Long-term effect of intrathecal baclofen treatment on bone health and body composition after spinal cord injury: A case matched report
Dora E Ifon, Mina P Ghatas, John C Davis, Refka E Khalil, Robert A Adler, Ashraf S Gorgey
Dora E Ifon, Mina P Ghatas, John C Davis, Refka E Khalil, Ashraf S Gorgey, Spinal Cord Injury and Disorders Center, Central Virginia VA Health Care System, Richmond, VA 23249, United States
Robert A Adler, Medical Service, Central Virginia VA Health Care System, Richmond, VA 23249, United States
Robert A Adler, Department of Internal Medicine, Division of Endocrinology, Diabetes and Metabolism, Virginia Commonwealth University, Richmond, VA 23298, United States
Ashraf S Gorgey, Department of Physical Medicine and Rehabilitation, Virginia Commonwealth University, Richmond, VA 23298, United States
Abstract
Key Words: Intrathecal baclofen; Spasticity; Bone mineral density; Epiphysis; Metaphysis; Spinal cord injury; Case report
INTRODUCTION
Spasticity is a sequela of spinal cord injury (SCI) associated with upper motor neuron disorder[1-3]. Seventy percent or more of individuals with chronic SCI experience spasticity[1-3]. Spasticity is more prevalent in individuals with motor complete or incomplete injury of cervicothoracic origin[3-6]. The most commonly cited definition of spasticity was proposed by Lance in 1980[3,4]and it is defined as, “a motor disorder characterized by velocity-dependent increase in the tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflexes as one component of the upper motor neuron syndrome”[1-3].
Spasticity is recognized for its deleterious as well as beneficial effects on health outcomes after SCI[5]. A mild or moderate spasticity may provide stability for sitting, bed mobility, transfers, standing for assistance with self-care, walking, and grip on assistive devices for self-care or feeding[3,5]. Furthermore, spasticity has been reported to have a positive influence on muscle mass and body composition in persons with SCI[6,7]. Two studies reported preservation of skeletal muscle size, fat-free mass (FFM) and increased resting energy expenditure[6,7]. Gorgeyet al[7]suggested that spasticity maintained FFM and decreased fat mass (FM) in persons with motor complete SCI. Increased FFM has been associated with cardiovascular benefits, improved metabolism, improved muscle strength, and healthier physique[2]. Another study noted that severe spasticity lowered fasting plasma glucose[8]. However, the findings are still not universal and additional studies with large sample size are required to elucidate the role of spasticity on obesity and cardiovascular disease after SCI.
On the contrary, severe spasticity may result in negative consequences on several health variables including functional mobility, quality of life, and it may accentuate lower extremity pain[3,5]. Also, functional limitations from spasticity can increase the risk of contractures, pressure injuries, infections, and falls[3,5]thereby impacting quality of life and may result in social isolation for persons with SCI. A variety of pharmacological and non-pharmacological treatments are available for the control of severe spasticity[9]. However, there is no high-quality evidence for nonpharmacological treatments; hence, pharmacological treatment is favored over nonpharmacological treatment[9,10]. Oral baclofen is generally recommended as first line treatment for spasticity in individuals with SCI[9,10]. However, the relatively high oral doses of baclofen have been associated with undesirable side effects such as dizziness, fatigue, liver toxicity and excessive sedation, which can impair problem solving and intellectual judgement[9,10].
To mitigate these side effects, use of intrathecal baclofen treatment (IBT) has steadily improved over the last four decades and has become a preferred method of managing severe spasticity that is refractory to oral spasmolytic agents. IBT has been associated with fewer side effects compared to oral baclofen, especially when only a small concentrated dose is pumped into the intrathecal space to effectively manage spasticity[10,11]. Intrathecal baclofen treatment requires surgical implantation of a metallic disc (pump) and a catheter under the skin of the abdomen near the waistline. The disc (pump) holds liquid baclofen and a catheter exiting the pump carries the liquid baclofen into the intrathecal space where the other end of the catheter terminates[10]. Numerous studies have reported the efficacy of IBT in relieving symptoms of severe spasticity and improving quality of life after SCI[9-13]. Some have pointed out to the negative consequences of IBT on body composition. In one prospective longitudinal trial, participants experienced increases in fat mass after 12 mo of IBT[8]. In a case report, Nevinet al[11]noted that a 36-year-old woman, who previously lost weight, gained an additional 6 kg within 27 mo of commencement of IBT. This suggests that IBT may predispose an individual to changes in body composition[13], cardiovascular disease and metabolic syndrome, even as early as 12 mo into treatment.
Osteoporosis represents a significant social and economic problem in the SCI population because of the propensity for osteoporosis-related low impact fractures. It is estimated that more than 50% of individuals with SCI will develop low impact fracture during their lifetime[14]. These fractures commonly occur at the distal femur and proximal tibia. Advances in imaging techniques, including upgraded dual-energy X-ray (DXA) software, provide the opportunity to easily study bone mineral content (BMC) and bone mineral density (BMD) at the potential fracture sites. The relatively high prevalence of fractures in persons with SCI, necessitates further scrutiny of potential side effect of long-term IBT on BMC and BMD in that population. This report seeks to aid that process by measuring BMC, BMD and body composition parameters in a long-term IBT user (case) compared to two matched participants.
CASE PRESENTATION
Chief complaints
A 46-year old Caucasian male with C6 SCI American Spinal Injury Association impairment scale (AIS) A presented with inability to walk and lack of use of his arms.
History of present illness
The case has complete tetraplegia as a result of SCI sustained in 1999 from a motor vehicle accident. He had sustained multiple cervical vertebrae (C5-C7) fractures and was classified as having a C6 Level of injury according to the International Standards for Neurological Classification of SCI. An AIS A classification indicates that there is no motor or sensory preservation in the sacral segment S4-S5[15], suggesting no voluntary movement or feeling below the level of injury.
The injury was complicated by severe lower extremities spasticity and neuropathic pain. In 2000, the case was implanted with a Medtronic pump, which allowed the delivery of liquid medication directly into his intrathecal space for management of his spasticity and pain. The pump contains a liquid mixture of Baclofen (for IBT), Bupivacaine and Morphine for pain management. Since commencement of IBT, the case has undergone 6 pumps changes, including one exchange due to a mechanical failure and has refills every 90 d.
History of past illness
No significant past medical history prior to his accident.
Personal and family history
No relevant family history.
Physical examination
At the time of presentation, he weighed 45 kg, measured 1.6m in height and his body mass index (BMI) was 17.5.
Laboratory examinations
Serum 25 hydroxyvitamin D [25(OH)D] was 25.1 ng/mL.
Imaging examinations
DXA imaging were obtained for BMD and body composition parameters.
FINAL DIAGNOSIS
Tetraplegia with severe osteopenia.
TREATMENT
The case continued to receive IBT for management of spasticity. There was no interruption in his treatment regimen. We compared the radiological findings of the case with those of two other participants with SCI, with similar levels of injury, who had never received IBT.
OUTCOME AND FOLLOW-UP
Two matched participants with chronic SCI were identified to determine the effects of long-term IBT on BMC, BMD and leg body composition parameters after SCI.
Matched participant (1); male with C7 AIS B (sensory but no motor function preserved below the level of injury, including sacral segment S4-S5)[15]and with only 2 years since injury. Match (1) was identified to serve as a control to account for the lack of an initial DXA scan for the case report, on the assumption that the match participant (1) would have had similar knee BMD to that of the case at the time of commencement of IBT.
Match Participant (2); male with C7 AIS A (no motor or sensory function preserved below the level of injury, including sacral segments S4-S5), with 13 years since injury. The general consensus is that bone loss accelerates in the first 6 mo after SCI, slows down in 2 years and reaches steady state in 5-8 years[14,16]. The case and matches were matched on the basis of neurological levels of injury (plus or minus one level), approximate age, weight in kg, height in meter and BMI in kg/m2.
Table 1 shows demographics, neurological characteristics, and treatment for the three participants (case and matches). The three participants were recruited from a convenience pool as a part of a parent clinical trial registered at clinicaltrials.gov (NCT01652040)[17].
Procedure
DXA scans:DXA scans were conducted by a trained investigator using the total body and dual knee modules of GE Lunar iDXA system (GE Healthcare Lunar, platform WI). These scan modules were used to capture BMC and BMD data for total body, legs, distal femur, and proximal tibia. Calibration was performed using a phantom calibration box, simulating human tissues to certify scan reliability and precision. All metals were removed from all participants (case and matches) before placement in a supine position on the scanning table. Arms were internally rotated in with the palms facing medially. A trapezoidal foot positioner was used to hold the feet in place during knee scans. The trapezoidal foot positioner kept the legs internally rotated to ensure minimal overlap between the tibia and fibula. A foam block was placed underneath each knee joint to increase stability during scanning. The scan starting point was identified by placing the DXA laser pointer four fingers breadths (10 cm) from the distal border of the patella.

Table 1 Physical and spinal cord injury characteristics of the case report and case-match (1 and 2)
Knee images analysis
Bone mineral content (BMC) and bone mineral density (BMD) values were computed at regional knee areas which correspond to anatomical sites of knee fractures in persons with SCI, using the manufacturer software Lunar EnCore version 16 (GE Healthcare, Madison, WI)[18]. Previously published work showed controversies about anatomical sites that are likely to contribute to knee fractures after SCI. To obviate such controversies, we have decided to assess bone at metaphysis[19]and epiphysis[20]of the distal femur and proximal tibia.
The calculations of BMC and BMD were performed for the distal femur metaphysis, distal femur epiphysis, proximal tibia metaphysis and proximal tibia epiphysis (Figure 1B). For the proximal tibia metaphysis, a rectangular region of interest was drawn with its height set at 7% of femur length, width set to include only tibial bone (excluding fibula) and its proximal edge positioned at the uppermost point of contact between the fibular head and the tibia (Figure 1B). For proximal tibia epiphysis, a rectangular region of interest was drawn with its height set at 10% of tibial length, width to include bone at tibial and fibular heads with its lateral edge located at the tibial plateau.
For the distal femur metaphysis, a rectangular region of interest was drawn with its height set to match the proximal tibia metaphysis, width to include femoral bone and its lateral end positioned at a distance of 13% of femoral length extended up from the lateral condyle. For distal femur epiphysis, the rectangular region of interest was drawn with its height set at 10% of femoral length. The width included the femoral epiphysial bone with its lateral edge positioned at the tibial groove (Figure 1B).
Manual correction, using a brushing tool within the software was used to assign bone pixels that were not automatically detected by the software (Figure 1A). Previous work showed that in chronic SCI group, the root-mean-square coefficient of variation (RMS-CV) values for BMD were 3.12, 4.70 and 3.40%, for the distal femur epiphysis, distal femur metaphysis and proximal tibia epiphysis, respectively[21]. The RMS-CV values were determined for the three knee regions using an existing DXA forearm acquisition algorithm against quantitative computed tomography-derived volumetric BMD. In the current case report, we have applied the above thresholds to determine whether the magnitude of the changes in BMD are clinically meaningful or not following IBT.
Results
The physical and SCI characteristics of the case, and the matched controls are presented in Table 1. There were no remarkable differences in physical characteristics among the three participants, except for the age of one of the two matched participants based on the design of the study. All participants were of approximate level of injury and similar ethnicity. The case was matched to two participants with different times since injury (2 and 13 years) and similar neurological level of injury. Serum 25 hydroxyvitamin D [25(OH)D] was 25.1 and 50.2 ng/mL for the case and matched 2, respectively, before enrollment in the study.
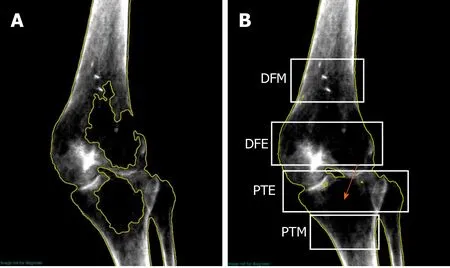
Figure 1 Regional left knee scan for case using GE software (Encore v.16).
Knee BMC and BMD
DXA-based BMC and BMD values of the three participants (case and matches) are presented in Table 2, Figures 2 and 3. Figure 2A and B displays the DXA-based BMC and BMD of the right and left distal femur metaphysis and epiphysis, respectively. Figure 3A and B displays the DXA-based BMC and BMD for the right and left proximal tibia metaphysis and epiphysis, respectively.
The case showed a remarkably lower BMD as compared to match 1 for the distal femur, with average percentage difference of 113% for both legs epiphyses and metaphyses; moreover the case showed lower BMD as compared to match 1 for the proximal tibia, with average percentage difference of 78.1% for both legs epiphyses and metaphyses.
Furthermore, the case showed lower BMD as compared to match 2 for the distal femur, with average percentage difference of 45% for both legs epiphyses and metaphyses, with no noticeable change in proximal tibia BMD.
Total body and leg composition assessments
Leg and total body BMC, lean mass and fat percentage for the case and matches are presented in Figure 4 respectively. Table 3 presents total body and leg composition assessment for the case compared to the matched participants with complete SCI. There were remarkable differences in leg and total body composition between the case and both matches, clearly in legs % fat where the case was 27.1% and 16.5% higher compared to match 1 and match 2 respectively. Moreover, the case was 17.4% and 11.8% lower in leg %lean mass compared to match 1 and match 2, respectively.
DISCUSSION
Knee BMC, BMD and body composition parameters were evaluated in a 46-year-old male with a C6 AIS A SCI, who had IBT implantation for approximately 20 years for management of severe spasticity. The case was matched to other two male participants with similar neurological level of injury and different times since injuries for the purpose of comparison. The first match with 2 years post-injury was selected with similar age and time since injury, to the case at the time of IBT implantation. This approach was adopted to account for the lack of initial DXA scan at the time of IBT implantation in the case. In addition, it presumably provides a reference for longitudinal changes in knee bone and body composition parameters compared to the case and match 1.
Despite the differences in the time since injury between the case and match 2 (20vs13), the observed changes in BMD and body composition support our hypothesis that long-term IBT may negatively influence bone and body composition parameters in persons with SCI. Bone tissue has dynamic turnover as determined by a delicate balance between osteoblastic and osteoclastic cells activities. This dynamic balance is disrupted following SCI and is likely to reach a steady state from 5-8 years postinjury[20]. Following the first 2 years of injury, bone loss continues with both cortical and trabecular bones reacting differently to injury, and probably contributing to low impact fracture risk at the distal femur or proximal tibia. Since the changes are speculated to reach a steady state at 5-8 years post injury, the rate of bone loss at 13 years compared to 20years post-injury should be reasonably comparable between the case and match 2.

Table 2 Bone mineral density and bone mineral content for knee metaphysis and bone epiphysis of distal femur and proximal tibia in a case report with intrathecal baclofen treatment implantation and the case-matches with complete spinal cord injury
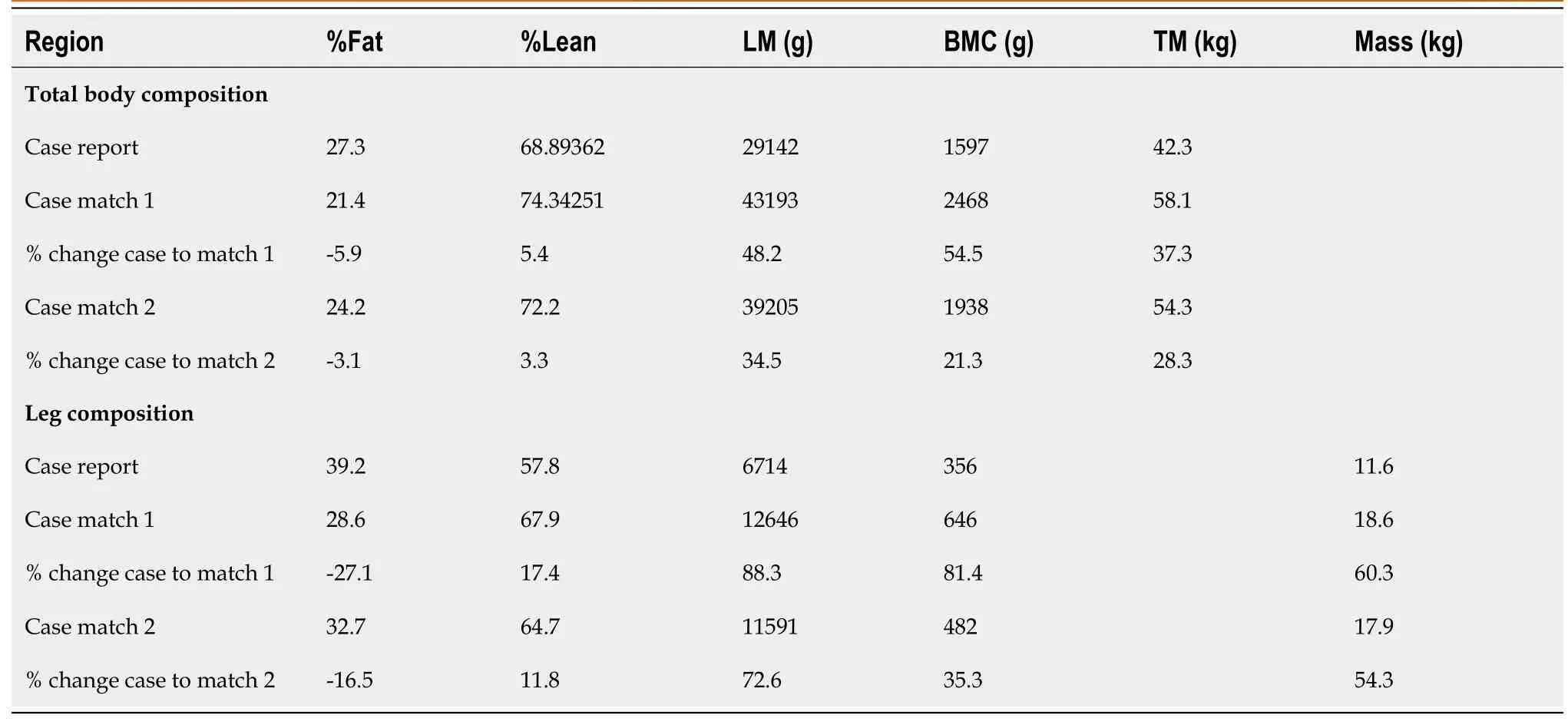
Table 3 Total body and leg composition assessments using dual-energy X-ray in a case report with IBT implantation and case-matches with complete spinal cord injury

Figure 2 Bar plots for bone mineral density (g/cm2) and bone mineral content (g) values of distal femur for the case and matched participants.
Major findings of the study
The major findings suggest that long-term IBT may have accelerated bone loss in the case as compared to the matched participants. Knee BMC and BMD were remarkably lower at the distal femur, with no observable changes in BMC in the proximal tibia. We have chosen to present BMC because the areas of the encompassed regions are extremely different between the case and the matches (Figure 1A). Despite the use of DXA-brushing tool, BMC was still lower in the case compared to the matched participants (Table 2). This may explain the unexpected results in BMD of proximal tibia in the case compared to the matches. The unexpected results in the tibial BMD are attributed to lower area of bone in the rectangular region of interest in the case and to his lower body weight. Furthermore, both legs and total body had greater % fat mass as well as lower absolute lean mass in the case compared to the matched participants.
The use of IBT in spasticity
Spasticity impacts about 70% of the entire SCI population[2]. The negative sequelae of spasticity are well recognized by clinicians, researchers and caregivers. In severe cases of SCI related spasticity, IBT has been recommended as a viable therapeutic approach to mitigate the negative consequences of spasticity, especially, in those who failed to respond to standard treatment[9,10]. In one study, participants treated for spasticity with IBT reported less frequent and milder spasticity compared to those treated with oral baclofen[21]. Another systematic review of 8 studies suggested that a recognizable number (162 individuals with a range of 7 to 75 persons in each study) are possibly using IBT for management of spasticity in the SCI population[22]. Nevertheless, the negative side effects of IBT on bone mass and body composition are not well recognized.

Figure 3 Bar plots for bone mineral density (g/cm2) and bone mineral content (g) values of proximal tibia for the case and matched participants.
Positive effects of spasticity
Over the years, researchers have identified positive aspects of maintaining spasticity on muscle mass, bone mass and body composition in persons with SCI. Gorgeyet al[6]noted that thigh muscle cross-sectional area as measured by magnetic resonance imaging was 22% greater in spastic individuals compared to non-spastic ones just six weeks post-injury. Furthermore, spasticity explained 54% of the variance in muscle size among individuals with incomplete SCI[6]. Another study demonstrated remarkable relationships between spasticity and parameters of body composition in persons with complete SCI[7]. Knee extensor spasticity was negatively related to abdominal circumferences and positively related to both total % fat-free mass and lower FM to FFM ratio[7]. Finally, spasticity may indirectly influence glucose homeostasis, lipid profile and basal metabolic rate by maintaining FFM[7]. It is worth noting that long-term administration of oral baclofen does not appear to attenuate the protective effects of spasticity on body composition and metabolic profile after SCI[23]. Researchers have suggested that spasticity possibly exerted positive effects on muscle mass and body compositionviaincreasing level of circulating insulin-like growth factors (IGF)[24]. Persons with modified Ashworth score greater than 2 had 44% greater plasma IGF-1 compared to those with lower scores[24].

Figure 4 Bar plot for total body and leg composition for the case and matched participants.
Effects of long-term IBT on bone and body composition parameters
There is currently a dearth of knowledge regarding the long-term effects of IBT on bone health in persons with SCI. It is estimated that 50 to 70% of individuals with SCI will sustain low impact fracture during their lifetime[25,26]. If the current findings hold true, then, the number of persons with SCI at risk of sustaining low impact fracture will naturally increase following IBT. The onset of scoliosis or worsening of preexisting scoliosis as a side effect of Lioresal intrathecal baclofen has been reported[27-29]. It has also been reported that BMD is lower in persons with scoliosis compared to controls of similar age group[30]. Therefore, it is possible to assume that IBT may contribute to worsening of BMD of the spine or long bones. However, additional studies are warranted to objectively determine the effect of long-term IBT on bone mass and body composition in persons with SCI.
Finally, recent work identified controversies about the anatomical sites that are likely to contribute to bone fractures in persons with SCI[19,20]. Cognizant of such controversies, we chose to measure BMD at both metaphysis and epiphysis of the distal femur and the proximal tibia. However, both epiphysis and metaphysis measurements of BMD at the distal femur indicated similar findings in the two participants (case and match).
Persons with SCI suffer dramatic changes in the parameters of body composition as characterized by decreasing lean mass and increasing fat mass[7]. In fact, the long-term consequences of altered body composition on metabolic profile is well recognized[17]. In the current study, long-term use of IBT may have negatively influenced body composition parameters in the case compared to the matched participants. It is apparent that the case has a remarkable decrease in total body and leg lean masses and increase in percentage fat mass compared to the matched participants. To determine whether these changes are of clinical relevance, we have contrasted these changes against the reported precision of the regional and whole-body composition of using DXA in persons with SCI[18]. Our findings suggested that the changes in body composition are of clinical relevance and may lead to serious cardio-metabolic comorbidities with aging in the case. Therefore, it is reasonable to speculate that longterm use of IBT may represent a potential risk factor for cardiovascular disease in the SCI population.
Limitations
Several limitations of this study should be highlighted. Firstly, this is a single matched case report; thus, findings should be treated with caution. Secondly, the study participants (case and matches) were all males and there may be gender differences in response to IBT. All efforts were made to match the case and other two participants as closely as possible in all demographics and level of injury, however we were limited to the participants previously enrolled in the parent clinical trial. The findings in this study may be considered a pilot study providing preliminary data for future robust studies to investigate the impact of long-term IBT on bone health and body composition parameters in persons with SCI. Therefore, large cohort prospective studies are warranted to monitor participants, prior to initiation of IBT, and to provide a reasonable follow-up period to objectively assess and further evaluate the changes that were observed in the current case report. Finally, the case was administered baclofen in conjunction with an opioid analgesic to treat aggravating neuropathic pain. The current case report cannot exclude the possible side effects of long-term use of opioid medications on bone health and body composition in persons with SCI.
CONCLUSION
The current case report clearly suggests that long-term use of IBT may affect bone health and body composition parameters in persons with complete SCI compared to matched controls. Compared to match 1, we were able to show longitudinal changes in knee BMD and body composition parameters of the case as well as lower knee BMD and worsened body composition profile compared to match 2.
This observation, though anecdotal, points to the need for larger prospective studies to objectively determine the long-term effect of IBT on bone health and body composition parameters. Recognizing the positive benefits of IBT for severe spasticity, clinicians may need to balance the potential benefits to the risk ratio, especially in young individuals who are likely to use IBT for an extended period of time. Clinicians need to be cautious in management of severe spasticity with IBT before exhausting other rehabilitation modalities; especially in young individuals with SCI. Regular screening of individuals on IBT for cardiovascular and metabolic diseases should be encouraged. Furthermore, it may be possible to consider modulating or titrating the dose of IBT to mitigate such negative consequences among IBT users.
 World Journal of Orthopedics2020年10期
World Journal of Orthopedics2020年10期
- World Journal of Orthopedics的其它文章
- Trochanteric bursitis information on the internet; can we trust the information presented?
- Conversion to reverse shoulder arthroplasty fifty-one years after shoulder arthrodesis: A case report
- Highly cross-linked versus conventional polyethylene inserts in total hip arthr oplasty, a five-year Roentgen stereophotogrammetric analysis randomised controlled trial
- Early clinical outcome and learning curve following unilateral primary total knee arthroplasty after introduction of a novel total knee arthroplasty system
- Mortality following combined fractures of the hip and proximal humerus
- Association of vitamin D and knee osteoarthritis in younger individuals
