistic infection in liver transplant patients.Its direct and indirect effects directly impact post liver transplant outcomes and patient mortality.Pre-transplant donor and recipient serological identification are key to determine posttransplant CMV risk and prophylaxis need.Current therapies have shown to be effective,however ongoing trials are focusing on prevention through vaccines and novel drugs for resistant cases.

