Radiographic evaluation of vascularity in scaphoid nonunions: A review
Hena S Cheema, Adnan N Cheema
Hena S Cheema, Department of Diagnostic Radiology, University of Pennsylvania, Philadelphia, PA 19104, United States
Adnan N Cheema, Department of Orthopaedic Surgery, University of Pennsylvania, Philadelphia, Pennsylvania 19104, United States
Abstract
Key Words: Vascularity; Perfusion; Scaphoid fracture; Scaphoid nonunion; Scaphoid open reduction and internal fixation; Bone graft
INTRODUCTION
As the most commonly fractured carpal bone, the treatment of scaphoid nonunions remains a significant clinical challenge for orthopaedic providers. Scaphoid nonunions are defined as fractures that fail to unite by six months after injury and comprise 5%-10% of all scaphoid fractures[1]. Misdiagnosis and under-treatment of scaphoid nonunions may result in continued pain, avascular necrosis (AVN), and chronic joint instability[1](Figure 1). Longstanding instability may increase the risk of developing scaphoid lunate advanced collapse and scaphoid nonunion advanced collapse (Figure 2). Other potential complications include permanent joint deformity, osteoarthritis, carpal tunnel syndrome, and sympathetic dystrophy[2,3]. Management of acute scaphoid fractures ranges are from non-operative immobilization to open reduction and internal fixation (ORIF). In nonunions, ORIF can be accompanied by vascularized bone grafting with reported union rates of 80%-91% in the absence of AVN and 43%-67% in the presence of AVN[4,5]. With non-vasculrized bone grafting, reported union rates have ranged between 90%-97% without AVN[6,7], and 40%-67% with AVN[8]. Fractures at the proximal pole of the scaphoid are at the highest risk for AVN due to its retrograde blood supply, whereas fractures at the relatively wellperfused distal pole typically unite without difficulty[9,10]. Eighty-three percent of the scaphoid bone is vascularized from a dorsal arterial network comprising of the radial artery, dorsal radial carpal arch, and dorsal scaphoid arteries at the proximal pole[9]. The remaining 17% of the vascular supply is provided by a volar network to the distal scaphoid[9]. In the pre- and postoperative setting, careful evaluation of the vascular status of the scaphoid is essential. Plain radiographs, computed tomography (CT), and magnetic resonance imaging (MRI) techniques are frequently used in assessing scaphoid viability, as well as bony bridging and graft integrity postoperatively. However, given the various limitations of these imaging modalities, assessing the viability of the scaphoid presents a diagnostic challenge.This review will summarize and evaluate the current literature on the radiologic evaluation of the vascularity of scaphoid nonunions, defined as greater than 6 mo without evidence of fracture healing, and imaging modalities used to assess and predict revascularization after surgical intervention.
ASSESSING PERFUSION IN SCAPHOID NONUNIONS
Plain radiographs are important in initially screening for nonunions, although further imaging is required for definitive diagnosis. The most common manifestation of poor blood flow to the scaphoid on plain radiographs is varying degrees of sclerosis (Figure 3). However, sclerosis may also signify new bone formation, dystrophic calcification, bone compaction, or even relative osteopenia of adjacent bones resulting from immobilization[10]. As such, the findings on plain radiographs are too non-specific for precise diagnosis.
Intraoperatively, perfusion to the scaphoid is assessed by puncturing the proximal pole to visually inspect for signs of bleeding; this serves as the gold standard to which imaging modalities are then compared. One study compared CT scans to punctate proximal pole bleeding and found that CT assessment of vascular status did not correlate with intraoperative findings[11]. While CT is not helpful in assessing vascularity, it is instrumental in assessing fine osseous details. As such CT is used to determine bony architectural changes, such as the degree of deformity (Figure 4) and level of bony bridging (Figure 5), which are helping in diagnosing nonunions and for operative planning.

Figure 1 Plain PA and lateral radiographs demonstrating a post-traumatic deformity of a proximal right scaphoid with moderate to severe radiocarpal joint narrowing as well as dorsal intercalated segmental instability.
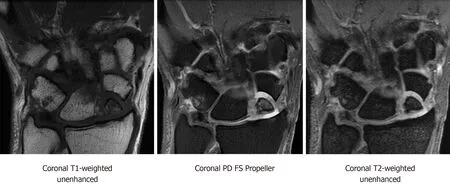
Figure 2 Coronal magnetic resonance imaging T1-weighted unenhanced, coronal proton density fat-saturation propeller, and coronal T2-weighted sequences showing scaphoid nonunion advanced collapse with chronic ununited comminuted scaphoid fracture involving the proximal pole and mid waist.
Bone scintigraphy has also been used to assess scaphoid vascularity, but it lacks sensitivity due to the low resolution of detecting radiotracers (i.e., measures of perfusion), such as Tc-99m hydroxymethylene diphosphonate, in the small carpal bones[12]. Although not very helpful in assessing vascularity, bone scintigraphy may still play a role in detecting occult scaphoid fractures suspected clinically in which other imaging correlates are not available.
MRI is the most reliable imaging modalities for assessing perfusion to the scaphoid in nonunions. Viable bone is characterized by T1-weighted-imaging isointensity and T1-weighted-imaging enhancement with gadolinium (Figure 6). Diagnostic criteria for avascular necrosis includes the presence of hypointensity on T1-weighted and T2-weighted imaging and lack of enhancement on fat-suppressed T1-weighted postgadolinium sequences (Figure 7). In a head-to-head comparison of MRIvsthe gold standard of intraoperative punctate bleeding, hypointensity on non-contrast T1-weighted-imaging of the proximal pole was 72% sensitive and 100% specific, in addition to having a 100% positive predictive value, and 73% negative predictive value for osteonecrosis[11]. Furthermore, 90% of scaphoids with MRI findings in keeping with osteonecrosis were confirmed to have the same finding during surgery[11,13].

Figure 3 Plain PA, lateral and navicular view radiographs demonstrating sclerosis of the scaphoid at the waist and resorption of the proximal pole.

Figure 4 Coronal and sagittal unenhanced computed tomography image demonstrating a chronic fracture involving the distal scaphoid with moderate humpback deformity and dorsal intercalated segmental instability.
If MRI is the best modality in assessing scaphoid vascularity, the logical next question is whether its utility can be enhanced with contrast. One study compared unenhanced MRI to the gold standard of intraoperative punctate bleeding and determined a sensitivity of 36% and specificity of 78%[14,15]. Another study used gadolinium-enhanced MRI and reported a significantly better sensitivity of 63%-66% and 77%-88% specificity[14,15]. As such, contrast enhancement improves the ability of MRI to assess scaphoid vascularity.
Staticvsdynamic contrast-enhancement protocols have also been compared to assess scaphoid vascularity. In one study, 28 scaphoid nonunions were evaluated and found that dynamic contrast-enhancement in fact led to inferior sensitivity and specificity in detecting vascular compromise[16]. Another study evaluated 35 scaphoid nonunions and determined that dynamic contrast-enhancement was superior to static contrast-enhanced MRIs[17]. Given these contradictory findings, the authors postulate that the utility of dynamic contrast-enhancement is dependent on the time from initial injury. Non-unions closer to the 6-mo post-injury timepoint have less contrasting signals between the proximal and distal fragments (i.e., flattening of the time-intensity curves); however, in nonunions several years old, the difference between the proximal and distal fragments is more evident and therefore more reliably assessed on dynamic contrast imaging[17]. Further research is needed to determine the timepoint whereafter dynamic protocols may be more helpful than static protocols in assessing scaphoid nonunions.
POST-OPERATIVE ASSESSMENT OF PERFUSION IN SCAPHOID NONUNIONS

Figure 5 Coronal, sagittal, and axial unenhanced computed tomography images status post treatment of scaphoid nonunion with vascularized bone graft from the distal radius and single threaded screw. There is evidence of bony bridging and integration of the graft.

Figure 6 T1-weighted fat saturated pre-contrast, T1-weighted fat saturated post-contrast, and T2-weighted post-contrast sequences showing preserved vascularity in a proximal pole scaphoid fracture.
Surgical fixation of scaphoid fractures may be performed using internal lag screws, plates, or staples. In nonunions, fixation often requires augmentation with bone grafts, which may or may not be vascularized, to provide the osteoinductive and osteoconductive growth factors important for healing[18,19]. Postoperatively, patients are followed with imaging to confirm graft integration and fracture union.
In the post-operative period, CT is the gold standard for evaluating bone graft integration; however, CT scan does not reliably demonstrate revascularization after vascularized bone grafting[20]. While the presence of a persistent pseudo-arthrosis on CT may be a reflection of inadequate vascular restoration, CT does not directly display the vascular compromise itself[20]. It is also important to remember that failure to achieve union is not necessarily a consequence of inadequate vascularity. For example, conditions such as in hypovitaminosis D or abnormal parathyroid hormone levels can lead to union despite ample vascularity. As such, presence of pseudo-arthrosis on CT does not necessitate inadequate vascularity.

Figure 7 Coronal T1-weighted proton density fat saturation pre-contrast, Coronal T1-weighted fat saturation post-contrast, and coronal T2-weighted fat saturation pre-contrast magnetic resonance imaging sequences demonstrating nondisplaced ununited fracture of the proximal pole of the scaphoid.
Rather than CT, MRI is the primary diagnostic modality in evaluating postoperative revascularization of scaphoid nonunions or identifying vascularized graft failures[21]. One study treated 77 scaphoid nonunions with distal radius vascularized bone grafts and then prospectively followed those patients with enhanced MRIs at 3-mo post-op. In all patients who had clinical signs of union,i.e.,complete to almost complete relief of pain, the MRIs showed restoration of signal in the proximal pole[22]. Another similar study also evaluated post-operative revascularization of 13 scaphoid nonunions after distal radius vascularized graft and compared MRI findings to CT scans, rather than clinical relief of pain. With a minimum of three year follow up, they reported that all patients who did not have restoration of bone marrow signal in the proximal pole on MRI had persistent nonunion on CT scan[20]. These studies confirm the ability of enhanced MRI to detect scaphoid revascularization post-operatively.
PREDICTING NONUNION FORMATION AND POST-OPERATIVE HEALING WITH MRIS
It remains unclear whether imaging may be used to detect acute scaphoid fractures at risk for progression to nonunion. In one study, scaphoid fractures were imaged using fat-suppressed T1-weighted gradient-echo MRI within 2 wk of injury and the proximal pole vascularity was quantified using a custom grading system[23]. The fractures were treated non-operatively and then underwent CT imaging 12 wk post-injury to assess for union. They reported that the quantitative severity of vascular compromise measured on pre-treatment MRIs had no correlation with development of nonunion on follow up CT imaging[23]. As such, the authors concluded that MRIs could not reliably predict which acute fractures would develop nonunion after non-operative treatment.
Similarly, other studies suggest that MRIs also cannot predict which scaphoid nonunions will heal after operative intervention. In a study of nonunions that underwent surgical management, those with more than 50% enhancement of the proximal pole on pre-operative MRI had union rates of 67%. However, nonunions with less than 50% enhancement pre-operatively actually had higher a union rate of 75%, and those with less than 25% enhancement had 50% union rates[24]. As such, no definitive correlation exists between preoperative vascularity and the ability to predict of union in the post-treatment (non-operative and operative) settings. Further research is needed before imaging can be reliably used to make predictions of nonunion.
CONCLUSION
This review provides an overview of the role of various imaging modalities used to assess the vascular status of scaphoid nonunions at either clinical presentation or after surgical intervention. In nonunions, changes associated with vascular compromise are too non-specific for accurate detection by plain radiographs or CT, and bone scans lack the resolution to adequately detect the extent of vascular insult. MRIs are useful for evaluation of vascular compromise, with slightly improved sensitivity and specificity with gadolinium administration. Dynamic contrast-enhancement does not necessarily provide additional advantages over static contrast-enhancement, but this may be dependent on the age of the nonunion. Postoperatively, plain radiographs and CTs can be used to assess bony union. However, MRI is preferred in detecting signal recovery as evidence of bone graft viability. None of our current modalities have been shown to reliably predict scaphoid fractures at risk for progression to nonunion, or the success of operative intervention on achieving bony bridging in nonunions. There is still a need for development of an accurate prognosticator.
A promising tool may be found in T1-rho MRI sequences, which are sensitive in detecting changes in cartilage vascularity and structure. Perhaps, T1-rho MRIs can be used to assess scaphoid cartilage after fractures, and thresholds can be set that define the degree of cartilage change needed to predict which fractures will go on to union or nonunion. Further research is needed to ascertain the role of new imaging modalities like T1-rho MRIs and delineate what constitutes normal or abnormal cartilage blood flow in the setting of acute scaphoid fractures and nonunions.
 World Journal of Orthopedics2020年11期
World Journal of Orthopedics2020年11期
- World Journal of Orthopedics的其它文章
- Does proximal femoral nail antirotation achieve better outcome than previous-generation proximal femoral nail?
- Patients’ perspectives on the conventional synthetic cast vs a newly developed open cast for ankle sprains
- Proximal fibular osteotomy: Systematic review on its outcomes
- Müller-Weiss disease: Four case reports
- Treatment of a rotator cuff tear combined with iatrogenic glenoid fracture and shoulder instability: A rare case report
- Modified surgical treatment for a patient with neurofibromatosis scoliosis: A case report
