Current Management Strategies in Patients with Heart Failure and Atrial Fibrillation:A Review of the Literature
Alex M.Parker,MD ,Juan R.Vilaro,MD ,Mustafa M.Ahmed,MD and Juan M.Aranda,Jr,MD
1 Division of Cardiology,Department of Medicine,University of Florida,Gainesville,FL 32611,USA
Abstract
Keywords:heart failure; atrial f ibrillation; cardiology; electrophysiology
Introduction
Atrial f ibrillation (AF) complicates the assessment and treatment of heart failure (HF) across the spectrum of left ventricular ejection fraction[1,2].While common and challenging in HF with reduced ejection fraction (HFrEF),management of AF in HF with preserved ejection fraction (HFpEF)is particularly diff icult because of the overlap in symptoms [1- 4].Symptoms such as dyspnea,exercise intolerance,and fatigue in the presence of multiple comorbid conditions make assessment of AF and its impact on prognosis more diff icult.While each disease state on its own is inexplicably complex from pathophysiology to treatment,when presenting in tandem the complexity mounts exponentially.
The aim of this article is to present a concise review of the literature and current understanding of the underlying mechanisms of and management strategies for AF in patients with HF.
Mechanisms
The HF syndrome is characterized by elevated left ventricular end-diastolic pressures and left atrial pressures.Long-standing increase in left atrial pressure leads to chamber dilatation and stretching of atrial tissue and pulmonary arteries,which in turn leads to local inf lammation [5- 7].Particularly in patients with HFpEF,accumulation and inf lammation of epicardial adipose tissue may play a key role in the abnormal cell signaling and electrical conduction in the atria [5,8,9].
AF is known to lead to increases in left atrial volume,abnormal left ventricular f illing,and a proinf lammatory state leading to atrial f ibrosis [10,11].Abnormal ventricular f illing and abnormal atrial function can ultimately lead to elevated left ventricular f illing pressures,thus leading to an HF syndrome [12,13].
Tachycardia from AF with rapid ventricular response is known to at times lead to development of a cardiomyopathy [14].Tachycardia-mediated cardiomyopathy is often induced by rapid pacing as a mechanism of developing an animal model of HF[15].This leads to both a decrease in the number of myocytes and intracellular disorganization.In animal models the intracardiac pressures and cardiac output seem to be reversible approximately 48 hours after cessation of rapid pacing; however,even after 4 weeks of cessation of rapid pacing,myocyte dysfunction and diastolic dysfunction can persist.At times it may be diff icult to know which disease developed f irst when both AF and HF are both present at the time of initial diagnosis.
Prognosis
Of all patients with AF,those with HF have decreased quality of life,higher risk of admission to the hospital,and higher risk of death than those who do not have HF [4].In patients with HF,AF is associated with increased risk of poorer outcomes(stroke,HF hospitalization,and death) when compared with those in sinus rhythm [2].This association persists across the spectrum of HF when it is stratif ied by ejection fraction.
In patients with HFrEF,those with paroxysmal AF and new-onset AF appear to be at the highest risk of stroke,HF hospitalization,and death when compared with patients with persistent or permanent AF [1].
Epidemiology
AF and HF represent a conf luence of two common disease states.AF has a prevalence of approximately 3% in the general population and approximately 16.8% in patients aged 75 years or older [16].The lifetime risk of developing AF is estimated at approximately 20% [17].Similarly,approximately 20% of people will develop HF over their lifetime[18].In one study,AF was diagnosed in 35- 40%of patients with HF and reduced ejection fraction[1].AF is estimated to be present in 60% of patients with HFpEF [2].In one population study of patients with AF,the incidence of developing HF was 24%over a follow-up period of 6.1 years on average[19].In patients with a pacemaker or def ibrillator,the presence of subclinical AF is associated with an increased rate of HF hospitalization when compared with patients without subclinical AF even when patients with a history of HF are excluded [20].
Prevention of Atrial Fibrillation in Heart Failure
Multiple risk factors have been identif ied as potential targets for prevention of the development of AF.Diabetes,hypertension,smoking,HF,and myocardial infarction (in men) have been identif ied as independent risk factors for the development of AF[21].
Considering the worse prognosis associated with AF,preventing AF seems to be a reasonable goal when one is treating patients with HF.There appears to be a reduction in the development of new-onset AF in In patients with HFrEF who are treated with beta-blockers and agents targeting the renin-angiotensin-aldosterone pathway [22- 24].This reduction in development of AF is likely due to inhibition of pathologic neurohormonal signaling that occurs in HF.Additionally,increases in left ventricular end-diastolic pressures leading to increase in left atrial pressure and thus stretching may lead to reduced abnormal pulmonary vein electrical activity that leads to AF [25].Thus far,treatment with aldosterone antagonism has not been demonstrated to reduce the development of new-onset AF in HFpEF[26].In patients with reduced ejection fraction,there are multiple reasons,supported by guidelines,to aggressively titrate neurohormonal blockers.
Obstructive sleep apnea has been proposed as a possible contributing risk factor for development of AF,and treating obstructive sleep apnea has been hypothesized as preventative therapy for AF[27,28].Given other benef its from treatment of obstructive sleep apnea in patients with HF,screening and treating patients with HF for obstructive sleep apnea is likely benef icial [29].Whereas treatment of obstructive sleep apnea appears to be benef icial in HF,treatment of central sleep apnea in HF with adaptive servo ventilation may be harmful and should be avoided [30].
Medical Therapy
The initial decision that must be made in a patient with HF who develops AF is whether to perform cardioversion to restore sinus rhythm or maintain a controlled ventricular rate in AF.The AFFIRM trial from 2002 found no difference in overall mortality for rate control versus rhythm control in patients with nonvalvular AF [31].Of note,most patients in the study had normal left ventricular systolic function.The AF-CHF trial was designed to answer whether a rate control stagey or a rhythm control strategy is superior in patients with HF [32].This trial randomized 1376 patients with left ventricular ejection fraction of 35% or less and nonvalvular AF to a rate control strategy with a beta-blocker and digitalis with a targeted heart rate of less than 80 beats per minute,or a rhythm control strategy using electrical cardioversion and antiarrhythmic agents(with amiodarone being the f irst-line agent).While this trial did not identify a difference in all-cause mortality,there was a signif icant degree of crossover as 21% of patients crossed from the rhythm control arm to rate control arm because of inability to maintain sinus rhythm.Approximately 10%of patients assigned to the rate control arm crossed over to the rhythm control arm.As in many other trials evaluating the effectiveness of rhythm control,amiodarone was used frequently.At the 12 month follow-up,82% of the patients in the rhythm control group were being treated with amiodarone.
Of note,the AFFIRM trial (while not a trial of patients specif cially with HF) found that those assigned to a rhythm control strategy had fewer symptoms of HF [33].Indices of right ventricular function,which itself is a powerful prognostic marker in HFpEF,are signif ciantly better in HFpEF patients in sinus rhythm compared with those with AF [34,35].This suggests that maintenance of sinus rhythm may be appropriate in carefully selected patients.
There are other antiarrhythmics that should be avoided in HF for the purposes of maintaining sinus rhythm.Flecainide and propafenone are contraindicated in HF because of negative inotropic properties,increased risk of death,and increased risk of ventricular arrhythmia [36,37].Likewise,dronedarone is associated with increased risk of HF symptoms and death in patients with permanent AF or HF with reduced systolic function [38,39].
The DIAMOND-CHF trial showed evidence that dofetilide is effective in cardioverting AF and maintaining sinus rhythm in patients with HFrEF [40].There was no difference in the primary end point of all-cause mortality.However,although there was no difference in overall mortality,there were 25 cases of torsade de pointes in the dofetilide arm of the trial,15 patients required direct current cardioversion,and two patients died.There were no patients in the placebo group with torsade de pointes.This demonstrates the importance of vigilance in starting dofetilide therapy in a safe monitored environment,using creatinine clearance- adjusted dosing,and monitoring the patient for evidence of corrected QT prolongation.In HF patients with high diuretic requirements who have signif icant f luctuations of their creatinine clearance over short periods,the risks associated with dofetilide as a rhythm control agent may be prohibitive.Dofetilide does appear to be effective in converting AF and preventing its recurrence even in patients with HF when compared with placebo (12% vs.1%).Therefore,in carefully selected patients with adequate renal function,loading with dofetilide in a carefully monitored setting may be an acceptable method of treating symptoms of AF in HF patients.
There are limited data to guide the decision as to when to start treatment with an oral antiarrhythmic for AF in HF patients.A recent retrospective analysis of patients who underwent electrical cardioversion did not identify a statistically signif icant difference among antiarrhythmic agents,although there was a trend toward improved maintenance of sinus rhythm with amiodarone [41].At this point there is insuff icient evidence to recommend that all patients undergoing electrical cardioversion be treated with an antiarrhythmic for initial cardioversion.In general,prior American College of Cardiology/American Heart Association/European Society of Cardiology guidelines for the management of AF have recommended careful consideration of prophylactic drug therapy before electrical cardioversion [42].In patients that are at lower risk of recurrence,it may be reasonable to not use prophylactic antiarrhythmic medications with an initial cardioversion attempt.There is a signif icant absence of data to suggest if this strategy is valid in patients with HF.
Catheter Ablation
Given the potential for adverse effects and drugdrug interactions of antiarrhythmic therapies,catheter ablation of AF offers an appealing option for patients who wish to reduce exposure to these drugs.Catheter ablation of AF typically involves delivery of radiofrequency current or freezing temperatures to the ostia of the pulmonary veins [43].These two technologies appear to be comparable in terms of eff icacy and safety,although in one trial there were a higher incidence of phrenic nerve palsies with cryoablation [43,44].
Randomized trials such as CASTLE-AF aimed to answer whether catheter ablation of AF is superior to medical therapy for patients with HF and AF.In CASTLE-AF,398 patients with AF and New York Heart Association class II or higher HF with left ventricular ejection fraction of 35% or less were randomized to receive catheter ablation of AF or medical therapy [45].Medical therapy consisted of rate or rhythm control,with aggressive attempts to maintain sinus rhythm.The investigators identif ied signif icantly better outcomes in the primary end point (combined death or HF hospitalization)in patients who were assigned to undergo catheter ablation.In this trial,patients in both arms received aggressive guideline-directed medical therapy,with more than 90% receiving renin-angiotensinaldosterone system inhibition and more than 90%receiving beta-blockers.In both arms,approximately 30% of patients received an antiarrhythmic drug,which was primarily amiodarone.
A recent meta-analysis by AlTurki et al.[46] evaluated available randomized controlled trials evaluating the eff icacy and safety of catheter ablation in AF with HFrEF.Their analysis of the seven trials that met the criteria for evaluation suggests better outcomes with catheter ablation compared with medical therapy in terms of all-cause mortality,HF hospitalization,changes in left ventricular ejection fraction,and functional parameters such as the 6 minute walk time and changes in the Minnesota Living with Heart Failure Questionnaire score.
In patients with refractory AF or when rhythm control is not effective,catheter ablation of the atrioventricular node is an option for patients in whom rate control is diff icult or who do not have optimal biventricular pacing after placement of a coronary sinus lead [47].This method of rate control leads to a pacemaker-dependent state by induced complete heart block.For patients without a pacemaker,this treatment option requires the simultaneous insertion of a permanent pacemaker.
Figure1 shows a proposed algorithm for the appropriate treatment of AF in HF patients.It shows the importance of guideline-directed medical therapy and anticoagulation as the key f irst step for treating symptoms and preventing long-term consequences of AF.This is followed by a patientspecif ic decision tree leading to options for rhythm control.
Stroke Prevention
Stroke is perhaps the most dreaded complication of AF,and therefore anticoagulation should be considered in appropriately selected patients with AF[48].Stroke risk reduction must also be balanced by the increased risk of bleeding associated with systemic anticoagulation and must be approached on an individual basis.Risk models to predict the risk of stroke and benef it of systemic anticoagulation includetheCHADS2and CHA2DS2-VASctools[49].Theprincipleofthesetoolsisthat systemic anticoagulation is recommended for patients with a score of 2 or greater in men and 3 or greater in women and should be considered if the score is 1 in men or 2 in women [50].Patients with HF (either with symptoms or with left ventricular ejection fraction of 40% or less) have at least a score of 1;therefore,anticoagulation should at least be considered when AF is present.
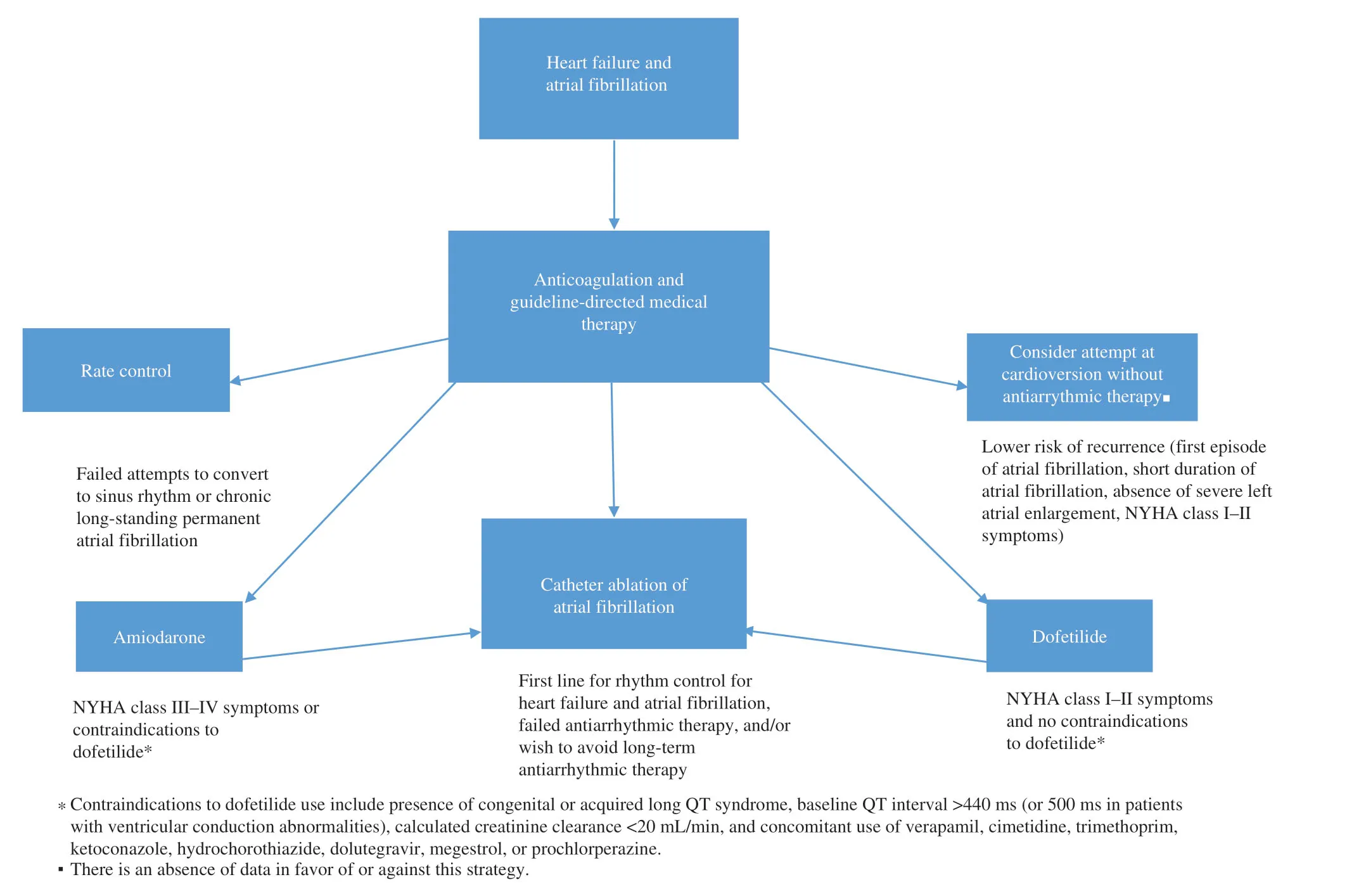
Figure1 Proposed Algorithm for Atrial Fibrillation in patients with Heart Failure.Catheter ablation is superior to medical therapy with improved quality of life,and mortality benef it in patients refractory to initial therapies.
For many years the only available oral anticoagulant with demonstrated eff icacy in reducing stroke in AF was warfarin.When titrated to an international normalized ratio of 2.0- 3.0 warfarin reduced stroke rate by nearly two-thirds [51].Over the past decade,novel oral anticoagulants (namely,the direct thrombin inhibitor dabigatran and the factor Xa inhibitors apixaban,rivaroxaban,and edoxaban)have been developed that in randomized controlled trials have been demonstrated to be noninferior to warfarin for prevention of stroke in AF [52- 54].These agents have an advantage over warfarin in the decreased need for laboratory monitoring,no need for bridging,and decreased food-drug interactions.Meta-analysis of the clinical trials studying these novel anticoagulants suggests that overall these agents have similar eff icacy in reducing stroke and systemic embolic events and reduced rates of major bleeding [55].Despite the benef it seen in patients with AF and HF,patients with HF,coronary artery disease,and no AF did not seem to benef it from low-dose anticoagulation with rivaroxaban at 2.5 mg twice per day in the COMMANDER HF trial,with no signif icantly lower rate of death,myocardial infarction,or stroke when compared with placebo [56].
Summary
Patients with HF and AF suffer from two common but very complex illnesses that when present lead to increased morbidity and mortality.Treatment of AF requires careful thought and expert opinion,even more so in patients with HF.Emerging therapies such as novel anticoagulants and catheter ablation offer new and exciting ways of treating AF and preventing strokes.
Conf ilct of Interest
The authors conf irm that there is no conf lict of interest.
 Cardiovascular Innovations and Applications2020年4期
Cardiovascular Innovations and Applications2020年4期
- Cardiovascular Innovations and Applications的其它文章
- Comparison of Clinical Value between Right Distal Radial Artery Access and Right Radial Artery Access in Patients Undergoing Coronary Angiography or Percutaneous Coronary Intervention
- Epicardial Adipose Tissue in Patients with Obstructive Sleep Apnea:A Systematic Review and Meta-analysis
- Comparison of Segmentation Algorithms for Detecting Myocardial Infarction Using Late Gadolinium Enhancement Magnetic Resonance Imaging
- In-Stent Thrombosis after Antiplatelet Therapy Conversion while Awaiting Coronary Bypass
- lmpact of MitraClip Program on the Volume and Outcomes of Mitral Valve Surgery:A Single-Center Retrospective Study
- Clinical Analysis of Transcatheter Embolotherapy for Congenital Pulmonary Arteriovenous Fistulas in Children
